Orphenadrine (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Orphenadrine (injection) is an antimuscarinic agent that is FDA approved for the treatment of the relief of discomfort associated with acute painful musculoskeletal conditions. Common adverse reactions include transient syncope, nausea, vomiting, xerostomia, dizziness and blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Orphenadrine citrate injection is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions. The mode of action of the drug has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate does not directly relax tense skeletal muscles in man.
Dosing Information
- INJECTION: Adults - One 2 mL vial (60 mg) intravenously or intramuscularly; may be repeated every 12 hours.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Orphenadrine (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Orphenadrine (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Orphenadrine (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Orphenadrine (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Orphenadrine (injection) in pediatric patients.
Contraindications
- Contraindicated in patients with glaucoma, pyloric or duodenal obstruction, stenosing peptic ulcers, prostatic hypertrophy or obstruction of the bladder neck, cardio-spasm (megaesophagus) and myasthenia gravis.
- Contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
Warnings
- Some patients may experience transient episodes of light-headedness, dizziness or syncope. Orphenadrine citrate may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore be cautioned accordingly.
- Orphenadrine citrate injection contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than nonasthmatic people.
PRECAUTIONS:
- Confusion, anxiety and tremors have been reported in few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
- Orphenadrine citrate should be used with caution in patients with tachycardia, cardiac decompensation, coronary insufficiency, cardiac arrhythmias.
- Safety of continuous long-term therapy with orphenadrine has not been established. Therefore, if orphenadrine is prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
DRUG ABUSE AND DEPENDENCE:
- Orphenadrine has been chronically abused for its euphoric effects. The mood elevating effects may occur at therapeutic doses of orphenadrine.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions of orphenadrine are mainly due to the mild anticholinergic action of orphenadrine, and are usually associated with higher dosage. Dryness of the mouth is usually the first adverse effect to appear. When the daily dose is increased, possible adverse effects include: tachycardia, palpitation, urinary hesitancy or urinary retention, blurred vision, dilatation of pupils, increased ocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, hypersensitivity reactions, pruritus, hallucinations, agitation, tremor, gastric irritation, and rarely urticaria and other dermatoses. Infrequently, an elderly patient may experience some degree of mental confusion.
- These adverse reactions can usually be eliminated by reduction in dosage. Very rare cases of aplastic anemia associated with the use of orphenadrine tablets have been reported. No causal relationship has been established.
Postmarketing Experience
There is limited information regarding Orphenadrine (injection) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Orphenadrine (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Safe use of orphenadrine has not been established with respect to adverse effects upon fetal development. Therefore, orphenadrine should be used in women of childbearing potential and particularly during early pregnancy only when in the judgment of the physician the potential benefits outweigh the possible hazards.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Orphenadrine (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Orphenadrine (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Orphenadrine (injection) in women who are nursing.
Pediatric Use
- Safety and effectiveness in children have not been established; therefore, this drug is not recommended for use in the pediatric age group.
Geriatic Use
There is no FDA guidance on the use of Orphenadrine (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Orphenadrine (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Orphenadrine (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Orphenadrine (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Orphenadrine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Orphenadrine (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Orphenadrine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Injection
Monitoring
There is limited information regarding Orphenadrine (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Orphenadrine (injection) and IV administrations.
Overdosage
- Orphenadrine is toxic when overdosed and typically induces anticholinergic effects. In a review of orphenadrine toxicity, the minimum lethal dose was found to be 2-3 grams for adults; however, the range of toxicity is variable and unpredictable. Treatment for orphenadrine overdose is evacuation of stomach contents (when necessary), charcoal at repeated doses, intensive monitoring, and appropriate supportive treatment of any emergent anticholinergic effects.
Pharmacology
Mechanism of Action
- The mode of therapeutic action has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate does not directly relax tense muscles in man. Orphenadrine citrate also possesses anti-cholinergic actions.
Structure
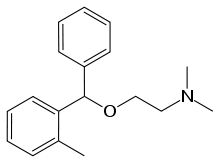
- Orphenadrine citrate is the citrate salt of orphenadrine ((±)-N, N-Dimethyl- 2-[(o-methyl-a-phenylbenzyl)oxy]ethylamine citrate (1:1)) with a molecular weight of 461.50; empirical formula C18H23NO•C6H8O7. The structural formula of orphenadrine citrate is:

- It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol.
- Orphenadrine citrate injection contains 60 mg of orphenadrine citrate in aqueous solution in each vial. Orphenadrine citrate injection also contains: sodium chloride USP 5.8 mg; sodium metabisulfite NF, 2.0 mg; sodium hydroxide NF, to adjust pH (5.0 to 6.0); and water for injection USP, q.s. to 2 mL.
Pharmacodynamics
There is limited information regarding Orphenadrine (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Orphenadrine (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Orphenadrine (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Orphenadrine (injection) Clinical Studies in the drug label.
How Supplied
INJECTION: Boxes of 10 (NDC 17478-538-02) 2 mL vials, each vial containing 60 mg of orphenadrine citrate in aqueous solution.
Single dose vial. Discard unused portion.
AKORN
Manufactured by: Akorn, Inc.
Lake Forest, IL 60045
RF00N Rev. 03/12
Storage
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Images
Drug Images
{{#ask: Page Name::Orphenadrine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Principal Display Panel Text for Container Label:
NDC 17478-538-02
Orphenadrine
Citrate
Injection, USP
60 mg (30 mg/mL)
Rx only 2 mL Sterile
For I.M. or I.V. Use.
Single dose vial.

Principal Display Panel Text for Carton Label:
NDC 17478-538-02
Orphenadrine Citrate Injection, USP
60 mg (30 mg/mL)
For Intravenous or Intramuscular Use
Single-dose Vial
10 Sterile Vials (2 ml each)
Rx only Akorn Logo

{{#ask: Label Page::Orphenadrine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Orphenadrine (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Orphenadrine (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ORPHENADRINE CITRATE®
Look-Alike Drug Names
There is limited information regarding Orphenadrine (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Labout, JJ; Thijssen, C; Keijser, GG; Hespe, W (1982). "Difference between single and multiple dose pharmacokinetics of orphenadrine hydrochloride in man". European journal of clinical pharmacology. 21 (4): 343–50. doi:10.1007/BF00637624. PMID 7056281.