Obinutuzumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: HEPATITIS B VIRUS REACTIVATION AND PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete Boxed Warning.
* Hepatitis B Virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients receiving CD20-directed cytolytic antibodies, including GAZYVA. Screen all patients for HBV infection before treatment initiation. Monitor HBV-positive patients during and after treatment with GAZYVA. Discontinue GAZYVA and concomitant medications in the event of HBV reactivation.
|
Overview
Obinutuzumab is an antineoplastic agent that is FDA approved for the treatment of chronic lymphoid leukemia,previously untreated. There is a Black Box Warning for this drug as shown here. Common adverse reactions include anemia, neutropenia,thrombocytopenia, musculoskeletal system disorder,cough, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Obinutuzumab , in combination with chlorambucil, is indicated for the treatment of patients with previously untreated chronic lymphocytic leukemia(CLL).
Dosage
Recommended Dosage Regimen
- Premedicate before each infusion.
- Administer only as an intravenous infusion through a dedicated line.
- Do not administer as an intravenous push or bolus.
- Monitor blood counts at regular intervals.
- Obinutuzumab should only be administered by a healthcare professional with appropriate medical support to manage severe infusion reactions that can be fatal if they occur.
Recommended Dose:
- Each dose of Obinutuzumab is 1000 mg, administered intravenously, with the exception of the first infusions in Cycle 1, which are administered on day 1 (100 mg) and day 2 (900 mg).

- If a planned dose of Obinutuzumab is missed, administer the missed dose as soon as possible and adjust dosing schedule accordingly. If appropriate, patients who do not complete the Day 1 Cycle 1 dose may proceed to the day 2 Cycle 1 dose.
- If a patient experiences an infusion reaction of any grade during infusion, adjust the infusion as follows:
- Grade 4 (life-threatening): Stop infusion immediately and permanently discontinue Obinutuzumab therapy.
- Grade 3 (severe): Interrupt infusion and manage symptoms. Upon resolution of symptoms, consider restarting Obinutuzumab infusion at no more than half the previous rate (the rate being used at the time that the infusion reaction occurred) and, if patient does not experience any further infusion reaction symptoms, infusion rate escalation may resume at the increments and intervals as appropriate for the treatment cycle dose. Day 1 infusion rate may be increased back up to 25 mg/hr after 1 hour but not increased further. Permanently discontinue treatment if patients experience a Grade 3 infusion-related symptom at rechallenge.
- Grade 1–2 (mild to moderate): Reduce infusion rate or interrupt infusion and treat symptoms. Upon resolution of symptoms, continue or resume infusion and, if patient does not experience any further infusion reaction symptoms, infusion rate escalation may resume at the increments and intervals as appropriate for the treatment cycle dose. Day 1 infusion rate may be increased back up to 25 mg/hr after 1 hour but not increased further.
Recommended Premedication
- Premedication is recommended to reduce the risk of infusion reactions as outlined in TABLE 2.
- Hypotension may occur during Obinutuzumab intravenous infusions. Consider withholding antihypertensive treatments for 12 hours prior to and throughout each Obinutuzumab infusion and for the first hour after administration.
- For patients with high tumor burden and/or high circulating absolute lymphocyte counts (greater than 25 × 109/L), premedicate with anti-hyperuricemics (e.g., allopurinol) beginning 12–24 hours prior to start of therapy and ensure adequate hydration for prophylaxis of tumor lysis syndrome.

Premedication for Antimicrobial Prophylaxis
- Patients with neutropenia are strongly recommended to receive antimicrobial prophylaxis throughout the treatment period. Antiviral and antifungal prophylaxis should be considered.
Treatment Interruption for Toxicity
Consider treatment interruption if patients experience an infection, Grade 3 or 4 cytopenia, or a ≥ Grade 2 non-hematologic toxicity.
DOSAGE FORMS AND STRENGTHS
- 1000 mg/40 mL (25 mg/mL) single-use vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Obinutuzumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Obinutuzumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Obinutuzumab in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Obinutuzumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Obinutuzumab in pediatric patients.
Contraindications
- None
Warnings
|
WARNING: HEPATITIS B VIRUS REACTIVATION AND PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete Boxed Warning.
* Hepatitis B Virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients receiving CD20-directed cytolytic antibodies, including GAZYVA. Screen all patients for HBV infection before treatment initiation. Monitor HBV-positive patients during and after treatment with GAZYVA. Discontinue GAZYVA and concomitant medications in the event of HBV reactivation.
|
Hepatitis B Virus Reactivation
- Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with anti-CD20 antibodies such as Obinutuzumab. HBV reactivation has been reported in patients who are hepatitis B surface antigen (HBsAg) positive and also in patients who are HBsAg negative but are hepatitis B core antibody (anti-HBc) positive. Reactivation has also occurred in patients who appear to have resolved hepatitis B infection (i.e., HBsAg negative, anti-HBc positive, and hepatitis B surface antibody [anti-HBs] positive).
- HBV reactivation is defined as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level or detection of HBsAg in a person who was previously HBsAg negative and anti-HBc positive. Reactivation of HBV replication is often followed by hepatitis, i.e., increase in transaminase levels and, in severe cases, increase in bilirubin levels, liver failure, and death.
- Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with Obinutuzumab. For patients who show evidence of hepatitis B infection (HBsAg positive [regardless of antibody status] or HBsAg negative but anti-HBc positive), consult physicians with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy.
- Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months following treatment with Obinutuzumab. HBV reactivation has been reported for other CD20-directed cytolytic antibodies following completion of therapy.
- In patients who develop reactivation of HBV while receiving Obinutuzumab, immediately discontinue Obinutuzumab and any concomitant chemotherapy and institute appropriate treatment. Resumption of Obinutuzumab in patients whose HBV reactivation resolves should be discussed with physicians with expertise in managing hepatitis B. Insufficient data exist regarding the safety of resuming Obinutuzumab in patients who develop HBV reactivation.
Progressive Multifocal Leukoencephalopathy
- JC virus infection resulting in progressive multifocal leukoencephalopathy (PML), which can be fatal, was observed in patients treated with Obinutuzumab. Consider the diagnosis of PML in any patient presenting with new onset or changes to preexisting neurologic manifestations. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture. Discontinue Obinutuzumab therapy and consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
Infusion Reactions
- Obinutuzumab can cause severe and life-threatening infusion reactions. Two thirds of patients experienced a reaction to the first 1000 mg infused of Obinutuzumab. Infusion reactions can also occur with subsequent infusions. Symptoms may include hypotension, tachycardia, dyspnea, and respiratory symptoms (e.g., bronchospasm, larynx and throat irritation, wheezing, laryngeal edema). Other common symptoms include nausea, vomiting, diarrhea, hypertension, flushing, headache, pyrexia, and chills.
- Premedicate patients with acetaminophen, antihistamine, and a glucocorticoid. Institute medical management (e.g., glucocorticoids, epinephrine, bronchodilators, and/or oxygen) for infusion reactions as needed. Closely monitor patients during the entire infusion. Infusion reactions within 24 hours of receiving Obinutuzumab have occurred .
- For patients with any Grade 4 infusion reactions, including but not limited to anaphylaxis, acute life-threatening respiratory symptoms, or other life-threatening infusion reaction: Stop the Obinutuzumab infusion. Permanently discontinue Obinutuzumab therapy.
- For patients with Grade 1, 2, or 3 infusion reactions: Interrupt Obinutuzumab for Grade 3 reactions until resolution of symptoms. Interrupt or reduce the rate of the infusion for Grade 1 or 2 reactions and manage symptoms.
- For patients with preexisting cardiac or pulmonary conditions, monitor more frequently throughout the infusion and the post-infusion period since they may be at greater risk of experiencing more severe reactions. Hypotension may occur as part of the Obinutuzumab infusion reaction. Consider withholding antihypertensive treatments for 12 hours prior to, during each Obinutuzumab infusion, and for the first hour after administration until blood pressure is stable. For patients at increased risk of hypertensive crisis, consider the benefits versus the risks of withholding their antihypertensive medication as is suggested here.
Tumor Lysis Syndrome
- Acute renal failure, hyperkalemia, hypocalcemia, hyperuricemia, and/or hyperphosphatemia from Tumor Lysis Syndrome (TLS) can occur within 12–24 hours after the first infusion. Patients with high tumor burden and/or high circulating lymphocyte count (> 25 × 109/L) are at greater risk for TLS and should receive appropriate tumor lysis prophylaxis with anti-hyperuricemics (e.g., allopurinol) and hydration beginning 12–24 hours prior to the infusion of Obinutuzumab. For treatment of TLS, correct electrolyte abnormalities, monitor renal function and fluid balance, and administer supportive care, including dialysis as indicated.
Infections
- Serious bacterial, fungal, and new or reactivated viral infections can occur during and following Obinutuzumab therapy. Fatal infections have been reported with Obinutuzumab. Do not administer Obinutuzumab to patients with an active infection. Patients with a history of recurring or chronic infections may be at increased risk of infection.
Neutropenia
- Obinutuzumab in combination with chlorambucil caused Grade 3 or 4 neutropenia in 33% of patients in the trial. Patients with Grade 3 to 4 neutropenia should be monitored frequently with regular laboratory tests until resolution. Anticipate, evaluate, and treat any symptoms or signs of developing infection.
- Neutropenia can also be of late onset (occurring more than 28 days after completion of treatment) and/or prolonged (lasting longer than 28 days).
- Patients with neutropenia are strongly recommended to receive antimicrobial prophylaxis throughout the treatment period. Antiviral and antifungal prophylaxis should be considered.
Thrombocytopenia
- Obinutuzumab in combination with chlorambucil caused Grade 3 or 4 thrombocytopenia in 10% of patients in the trial. In 4% of patients, Obinutuzumab caused acute thrombocytopenia occurring within 24 hours after the Obinutuzumab infusion. Fatal hemorrhagic events during Cycle 1 have also been reported in patients treated with Obinutuzumab.
- Monitor all patients frequently for thrombocytopenia and hemorrhagic events, especially during the first cycle. In patients with Grade 3 or 4 thrombocytopenia, monitor platelet counts more frequently until resolution and consider subsequent dose delays of Obinutuzumab and chlorambucil or dose reductions of chlorambucil. Transfusion of blood products (i.e., platelet transfusion) may be necessary. Consider withholding concomitant medications which may increase bleeding risk (platelet inhibitors, anticoagulants), especially during the first cycle.
Immunization
- The safety and efficacy of immunization with live or attenuated viral vaccines during or following Obinutuzumab therapy has not been studied. Immunization with live virus vaccines is not recommended during treatment and until B-cell recovery.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the label:
- Hepatitis B reactivation
- Progressive multifocal leukoencephalopathy
- Infusion reactions
- Tumor lysis syndrome
- Infections
- Neutropenia
- Thrombocytopenia
- The most common adverse reactions (incidence ≥ 10%) were infusion reactions, neutropenia, thrombocytopenia, anemia, pyrexia, cough, nausea, and diarrhea.
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described in Tables 3–6 below are based on a safety population of 773 previously untreated patients with CLL. Patients were treated with chlorambucil alone, Obinutuzumab in combination with chlorambucil, or rituximab in combination with chlorambucil. The Stage 1 analysis compared Obinutuzumab in combination with chlorambucil vs. chlorambucil alone, and Stage 2 compared Obinutuzumab in combination with chlorambucil vs. rituximab in combination with chlorambucil. Patients received three 1000 mg doses of Obinutuzumab on the first cycle and a single dose of 1000 mg once every 28 days for 5 additional cycles in combination with chlorambucil (6 cycles of 28 days each in total). In the last 140 patients enrolled, the first dose of Obinutuzumab was split between day 1 (100 mg) and day 2 (900 mg).In total, 81% of patients received all 6 cycles (of 28 days each) of Obinutuzumab-based therapy.

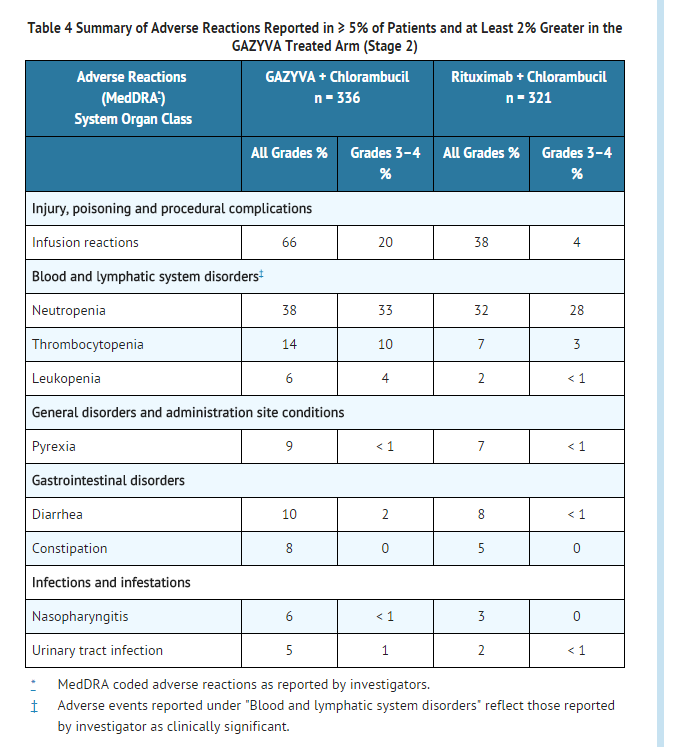


- Infusion Reactions: The incidence of infusion reactions was 65% with the first infusion of Obinutuzumab. The incidence of Grade 3 or 4 infusion reactions was 20% with 7% of patients discontinuing therapy. The incidence of reactions with subsequent infusions was 3% with the second 1000 mg and < 1% thereafter. No Grade 3 or 4 infusion reactions were reported beyond the first 1000 mg infused.
Of the first 53 patients receiving Obinutuzumab on the trial, 47 (89%) experienced an infusion reaction. After this experience, study protocol modifications were made to require pre-medication with a corticosteroid, antihistamine, and acetaminophen. The first dose was also divided into two infusions (100 mg on day 1 and 900 mg on day 2). For the 140 patients for whom these mitigation measures were implemented, 74 patients (53%) experienced a reaction with the first 1000 mg (64 patients on day 1, 3 patients on day 2, and 7 patients on both days) and < 3% thereafter.
- Neutropenia: The incidence of neutropenia reported as an adverse reaction was 38% in the Obinutuzumab treated arm and 32% in the rituximab treated arm, with the incidence of serious adverse events being 1% and < 1%, respectively (TABLE 4). Cases of late-onset neutropenia (occurring 28 days after completion of treatment or later) were 16% in the Obinutuzumab treated arm and 12% in the rituximab treated arm.
- Infection: The incidence of infections was similar between Obinutuzumab and rituximab treated arms. Thirty-eight percent of patients in the Obinutuzumab treated arm and 37% in the rituximab treated arm experienced an infection, with Grade 3–4 rates being 11% and 13%, respectively. Fatal events were reported in 1% of patients in both arms.
- Thrombocytopenia: The overall incidence of thrombocytopenia reported as an adverse reaction was higher in the Obinutuzumab treated arm (14%) compared to the rituximab treated arm (7%), with the incidence of Grade 3–4 events being 10% and 3%, respectively (TABLE 4). The difference in incidences between the treatment arms is driven by events occurring during the first cycle. The incidence of thrombocytopenia (all grades) in the first cycle were 11% in the Obinutuzumab and 3% in the rituximab treated arms, with Grade 3–4 rates being 8% and 2%, respectively. Four percent of patients in the Obinutuzumab treated arm experienced acute thrombocytopenia (occurring within 24 hours after the Obinutuzumab infusion).
The overall incidence of hemorrhagic events and the number of fatal hemorrhagic events were similar between the treatment arms, with 3 in the rituximab and 4 in the Obinutuzumab treated arms. However, all fatal hemorrhagic events in patients treated with Obinutuzumab occurred in Cycle 1.
- Tumor Lysis Syndrome: The incidence of Grade 3 or 4 tumor lysis syndrome was 2% in the Obinutuzumab treated arm versus 0% in the rituximab treated arm.
- Musculoskeletal Disorders: Adverse events related to musculoskeletal disorders (all events from the System Organ Class), including pain, have been reported in the Obinutuzumab treated arm with higher incidence than in the rituximab treated arm (18% vs. 15%).
- Liver Enzyme Elevations: Hepatic enzyme elevations have occurred in patients who received Obinutuzumab in clinical trials and had normal baseline hepatic enzyme levels (AST, ALT, and ALP). The events occurred most frequently within 24-48 hours of the first infusion. In some patients, elevations in liver enzymes were observed concurrently with infusion reactions or tumor lysis syndrome. In the pivotal study, there was no clinically meaningful difference in overall hepatotoxicity adverse events between all arms (4% of patients in the Obinutuzumab treated arm). Medications commonly used to prevent infusion reactions (e.g., acetaminophen) may also be implicated in these events. Monitor liver function tests during treatment, especially during the first cycle. Consider treatment interruption or discontinuation for hepatotoxicity.
Immunogenicity
- Serum samples from patients with previously untreated CLL were tested during and after treatment for antibodies to Obinutuzumab. Of the Obinutuzumab treated patients, 7% (18/271) tested positive for anti-Obinutuzumab antibodies at one or more time points. Neutralizing activity of anti-Obinutuzumab antibodies has not been assessed.
- Immunogenicity data are highly dependent on the sensitivity and specificity of the test methods used. Additionally, the observed incidence of a positive result in a test method may be influenced by several factors, including sample handling, timing of sample collection, drug interference, concomitant medication, and the underlying disease. Therefore, comparison of the incidence of antibodies to Obinutuzumab with the incidence of antibodies to other products may be misleading. Clinical significance of anti-Obinutuzumab antibodies is not known.
Additional Clinical Trial Experience
- Worsening of Pre-existing Cardiac Conditions: Fatal cardiac events have been reported in patients treated with Obinutuzumab.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Obinutuzumab in the drug label.
Drug Interactions
- No formal drug interaction studies have been conducted with Obinutuzumab.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C
Risk Summary
- There are no adequate and well-controlled studies of Obinutuzumab in pregnant women. Women of childbearing potential should use effective contraception while receiving Obinutuzumab and for 12 months following treatment. Obinutuzumab should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Mothers who have been exposed to Obinutuzumab during pregnancy should discuss the safety and timing of live virus vaccinations for their infants with their child's healthcare providers.
Animal Data
- In a pre- and post-natal development study, pregnant cynomolgus monkeys received weekly intravenous doses of 25 or 50 mg/kg obinutuzumab from day 20 of pregnancy until parturition. There were no teratogenic effects in animals. The high dose results in an exposure (AUC) that is 2.4 times the exposure in patients with CLL at the recommended label dose. When first measured on day 28 postpartum, obinutuzumab was detected in offspring, and B cells were completely depleted. The B-cell counts returned to normal levels, and immunologic function was restored within 6 months after birth.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Obinutuzumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Obinutuzumab during labor and delivery.
Nursing Mothers
- It is not known whether obinutuzumab is excreted in human milk. However, obinutuzumab is excreted in the milk of lactating cynomolgus monkeys, and human IgG is known to be excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from obinutuzumab, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Obinutuzumab in pediatric patients has not been established.
Geriatic Use
- Of 336 previously untreated CLL patients who received Obinutuzumab in combination with chlorambucil, 273 patients (81%) were ≥ 65 years of age and 156 patients (46%) were ≥ 75 years of age. The median age was 74 years. Of the 156 patients ≥ 75 years of age, 72 (46%) experienced serious adverse events and 11 (7%) experienced adverse events leading to death. For 180 patients < 75 years of age, 59 (33%) experienced a serious adverse event and 4 (2%) an adverse event leading to death. No significant differences in efficacy were observed between patients ≥ 75 years of age and those < 75 years of age
Gender
There is no FDA guidance on the use of Obinutuzumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Obinutuzumab with respect to specific racial populations.
Renal Impairment
- Based on population pharmacokinetic analysis, a baseline creatinine clearance (CrCl) ≥ 30 mL/min does not affect the pharmacokinetics of Obinutuzumab. Obinutuzumab has not been studied in patients with a baseline CrCl < 30 mL/min
Hepatic Impairment
- Obinutuzumab has not been studied in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Obinutuzumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Obinutuzumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Preparation and Administration
Preparation
Prepare the solution for infusion, using aseptic technique, as follows:
- Inspect visually for any particulate matter and discoloration prior to administration.
- Dilute into a 0.9% sodium chloride PVC or non-PVC polyolefin infusion bag. Do not use other diluents such as dextrose (5%).
- Preparation of solution for infusion on day 1 (100 mg) and day 2 (900 mg) of Cycle 1:
- Withdraw 40 mL of Obinutuzumab solution from the vial.
- Dilute 4 mL (100 mg) of Obinutuzumab into a 100 mL 0.9% sodium chloride infusion bag for immediate administration.
- Dilute the remaining 36 mL (900 mg) into a 250 mL 0.9% sodium chloride infusion bag at the same time for use on day 2 and store at 2°C to 8°C (36°F to 46°F) for up to 24 hours. After allowing the diluted bag to come to room temperature, use immediately.
- Clearly label each infusion bag.
- Preparation of solution for infusion on day 8 and 15 of Cycle 1 and day 1 Cycles 2–6:
- Withdraw 40 mL of Obinutuzumab solution from the vial.
- Dilute 40 mL (1000 mg) into a 250 mL 0.9% sodium chloride infusion bag.
- Mix diluted solution by gentle inversion. Do not shake or freeze.
- For microbiological stability, the diluted Obinutuzumab infusion solution should be used immediately. Dilute under appropriate aseptic conditions. If not used immediately, the solution may be stored in a refrigerator at 2°C to 8°C (36°F to 46°F) for up to 24 hours prior to use.
The product can be administered at a final concentration of 0.4 mg/mL to 4 mg/mL.
Administration
- Administer as an intravenous infusion only.
- Do not administer as an intravenous push or bolus.
- Do not mix Obinutuzumab with other drugs.
- No incompatibilities between Obinutuzumab and polyvinylchloride (PVC) or non-PVC polyolefin bags and administration sets have been observed.
Monitoring
There is limited information regarding Monitoring of Obinutuzumab in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Obinutuzumab in the drug label.
Overdosage
- There has been no experience with overdose in human clinical trials. Doses ranging from 50 mg up to and including 2000 mg per infusion have been administered in clinical trials. For patients who experience overdose, treatment should consist of immediate interruption or reduction of Obinutuzumab and supportive therapy.
Pharmacology
Obinutuzumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | CD20 |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 146.1 kDa |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 28.4 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Obinutuzumab is a monoclonal antibody that targets the CD20 antigen expressed on the surface of pre B- and mature B-lymphocytes. Upon binding to CD20, obinutuzumab mediates B-cell lysis through (1) engagement of immune effector cells, (2) by directly activating intracellular death signaling pathways (direct cell death), and/or (3) activation of the complement cascade. The immune effector cell mechanisms include antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis.
- As an antibody with reduced fucose content, obinutuzumab induces greater ADCC activity than rituximab in vitro using human cancer cell lines. Obinutuzumab also demonstrated an increased ability to induce direct cell death when compared to rituximab. Obinutuzumab binds to FcγRIII using purified proteins with a higher affinity than rituximab. Obinutuzumab and rituximab bind with similar affinity to overlapping epitopes on CD20.
Structure
- Obinutuzumab is a humanized anti-CD20 monoclonal antibody of the IgG1 subclass. It recognizes a specific epitope of the CD20 molecule found on B cells. The molecular mass of the antibody is approximately 150 kDa.
- Obinutuzumab is produced by mammalian cell (CHO) suspension culture. Obinutuzumab was engineered for reduced fucose content as compared to a typical IgG1 produced in CHO cells. Obinutuzumab is a sterile, clear, colorless to slightly brown, preservative-free liquid concentrate for intravenous administration. Obinutuzumab is supplied at a concentration of 25 mg/mL in 1000 mg single-use vials. The product is formulated in 20 mM L-histidine/L-histidine hydrochloride, 240 mM trehalose, 0.02% poloxamer 188. The pH is 6.0.
Pharmacodynamics
- In clinical trials in patients with CLL, Obinutuzumab caused CD19 B-cell depletion (defined as CD19 B-cell counts < 0.07 × 109/L). Initial CD19 B-cell recovery was observed in some patients approximately 9 months after the last Obinutuzumab dose. At 18 months of follow-up, some patients remain B-cell depleted.
- Although the depletion of B cells in the peripheral blood is a measurable pharmacodynamic effect, it is not directly correlated with the depletion of B cells in solid organs or in malignant deposits. B-cell depletion has not been shown to be directly correlated to clinical response.
Cardiac Electrophysiology
- The potential effects of Obinutuzumab on the QTc interval have not been studied.
Pharmacokinetics
- Based on a population pharmacokinetic (pop-PK) analysis, the geometric mean (CV%) volume of distribution of obinutuzumab at steady state is approximately 3.9 (21) L.
- The elimination of obinutuzumab is comprised of a linear clearance pathway and a time-dependent non-linear clearance pathway. As Obinutuzumab treatment progresses, the impact of the time-dependent pathway diminishes in a manner suggesting target-mediated drug disposition (TMDD). Based on a pop-PK analysis, the geometric mean (CV%) terminal obinutuzumab clearance and half-life are approximately 0.09 (37%) L/day and 29.7 (35%) days, respectively.
Specific Populations:
- Age: Age did not affect the pharmacokinetics of Obinutuzumab.
- Body Weight: Volume of distribution and steady-state clearance both increased with body weight; however, the expected change in exposure does not warrant a dose modification.
- Renal Impairment: Based on the population pharmacokinetic analysis, a baseline creatinine clearance (CrCl) > 30 mL/min does not affect the pharmacokinetics of Obinutuzumab. Obinutuzumab has not been studied in patients with a baseline CrCl < 30 mL/min.
- Hepatic Impairment: Obinutuzumab has not been studied in patients with hepatic impairment.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity or genotoxicity studies have been conducted with obinutuzumab.
- No specific studies have been conducted to evaluate potential effects on fertility; however, no adverse effects on male or female reproductive organs were observed in the 26-week repeat-dose toxicity study in cynomolgus monkeys.
Clinical Studies
Chronic Lymphocytic Leukemia
- Obinutuzumab was evaluated in a three-arm, open-label, active-controlled, randomized, multicenter trial (Study 1) in 781 patients with previously untreated CD20+ chronic lymphocytic leukemia requiring treatment who had coexisting medical conditions or reduced renal function as measured by creatinine clearance (CrCl) < 70 mL/min. Patients with CrCl < 30 mL/min, active infections, positive hepatitis B (HBsAg or anti-HBc positive; patients positive for anti-HBc could be included if hepatitis B viral DNA was not detectable) and hepatitis C serology, or immunization with live virus vaccine within 28 days prior to randomization were excluded from the trial. Patients were treated with chlorambucil control (Arm 1), Obinutuzumab in combination with chlorambucil (Arm 2), or rituximab in combination with chlorambucil (Arm 3). The safety and efficacy of Obinutuzumab was evaluated in a Stage 1 comparison of Arm 1 vs. Arm 2 in 356 patients and a Stage 2 comparison of Arm 2 vs. Arm 3 in 663 patients.
- The majority of patients received 1000 mg of Obinutuzumab on days 1, 8, and 15 of the first cycle, followed by treatment on the first day of 5 subsequent cycles (total of 6 cycles, 28 days each). The first dose of Obinutuzumab was divided between day 1 (100 mg) and day 2 (900 mg), which was implemented in 140 patients. Chlorambucil was given orally at 0.5 mg/kg on day 1 and day 15 of all treatment cycles (1 to 6).
- In Study 1, the median age was 73 years, 62% were male, and 95% were Caucasian. Sixty-five percent had a CrCl < 70 mL/min and 76% had multiple coexisting medical conditions. Twenty-two percent of patients were Binet stage A, 42% were stage B, and 36% were stage C. The median estimated CrCl was 62 mL/min. Eighty-one percent of patients treated with Obinutuzumab in combination with chlorambucil received all 6 cycles compared to 89% of patients in the rituximab treated arm and 67% in the chlorambucil alone arm.
- In the Stage 1 analysis of Study 1, the median progression-free survival (PFS) in the Obinutuzumab in combination with chlorambucil arm was 27.2 months and 11.2 months in the chlorambucil alone arm (median observation time 22.8 months) as assessed by independent review and is consistent with investigator-assessed PFS. The median overall survival (OS) was not yet reached with a total of 46 deaths: 22 (9%) in the Obinutuzumab in combination with chlorambucil arm and 24 (20%) in the chlorambucil arm. The hazard ratio for OS was 0.41 (95% CI: 0.23-0.74).
- In the Stage 2 analysis of Study 1, the median PFS was 26.7 months in the Obinutuzumab arm and 14.9 months in the rituximab arm with a median observation time of 18.7 months (HR: 0.42, 95% CI: 0.33-0.54, p-value <0.0001). These results were assessed by independent review and are consistent with investigator-assessed PFS. Minimal Residual Disease (MRD) was evaluated using allele-specific oligonucleotide polymerase chain reaction (ASO-PCR). The cutoff for a negative status was one CLL cell per 104 leukocytes in the sample (i.e., an MRD value of <10-4 was considered negative). Among patients who achieved complete response (CR) and complete response with incomplete marrow recovery (CRi) (94 patients in the Obinutuzumab arm and 34 patients in the rituximab arm), 18 patients (19%) had negative MRD in the bone marrow in the Obinutuzumab arm compared to 2 patients (6%) in the rituximab arm. Out of the patients who achieved CR and CRi, 39 patients (41%) in the Obinutuzumab arm and 4 patients (12%) in the rituximab arm were MRD negative in peripheral blood samples collected at least 3 months after the end of treatment.
- Efficacy results are shown in TABLE 7, and the Kaplan-Meier curves for Stage 1a Overall Survival and Stage 2 PFS are shown in FIGURES 1 and 2, respectively.



How Supplied
- Obinutuzumab 1000 mg/40 mL (25 mg/mL) single-use vials containing preservative-free solution (NDC 50242-070-01) are stable at 2°C to 8°C (36°F to 46°F). Do not use beyond expiration date stamped on carton. Obinutuzumab vials should be protected from light. DO NOT FREEZE. DO NOT SHAKE.
- For the diluted product, chemical and physical stability have been demonstrated in 0.9% NaCl at concentrations of 0.4 mg/mL to 20 mg/mL for 24 hours at 2°C to 8°C (36°F to 46°F) followed by 48 hours (including infusion time) at room temperature (≤ 30°C/86°F). Obinutuzumab does not contain antimicrobial preservatives. Therefore, care must be taken to ensure that the solution for infusion is not microbiologically compromised during preparation. The solution for infusion should be used immediately. If not used immediately, the prepared solution may be stored up to 24 hours at 2 to 8°C. No incompatibilities between Obinutuzumab and polyvinyl chloride or polyolefin infusion materials have been observed in concentration ranges from 0.4 mg/mL to 20.0 mg/mL after dilution of Obinutuzumab with 0.9% sodium chloride.
Storage
There is limited information regarding Obinutuzumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Obinutuzumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Obinutuzumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise patients to seek immediate medical attention for any of the following:
- Signs and symptoms of infusion reactions including dizziness, nausea, chills, fever, vomiting, diarrhea, breathing problems, or chest pain.
- Symptoms of tumor lysis syndrome such as nausea, vomiting, diarrhea, and lethargy.
- Signs of infections including fever and cough.
- Symptoms of hepatitis including worsening fatigue or yellow discoloration of skin or eyes.
- New or changes in neurological symptoms such as confusion, dizziness or loss of balance, difficulty talking or walking, or vision problems.
Advise patients of the need for:
- Periodic monitoring of blood counts.
- Avoid vaccinations with live viral vaccines.
- Patients with a history of hepatitis B infection (based on the blood test) should be monitored and sometimes treated for their hepatitis.
Precautions with Alcohol
- Alcohol-Obinutuzumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GAZYVA* ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "GAZYVA- obinutuzumab injection, solution, concentrate".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Obinutuzumab
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Obinutuzumab |Label Name=Obinutuzumab ingredients and appearance.png
}}
{{#subobject:
|Label Page=Obinutuzumab |Label Name=File:Obinutuzumab image1.jpg
}}