Protecting group
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
|
WikiDoc Resources for Protecting group |
|
Articles |
|---|
|
Most recent articles on Protecting group Most cited articles on Protecting group |
|
Media |
|
Powerpoint slides on Protecting group |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Protecting group at Clinical Trials.gov Trial results on Protecting group Clinical Trials on Protecting group at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Protecting group NICE Guidance on Protecting group
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Protecting group Discussion groups on Protecting group Patient Handouts on Protecting group Directions to Hospitals Treating Protecting group Risk calculators and risk factors for Protecting group
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Protecting group |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
A Protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminum hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acetal is removed (by reacting it with an aqueous acid), giving back the original carbonyl. This step is called deprotection.
Protecting groups are more commonly used in small-scale laboratory work and initial development than in industrial production processes because their use adds additional steps and material costs to the process.
Common protecting groups
Alcohol protecting groups
Protection of alcohols:
- Acetyl (Ac) - Removed by acid or base. (see Acetoxy_group)
- β-Methoxyethoxymethyl ether (MEM) - Removed by acid.
- Methoxymethyl ether (MOM) - Removed by acid.
- p-Methoxybenzyl ether (PMB) [2] - Removed by acid, hydrogenolysis, or oxidation.
- Methylthiomethyl ether - Removed by acid.
- Pivaloyl (Piv) - Removed by acid, base or reductant agents. It is stronger than other acyl protecting groups.
- Tetrahydropyran (THP) - Removed by acid.
- Silyl ether (most popular ones include trimethylsilyl (TMS), tert-butyldimethylsilyl (TBDMS), and triisopropylsilyl (TIPS) ethers) - Removed by acid or fluoride ion. (such as NaF or TBAF (Tetra-n-butylammonium fluoride))
- Methyl Ethers (cleavage is by TMSI in DCM or MeCN or Chloroform the other method to cleave methyl ethers is BBr3 in DCM)
Amine protecting groups
Protection of amines:
- Carbobenzyloxy (Cbz) group - Removed by hydrogenolysis
- tert-Butyloxycarbonyl (BOC) group (Common in solid phase peptide synthesis) - Removed by concentrated, strong acid. (such as HCl or CF3COOH)
- 9-Fluorenylmethyloxycarbonyl (FMOC) group (Common in solid phase peptide synthesis) - Removed by base, such as piperidine.
- Benzyl (Bn) group - Removed by hydrogenolysis
- p-methoxyphenyl (PMP) group - Removed by Ammonium cerium(IV) nitrate (CAN)
Carbonyl protecting groups
Protection of carbonyl groups:
- Acetals and Ketals - Removed by acid. Normally, the cleavage of acyclic acetals is easier than of cyclic acetals.
- Acylals - Removed by Lewis acids.
- Dithianes - Removed by metal salts or oxidizing agents.
Carboxylic acid protecting groups
Protection of carboxylic acids:
- Methyl esters - Removed by acid or base.
- Benzyl esters - Removed by hydrogenolysis.
- tert-Butyl esters - Removed by acid, base and some reductants.
- Silyl esters - Removed by acid, base and organometallic reagents
Miscellaneous
- Terminal alkynes can be protected as propargyl alcohols in the Favorskii reaction.
Orthogonal protection
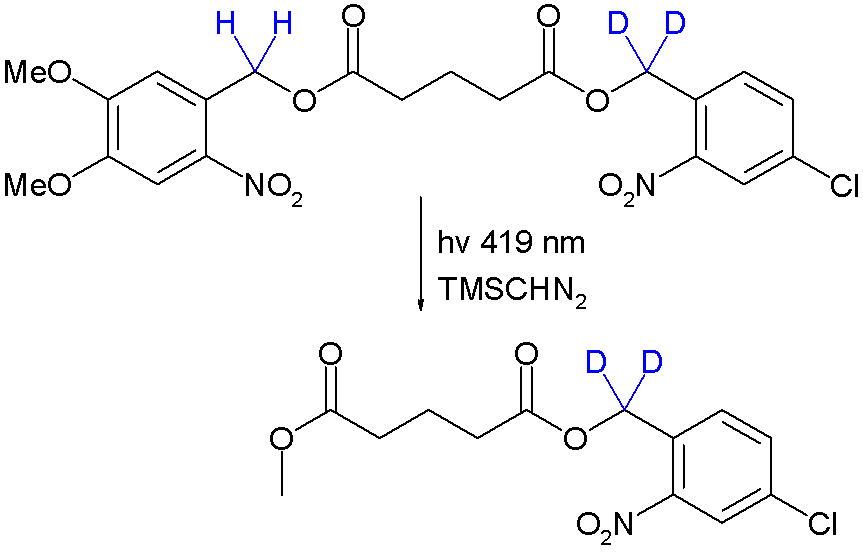
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one at the time each with a dedicated set of reaction conditions without affecting the other. It was introduced in the field of peptide synthesis by Robert Bruce Merrifield in 1977 [1]. As a proof of concept orthogonal deprotection is demonstrated in a photochemical transesterification by trimethylsilyldiazomethane utilizing the kinetic isotope effect [2]:
Due to this effect the quantum yield for deprotection of the right-side ester group is reduced and it stays intact. Significantly by placing the deuterium atoms next to the left-side ester group or by changing the wavelength to 254 nm the other monoarene is obtained.
External links
- Different Protecting groups with removal instructions
- Stability data for common protective groups
- Structure of Protecting groups
References
- ↑ Merrifield, R. B.; Barany, G.; Cosand, W. L.; Engelhard, M.; Mojsov, S. Pept.: Proc. Am. Pept. Symp. 5th 1977
- ↑ Isotope Effects in Photochemistry: Application to Chromatic Orthogonality Aurélien Blanc and Christian G. Bochet Org. Lett.; 2007; 9(14) pp 2649 - 2651; (Letter) doi: 10.1021/ol070820h
de:Schutzgruppe sv:Kemisk skyddsgrupp Template:WH Template:WS Template:Jb1