Rabies primary prevention
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies primary prevention On the Web |
|
American Roentgen Ray Society Images of Rabies primary prevention |
|
Risk calculators and risk factors for Rabies primary prevention |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Mahshid Mir, M.D. [2]
Overview
There is no known cure for symptomatic rabies, but it can be prevented by vaccination, both in humans and other animals. Virtually every infection with rabies was a death sentence, until Louis Pasteur and Emile Roux developed the first rabies vaccination in 1885. This vaccine was first used on a human on July 6, 1885 – nine-year old boy Joseph Meister (1876–1940) had been mauled by a rabid dog.Their vaccine consisted of a sample of the virus harvested from infected (and necessarily dead) rabbits, which was weakened by allowing it to dry. Similar nerve tissue-derived vaccines are still used now in some countries, and while they are much cheaper than modern cell culture vaccines, they are not as effective and carry a certain risk of neurological complications.The human diploid cell rabies vaccine (H.D.C.V.) was started in 1967. Human diploid cell rabies vaccines are made using the attenuated Pitman-Moore L503 strain of the virus. Human diploid cell rabies vaccines have been given to more than 1.5 million humans as of 2006. Newer and less expensive purified chicken embryo cell vaccine, and purified Vero cell rabies vaccine are now available. The purified Vero cell rabies vaccine uses the attenuated Wistar strain of the rabies virus, and uses the Vero cell line as its host.
Primary Prevention
Be a Responsible Pet Owner
- Keep vaccinations up to date for all dogs, cats, and ferrets. This requirement is important not only to keep your pets from getting rabies, but also to provide a barrier of protection for you, if your animal is bitten by a rabid wild animal.
- Keep your pets under direct supervision so they do not come in contact with wild animals. If your pet is bitten by a wild animal, seek veterinary assistance for the animal immediately.
- Call your local animal control agency to remove any stray animals from your neighborhood. They may be unvaccinated and could be infected by the disease.
- Spay or neuter your pets to help reduce the number of unwanted pets that may not be properly cared for or regularly vaccinated.
Avoid Direct Contact with Unfamiliar Animals
- Enjoy wild animals (raccoons, skunks, foxes) from afar. Do not handle, feed, or unintentionally attract wild animals with open garbage cans or litter.
- Never adopt wild animals or bring them into your home. Do not try to nurse sick animals to health. Call animal control or an animal rescue agency for assistance.
- Teach children never to handle unfamiliar animals, wild or domestic, even if they appear friendly. "Love your own, leave other animals alone" is a good principle for children to learn.
- Prevent bats from entering living quarters or occupied spaces in homes, churches, schools, and other similar areas, where they might come in contact with people and pets.
- When traveling abroad, avoid direct contact with wild animals and be especially careful around dogs in developing countries.
- Rabies is common in developing countries in Asia, Africa, and Latin America where dogs are the major reservoir of rabies.
- Tens of thousands of people die of rabies each year in these countries. Before traveling abroad, consult with a health care provider, travel clinic, or your health department about the risk of exposure to rabies, preexposure prophylaxis, and how you should handle an exposure, should it arise.
Pre-Exposure Prophylaxis
Currently pre-exposure immunization has been used on domesticated and normal non-human populations. In many jurisdictions, domestic dogs, cats, and ferrets are required to be vaccinated. A pre-exposure vaccination is also available for humans, most commonly given to veterinarians and those traveling to regions where the disease is common, such as India. Most tourists do not need such a vaccination, just those doing substantial non-urban activities. However, should a vaccinated human be bitten by a carrier, failure to receive subsequent post-exposure treatment could be fatal, although post-exposure treatment for a vaccinated human is far less extensive than which would normally be required by one with no pre-exposure vaccination.
In 1984 researchers at the Wistar Institute developed a recombinant vaccine called V-RG by inserting the glycoprotein gene from rabies into a vaccinia virus.[1] The V-RG vaccine has since been commercialized by Merial under the trademark Raboral. It is harmless to humans and has been shown to be safe for various species of animals that might accidentally encounter it in the wild, including birds (gulls, hawks, and owls).[2]
V-RG has been successfully used in the field in Belgium, France, and the United States to prevent outbreaks of rabies in wildlife. The virus is stable under relatively high temperatures and can be delivered orally, making mass vaccination of wildlife possible by putting it in tasty baits. The plan for immunization of normal populations involves dropping bait containing food wrapped around a small dose of the live virus. The bait would be dropped by helicopter concentrating on areas that have not been infected yet. Just such a strategy of oral immunization of foxes in Europe has already achieved substantial reductions in the incidence of human rabies. A strategy of vaccinating "neighborhood dogs" in Jaipur, India, (combined with a sterilization program) has also resulted in a large reduction in the number of human cases.[3]
Risk for travellers
- The risk to travelers in areas where rabies occurs is proportional to the probability of contact with rabid mammals. In most developing countries, the estimated ratio of dogs, both owned and ownerless, to human beings is 1:10 and an average 100 suspected rabid dog bites per 100 000 inhabitants are reported annually. As rabies is a lethal disease, medical advice should be sought immediately at a competent medical centre – ideally, the rabies treatment centre of a major city hospital. First-aid measures should also be started immediately.
- Travellers should avoid contact with free-roaming animals, especially dogs and cats, and with wild, free-ranging or captive animals. For travellers who participate in caving or spelunking, casual exposure to cave air is not a concern, but cavers should be warned not to handle bats. In most countries of the world, suspected contact with bats should be followed by post-exposure prophylaxis.
- The following map shows WHO’s categories of risk, from no risk (rabies-free) countries or areas, to countries or areas of low, medium and high risk. Categorization is based primarily on the animal host species in which the rabies virus is maintained, e.g. bats and/or other wildlife and/or dogs, and on the availability of reliable laboratory-based surveillance data on these reservoir species. Access to proper medical care and the availability of modern rabies vaccines have also been taken into consideration on a country basis. In countries or areas with even low risk of rabies, pre-exposure immunization against rabies is recommended for travellers with certain characteristics:
|
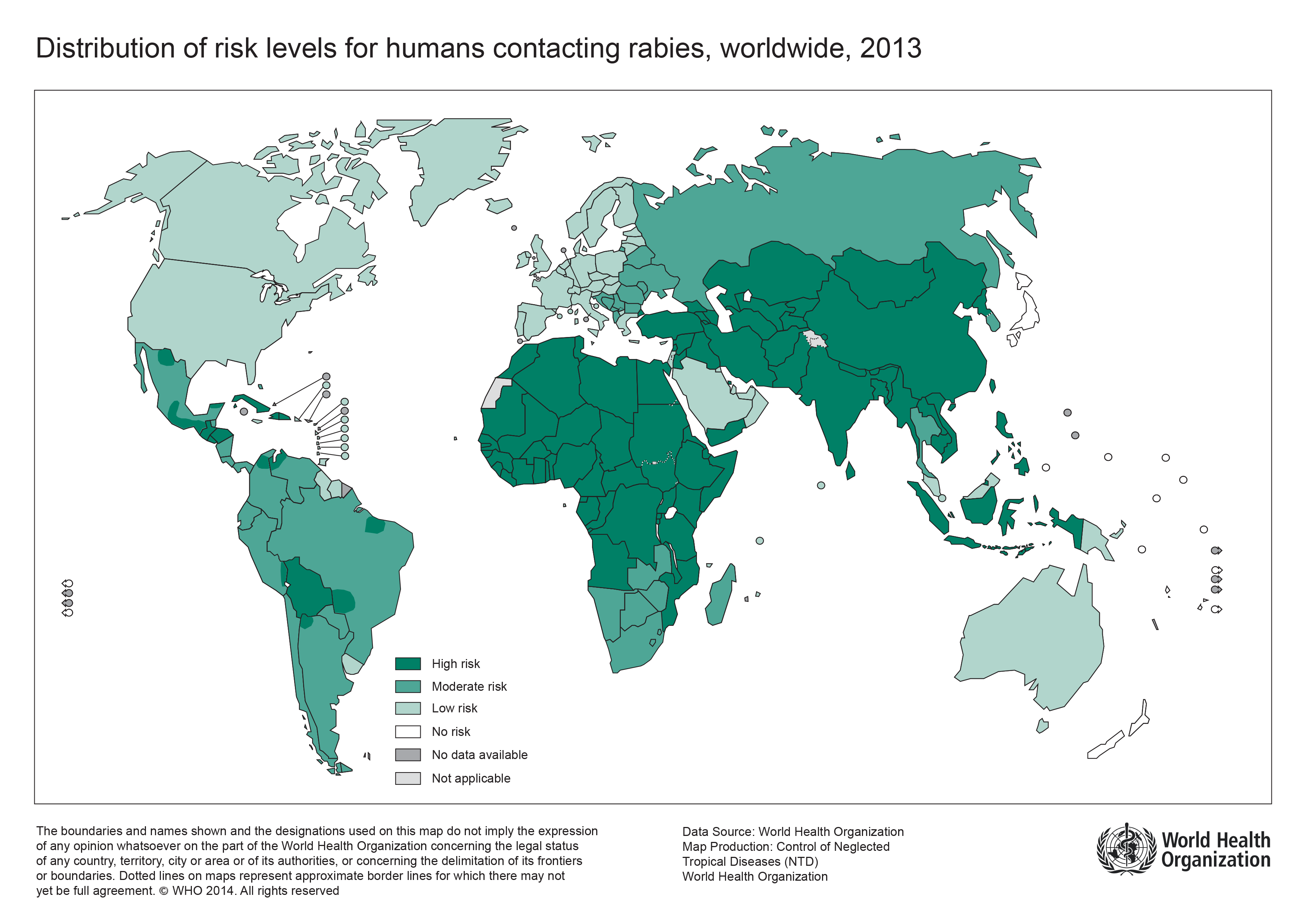 http://www.who.int/rabies/epidemiology/en/ |
Vaccination
Vaccination against rabies is used in two distinct situations:
- To protect those who are at risk of exposure to rabies, in other words pre-exposure vaccination.
- To prevent the development of clinical rabies after exposure has occurred, usually following the bite of an animal suspected of having rabies, in other words post-exposure prophylaxis.
The vaccines used for pre-exposure and post-exposure vaccination are the same, but the immunization schedule differs. Rabies immunoglobulin is used only for post-exposure prophylaxis. Modern vaccines of cell-culture or embryonated-egg origin are safer and more effective than the older vaccines, which were produced in mouse brain tissue. These modern rabies vaccines are now available in major urban centers of most countries of the developing world. Rabies immunoglobulin, on the other hand, is in short supply worldwide and may not be available, even in major urban centres, in many countries where canine rabies is prevalent.
Preexposure Vaccinations
People who work with rabies in laboratory settings and animal control and wildlife officers are just a few of the people who should consider rabies preexposure vaccinations. Consider preexposure vaccination in:
- The patient stay longer than 1 month in an area where rabies is common.
- The patient will be visiting remote areas where medical care is difficult to obtain or may be delayed.
- Rabies research laboratory workers and rabies biologics production workers.
- Veterinarians and terrestrial animal-control workers in areas where rabies is uncommon to rare.
- All persons who frequently handle rabies vector animals.
Although preexposure vaccination does not eliminate the need for additional therapy after a rabies exposure, it simplifies management by eliminating the need for rabies immune globulin and decreasing the number of doses of vaccine needed. This is of particular importance for persons at high risk for exposure to rabies in areas where immunizing products might not be available or where lesser quality biologics might be used which would place the exposed person at increased risk for adverse events. Preexposure prophylaxis may also protect people whose postexposure therapy is delayed and provide protection to people who are at risk for unapparent exposures to rabies.
Vaccine Structure and Guide
- Pre-exposure rabies vaccination consists of three full intramuscular (IM) doses of cell-culture- or embryonated-egg-based vaccine given on days 0, 7 and 21 (or day 28, if more convenient); a few days’ variation in the timing is not important. For adults and children aged ≥2 years, the vaccine should always be administered in the deltoid area of the arm; for children aged <2 years, the anterolateral area of the thigh is recommended. Rabies vaccine should never be administered in the gluteal area: administration in this manner results in lower neutralizing antibody titres.
- To reduce the cost of cell-derived vaccines for pre-exposure rabies vaccination, intradermal vaccination in doses of 0.1 ml on days 0, 7 and either 21 or 28 may be considered. This method of administration is an acceptable alternative to the standard intramuscular administration, but it is technically more demanding and requires appropriately trained staff and qualified medical supervision. Concurrent use of chloroquine can reduce the antibody response to intradermal application of cell-culture rabies vaccines. People who are currently receiving malaria prophylaxis or who are unable to complete the entire three-dose pre-exposure series before starting malarial prophylaxis should therefore receive pre-exposure vaccination by the intramuscular route.
- Periodic booster injections are not recommended for general travellers. However, in the event of exposure through the bite or scratch of an animal known or suspected to be rabid, individuals who have previously received a complete series of pre- or post-exposure rabies vaccine (with cell-culture or embryonated-egg-derived vaccine) should receive two booster doses of vaccine. Ideally, the first dose should be administered on the day of exposure and the second 3 days later. This should be combined with thorough wound treatment (see Post-exposure prophylaxis, below). Rabies immunoglobulin is not required for patients who have previously received a complete vaccination series.
- Pre-exposure immunization is recommended for all individuals living in or traveling to areas where rabies is highly enzootic, and for those exposed to rabies by nature of their occupation, including laboratory staff, veterinarians, animal handlers and wildlife officers. However, according to age-stratified studies of incidence, those at greatest risk are children living in rabies-enzootic regions of the developing world. Pre-exposure vaccination is therefore advisable for children living in or visiting areas with a high risk of rabies. Pre-exposure vaccination is also recommended for individuals travelling to isolated areas or to areas where immediate access to appropriate medical care is limited or to countries where modern rabies vaccines are in short supply and locally available rabies vaccines might be unsafe and/or ineffective.
Precautions and contraindications
Modern rabies vaccines are well tolerated. The frequency of minor adverse reactions (local pain, erythema, swelling and pruritus) varies widely from one report to another. Occasional systemic reactions (malaise, generalized aches and headaches) have been noted after intramuscular or intradermal injections.
| Risk Category | Nature of Risk | Typical Population | Preexposure Recommendations |
|---|---|---|---|
| Continuous | Countinous virus exposure:
Often in high concentrations with known or unknown source. |
|
Primary course. Serologic testing every 6 months; booster vaccination if antibody titer is below acceptable level. |
| Frequent | Episodic exposure:
With known or unknown source. |
|
Primary course. Serologic testing every 2 years; booster vaccination if antibody titer is below acceptable level. |
| Infrequent | Episidic exposure with recognized source.
Bite or nonbite exposure. |
|
Primary course. No serologic testing or booster vaccination. |
| Rare (population at large) | Exposure always episodic with recognized source.
Bite or nonbite exposure. |
|
No vaccination necessary. |
Primary vaccination
Three 1.0-mL injections of HDCV or PCEC vaccine should be administered intramuscularly (deltoid area) — one injection per day on days 0, 7, and 21 or 28. Vaccine preparations for intradermal administration are no longer available in the United States.
Summary of vaccine data
| Considerations for travellers for Rabies vaccination | |
|---|---|
| Type of vaccine |
|
| Number of doses |
|
| Boosters |
|
| Contraindications | |
| Adverse reactions |
|
| Before departure |
|
| Indication |
|
| Special precautions |
|
Booster doses
Continuous risk
Serum testing for rabies antibody should be tested for the following population every 6 months:
- People who work with rabies virus in research laboratories or vaccine production facilities
- Due to be at the highest risk for unapparent exposures.
The goal of booster administration in this group is to maintain a serum titer corresponding to at least complete neutralization at a 1:5 serum dilution by the Rapid Fluorescent Focus Inhibition Test (RFFIT). Intramuscular booster doses of vaccine should be
Frequent risk
This group includes other laboratory workers such as those performing rabies diagnostic testing, spelunkers, veterinarians and staff, and animal-control and wildlife officers in areas where animal rabies is enzootic. The frequent-risk category also includes persons who frequently handle bats, regardless of location in the Unites States. Persons in the frequent risk group should have a serum sample tested for rabies antibody every 2 years; if the titer is less than complete neutralization at a 1:5 serum dilution by the RFFIT, the person also should receive a single booster dose of vaccine.
Infrequent risk
Veterinarians, veterinary students, and terrestrial animal-control and wildlife officers working in areas where rabies is uncommon to rare (infrequent exposure group) and at-risk international travelers fall into this category and do not routine preexposure booster doses of vaccine after completion of primary preexposure vaccination.
| Type | Name | Route | Indications |
|---|---|---|---|
| Human Diploid Cell Vaccine (HDCV) | Imovax® Rabies | Intramuscular | Preexposure or Postexposure |
| Purified Chick Embryo Cell Vaccine (PCEC) | RabAvert® | Intramuscular | Preexposure or Postexposure |
References
- ↑ Wiktor TJ, Macfarlan RI, Reagan KJ, Dietzschold B, Curtis PJ, Wunner WH, Kieny MP, Lathe R, Lecocq JP, Mackett M (1984). "Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene". Proc. Natl. Acad. Sci. U.S.A. 81 (22): 7194–8. PMID 6095272.
- ↑ Artois M, Charlton KM, Tolson ND, Casey GA, Knowles MK, Campbell JB (1990). "Vaccinia recombinant virus expressing the rabies virus glycoprotein: safety and efficacy trials in Canadian wildlife". Can. J. Vet. Res. 54 (4): 504–7. PMID 2249183.
- ↑ Reece JF, Chawla SK. (2006). "Control of rabies in Jaipur, India, by the sterilisation and vaccination of neighbourhood dogs". Vet Rec. 159: 379&ndash, 83.