Nitroglycerin (Injection solution in 5% dextrose)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nitroglycerin (Injection solution in 5% dextrose) is an antianginal that is FDA approved for the treatment of peri-operative hypertension; for control of congestive heart failure in the setting of acute myocardial infarction; for treatment of angina pectoris in patients who have not responded to sublingual nitroglycerin and β-blockers; and for induction of intraoperative hypotension. Common adverse reactions include headache, lightheadedness and hypotension.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Nitroglycerin in 5% Dextrose Injection is indicated for treatment of peri-operative hypertension; for control of congestive heart failure in the setting of acute myocardial infarction; for treatment of angina pectoris in patients who have not responded to sublingual nitroglycerin and β-blockers; and for induction of intraoperative hypotension.
Dosage
- Nitroglycerin in 5% Dextrose Injection is intended for intravenous administration using sterile equipment. It should be administered only via an infusion pump that can maintain a constant infusion rate. A container which has lost its vacuum, or one in which particulate matter is visible, should not be used.
- Dosage is affected by the type of infusion set used. Although the usual adult starting dose in published studies has been 25 mcg/min or more, these studies used PVC tubing, so the delivered doses were less than those reported. When nonabsorptive tubing is used, doses must be reduced.
- Even using nonabsorptive tubing, the dose necessary to achieve a given response will vary greatly from patient to patient. Patients with normal or low left-ventricular filling pressure (e.g., patients with uncomplicated angina pectoris) may respond fully to as little as 5 mcg/min, while other patients may require a dose that is one or even two orders of magnitude higher. Continuous monitoring of blood pressure and heart rate is necessary in all patients receiving this medication; in many cases, invasive monitoring of pulmonary capillary wedge pressure will also be indicated.
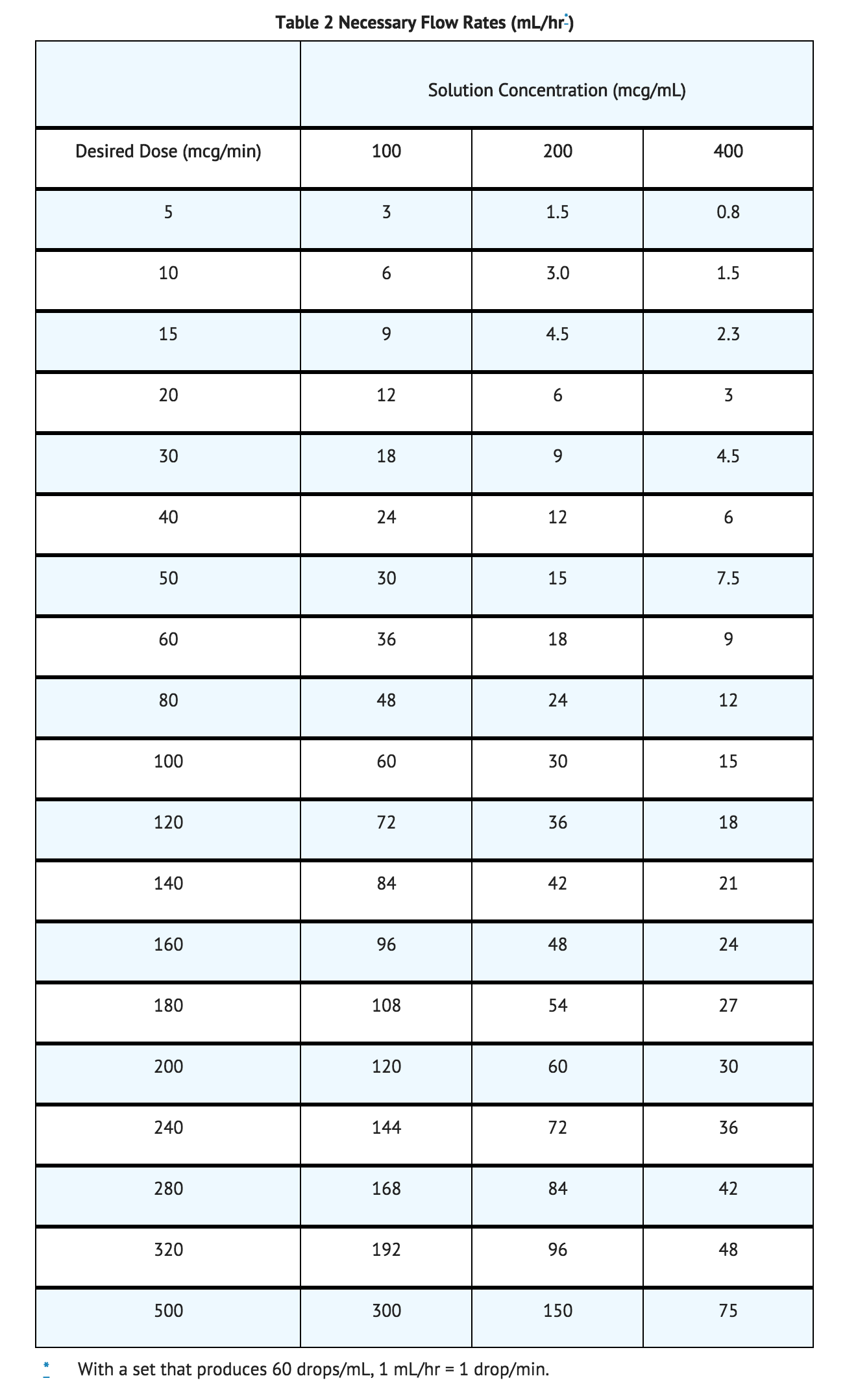
- Lower concentrations of Nitroglycerin in 5% Dextrose Injection increase the potential precision of dosing, but these concentrations increase the total fluid volume that must be delivered to the patient. Total fluid load may be a dominant consideration in patients with compromised function of the heart, liver, and/or kidneys. The necessary flow rates to achieve various dose rates with the available concentrations are shown in the following table.
- Using nonabsorptive tubing, the initial adult dosage of Nitroglycerin in 5% Dextrose Injection should be 5 mcg/min. Subsequent titration must be guided by the clinical results, with dose increments becoming more cautious as partial response is seen. Initial titration should be in 5 mcg/min increments at intervals of 3 to 5 minutes. If no response is seen at 20 mcg/min, increments of 10 and even 20 mcg/min can be used. Once some hemodynamic response is observed, dosage increments should be smaller and less frequent.
- When the concentration is changed, the tubing must be disconnected from the patient and flushed with the new solution before therapy is continued. If this precaution is not taken, then depending upon the tubing, pump, and flow rate used, it might be several hours before nitroglycerin is delivered at the desired rate.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not add supplementary medication to Nitroglycerin in 5% Dextrose Injection.
- Do not use unless vacuum is present and solution is clear.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Injection solution in 5% dextrose) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Injection solution in 5% dextrose) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Safety and effectiveness in the pediatric population have not been established. However, the relationship between hemodynamic effects of nitroglycerin and dose in the pediatric population have been documented in the literature.
Dosage
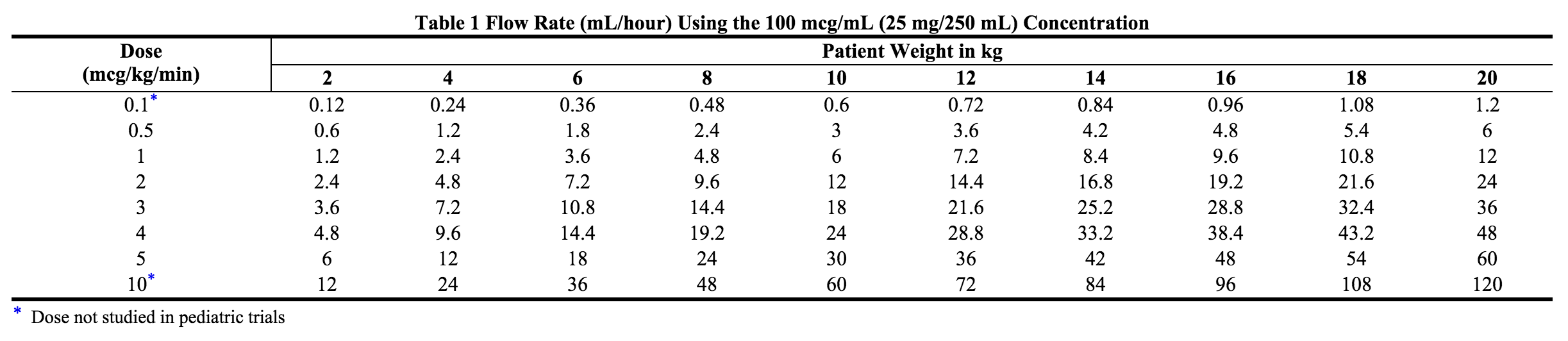
- Studies in the literature used doses of nitroglycerin injection in pediatric patients ranging from 0.5 to 5 mcg/kg/min. The following chart can be used to calculate the flow rate in mL/hour of nitroglycerin using the 100 mcg/mL (25 mg/250 mL) concentration of nitroglycerin.
- Note: Very low infusion rates may require that a more dilute concentration of nitroglycerin infusion solution be prepared.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Injection solution in 5% dextrose) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Injection solution in 5% dextrose) in pediatric patients.
Contraindications
- Nitroglycerin in 5% Dextrose Injection is contraindicated in patients who are allergic to it.
- In patients with pericardial tamponade, restrictive cardiomyopathy, or constrictive pericarditis, cardiac output is dependent upon venous return. Intravenous nitroglycerin is contraindicated in patients with these conditions.
- Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
- Do not use Nitroglycerin in 5% Dextrose Injection in patients who are taking certain drugs for erectile dysfunction (phosphodiesterase inhibitors) such as sildenafil, tadalafil, or vardenafil. Concomitant use can cause severe hypotension, syncope, or myocardial ischemia.
- Do not use nitroglycerin in 5% dextrose in patients who are taking the soluble guanylate cyclase stimulator riociguat. Concomitant use can cause hypotension.
Warnings
- Nitroglycerin readily migrates into many plastics, including the polyvinyl chloride (PVC) plastics commonly used for intravenous administration sets. Nitroglycerin absorption by PVC tubing is increased when the tubing is long, the flow rates are low, and the nitroglycerin concentration of the solution is high. The delivered fraction of the solution’s original nitroglycerin content has been 20-60% in published studies using PVC tubing; the fraction varies with time during a single infusion, and no simple correction factor can be used. PVC tubing has been used in most published studies of intravenous nitroglycerin, but the reported doses have been calculated by simply multiplying the flow rate of the solution by the solution’s original concentration of nitroglycerin. The actual doses delivered have been less, sometimes much less, than those reported.
- Relatively nonabsorptive intravenous administration sets are available. If intravenous nitroglycerin is administered through nonabsorptive tubing, doses based upon published reports will generally be too high.
- Some in-line intravenous filters also absorb nitroglycerin; these filters should be avoided.
- Solutions containing dextrose without electrolytes should not be administered through the same administration set as blood, as this may result in pseudoagglutination or hemolysis.
- The intravenous administration of solutions may cause fluid overloading resulting in dilution of serum electrolyte concentrations, overhydration and congested states of pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentrations of the injections. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration of the injections.
Precautions
- General: Severe hypotension and shock may occur with even small doses of nitroglycerin. This drug should therefore be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris.
- Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
- As tolerance to other forms of nitroglycerin develops, the effect of sublingual nitroglycerin on exercise tolerance, although still observable, is somewhat blunted.
- In industrial workers who have long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence.
- Some clinical trials in angina patients have provided nitroglycerin for about 12 continuous hours of every 24-hour day. During the nitrate-free intervals in some of these trials, anginal attacks have been more easily provoked than before treatment, and patients have demonstrated hemodynamic rebound and decreased exercise tolerance. The importance of these observations to the routine, clinical use of intravenous nitroglycerin is not known.
- Lower concentrations of Nitroglycerin in 5% Dextrose Injection increase the potential precision of dosing, but these concentrations increase the total fluid volume that must be delivered to the patient. Total fluid load may be a dominant consideration in patients with compromised function of the heart, liver, and/or kidneys.
- Nitroglycerin in 5% Dextrose Injection should be administered only via an infusion pump that can maintain a constant infusion rate.
- Intracoronary injection of Nitroglycerin in 5% Dextrose Injection has not been studied.
- Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
- Laboratory Tests: Because of the propylene glycol content of intravenous nitroglycerin, serum triglyceride assays that rely on glycerol oxidase may give falsely elevated results in patients receiving this medication.
- Concomitant use of Nitroglycerin in 5% Dextrose Injection with phosphodiesterase inhibitors in any form is contraindicated.
- Concomitant use of nitroglycerin in 5% dextrose with riociguat, a soluble guanylate cyclase stimulator, is contraindicated.
- Marked symptomatic orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used in combination.
- Intravenous nitroglycerin interferes, at least in some patients, with the anticoagulant effect of heparin. In patients receiving intravenous nitroglycerin, concomitant heparin therapy should be guided by frequent measurement of the activated partial thromboplastin time.
- Administration of Nitroglycerin in 5% Dextrose Injection through the same infusion set as blood can result in pseudoagglutination and hemolysis. More generally, Nitroglycerin in 5% Dextrose Injection should not be mixed with any other medication of any kind.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions to nitroglycerin are generally dose-related and almost all of these reactions are the result of nitroglycerin’s activity as a vasodilator. headache, which may be severe, is the most commonly reported side effect. Headache may be recurrent with each daily dose, especially at higher doses. Transient episodes of lightheadedness, occasionally related to blood pressure changes, may also occur. Hypotension occurs infrequently, but in some patients it may be severe enough to warrant discontinuation of therapy.Syncope, crescendo angina, and rebound hypertension have been reported but are uncommon.
- Allergic reactions to nitroglycerin are also uncommon, and the great majority of those reported have been cases of contact dermatitis or fixed drug eruptions in patients receiving nitroglycerin in ointments or patches. There have been a few reports of genuine anaphylactoid reactions, and these reactions can probably occur in patients receiving nitroglycerin by any route.
- Extremely rarely, ordinary doses of organic nitrates have caused methemoglobinemia in normal-seeming patients. Methemoglobinemia is so infrequent at these doses that further discussion of its diagnosis and treatment is deferred.
- Data are not available to allow estimation of the frequency of adverse reactions during treatment with Nitroglycerin in 5% Dextrose Injection.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Nitroglycerin (Injection solution in 5% dextrose) in the drug label.
Drug Interactions
- The vasodilating effects of nitroglycerin may be additive with those of other vasodilators.
Use in Specific Populations
Pregnancy
- Animal teratology studies have not been conducted with nitroglycerin injection. Teratology studies in rats and rabbits were conducted with topically applied nitroglycerin ointment at doses up to 80 mg/kg/day and 240 mg/kg/day, respectively, and no toxic effects on dams or fetuses were seen. There are no adequate and well-controlled studies in pregnant women. Nitroglycerin should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitroglycerin (Injection solution in 5% dextrose) in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) during labor and delivery.
Nursing Mothers
- It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nitroglycerin is administered to a nursing woman.
Pediatric Use
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) with respect to pediatric patients.
Geriatic Use
- Clinical studies of nitroglycerin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance on the use of Nitroglycerin (Injection solution in 5% dextrose) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- Continuous monitoring of blood pressure and heart rate is necessary in all patients receiving this medication; in many cases, invasive monitoring of pulmonary capillary wedge pressure will also be indicated.
IV Compatibility
There is limited information regarding IV Compatibility.
Overdosage
- Hemodynamic Effects: The ill effects of nitroglycerin overdose are generally the results of nitroglycerin’s capacity to induce vasodilation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); air hunger and dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures; and death.
- Laboratory determinations of serum levels of nitroglycerin and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of nitroglycerin overdose.
- No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of nitroglycerin and its active metabolites. Similarly, it is not known which - if any - of these substances can usefully be removed from the body by hemodialysis.
- No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient’s legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
- The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
- In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
- Methemoglobinemia: Nitrate ions liberated during metabolism of nitroglycerin can oxidize hemoglobin into methemoglobin. Even in patients totally without cytochrome b5 reductase activity, however, and even assuming that the nitrate moieties of nitroglycerin are quantitatively applied to oxidation of hemoglobin, about 1 mg/kg of nitroglycerin should be required before any of these patients manifests clinically significant (≥10%) methemoglobinemia. In patients with normal reductase function, significant production of methemoglobin should require even larger doses of nitroglycerin. In one study in which 36 patients received 2-4 weeks of continuous nitroglycerin therapy at 3.1 to 4.4 mg/hr, the average methemoglobin level measured was 0.2%; this was comparable to that observed in parallel patients who received placebo.
- Notwithstanding these observations, there are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. None of the affected patients had been thought to be unusually susceptible.
- Methemoglobin levels are available from most clinical laboratories. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial pO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
- When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1-2 mg/kg intravenously.
Pharmacology
Mechanism of Action
- The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined.
Structure
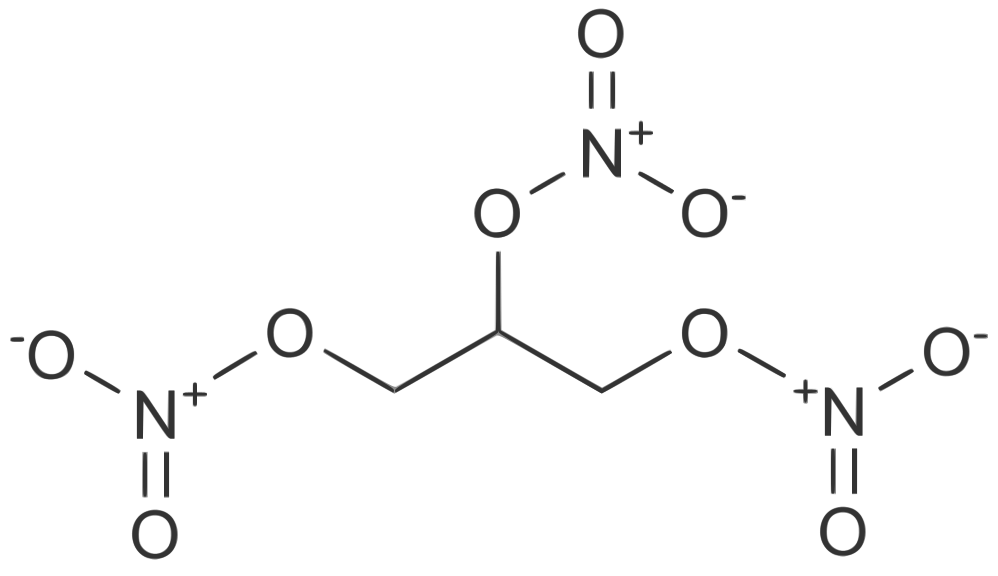
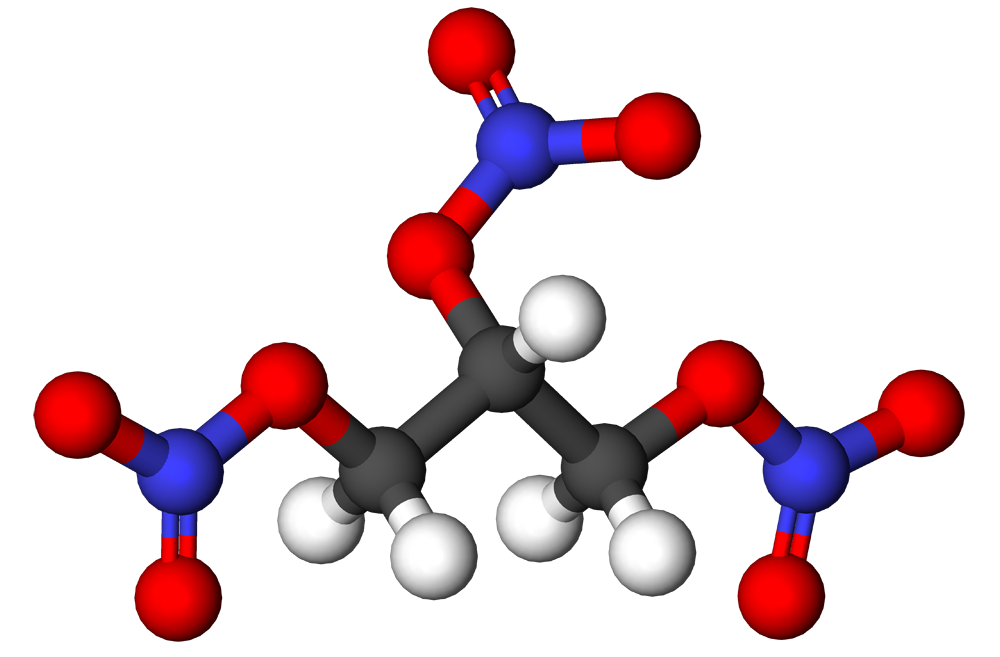
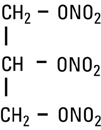
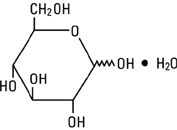
- Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:
- Nitroglycerin in 5% Dextrose Injection is a sterile, nonpyrogenic solution of nitroglycerin and dextrose in water for injection. The solution is clear and practically colorless. Each mL of 100 mcg/mL Nitroglycerin in 5% Dextrose Injection contains nitroglycerin 100 mcg; propylene glycol 0.9 mg and dextrose, hydrous 50 mg in water for injection. May contain nitric acid for pH adjustment. The pH is 4.0 (3.0 to 6.5) and the calculated osmolarity is 265 mOsmol/L.*
- Each mL of 200 mcg/mL Nitroglycerin in 5% Dextrose Injection contains nitroglycerin 200 mcg, propylene glycol 1.80 mg and dextrose, hydrous 50 mg in water for injection. May contain nitric acid for pH adjustment. The pH is 4.0 (3.0 to 6.5) and the calculated osmolarity is 277 mOsmol/L.*
- Each mL of 400 mcg/mL Nitroglycerin in 5% Dextrose Injection contains nitroglycerin 400 mcg, propylene glycol 3.60 mg and dextrose, hydrous 50 mg in water for injection. May contain nitric acid for pH adjustment. The pH is 4.0 (3.0 to 6.5) and the calculated osmolarity is 301 mOsmol/L.*
- Normal physiologic osmolarity range is approximately 280 to 310 mOsmol/L. Administration of substantially hypertonic solutions (≥600 mOsmol/L) may cause vein damage.
- Although dry nitroglycerin is explosive, nitroglycerin in 5% dextrose is not.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Nitroglycerin (Injection solution in 5% dextrose) in the drug label.
Pharmacokinetics
- The volume of distribution of nitroglycerin is about 3 L/kg, and nitroglycerin is cleared from this volume at extremely rapid rates, with a resulting serum half-life of about 3 minutes. The observed clearance rates (close to 1 L/kg/min) greatly exceed hepatic blood flow; known sites of extrahepatic metabolism include red blood cells and vascular walls.
- The first products in the metabolism of nitroglycerin are inorganic nitrate and the 1,2- and 1,3-dinitroglycerols. The dinitrates are less effective vasodilators than nitroglycerin, but they are longer-lived in the serum, and their net contribution to the overall effect of chronic nitroglycerin regimens is not known. The dinitrates are further metabolized to (non-vasoactive) mononitrates and, ultimately, to glycerol and carbon dioxide.
- To avoid development of tolerance to nitroglycerin, drug-free intervals of 10-12 hours are known to be sufficient; shorter intervals have not been well studied. In one well-controlled clinical trial, subjects receiving nitroglycerin appeared to exhibit a rebound or withdrawal effect, so that their exercise tolerance at the end of the daily drug-free interval was less than that exhibited by the parallel group receiving placebo.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis and Impairment of Fertility
- Animal carcinogenesis studies with injectable nitroglycerin have not been performed.
- Rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At high dose, the incidences of hepatocellular carcinomas in both sexes were 52% vs. 0% in controls, and incidences of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
- Nitroglycerin was weakly mutagenic in Ames tests performed in two different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, p.o., or in in vitro cytogenetic tests in rat and dog tissues.
- In a three-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for six months prior to mating of the F0 generation with treatment continuing through successive F1 and F2 generations. The high-dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high dose males. In this three-generation study there was no clear evidence of teratogenicity.
Clinical Studies
- Blinded, placebo-controlled trials of intravenous nitroglycerin have not been reported, but multiple investigators have reported open-label studies, and there are scattered reports of studies in which intravenous nitroglycerin was tested in blinded fashion against sodium nitroprusside.
- In each of these studies, therapeutic doses of intravenous nitroglycerin were found to reduce systolic and diastolic arterial blood pressure. The heart rate was usually increased, presumably as a reflexive response to the fall in blood pressure. Coronary perfusion pressure was usually, but not always, maintained.
- Intravenous nitroglycerin reduced central venous pressure (CVP), right atrial pressure (RAP), pulmonary arterial pressure (PAP), pulmonary-capillary wedge pressure (PCWP), pulmonary vascular resistance (PVR), and systemic vascular resistance (SVR). When these parameters were elevated, reducing them toward normal usually caused a rise in cardiac output. Conversely, intravenous nitroglycerin usually reduced cardiac output when it was given to patients whose CVP, RAP, PAP, PCWP, PVR, and SVR were all normal.
- Most clinical trials of intravenous nitroglycerin have been brief; they have typically followed hemodynamic parameters during a single surgical procedure. In one careful study, one of the few that lasted more than a few hours, continuous intravenous nitroglycerin had lost almost all of its hemodynamic effect after 48 hours. In the same study, patients who received nitroglycerin infusions for only 12 hours out of each 24 demonstrated no similar attenuation of effect. These results are consistent with those seen in multiple large, double-blind, placebo-controlled trials of other formulations of nitroglycerin and other nitrates.
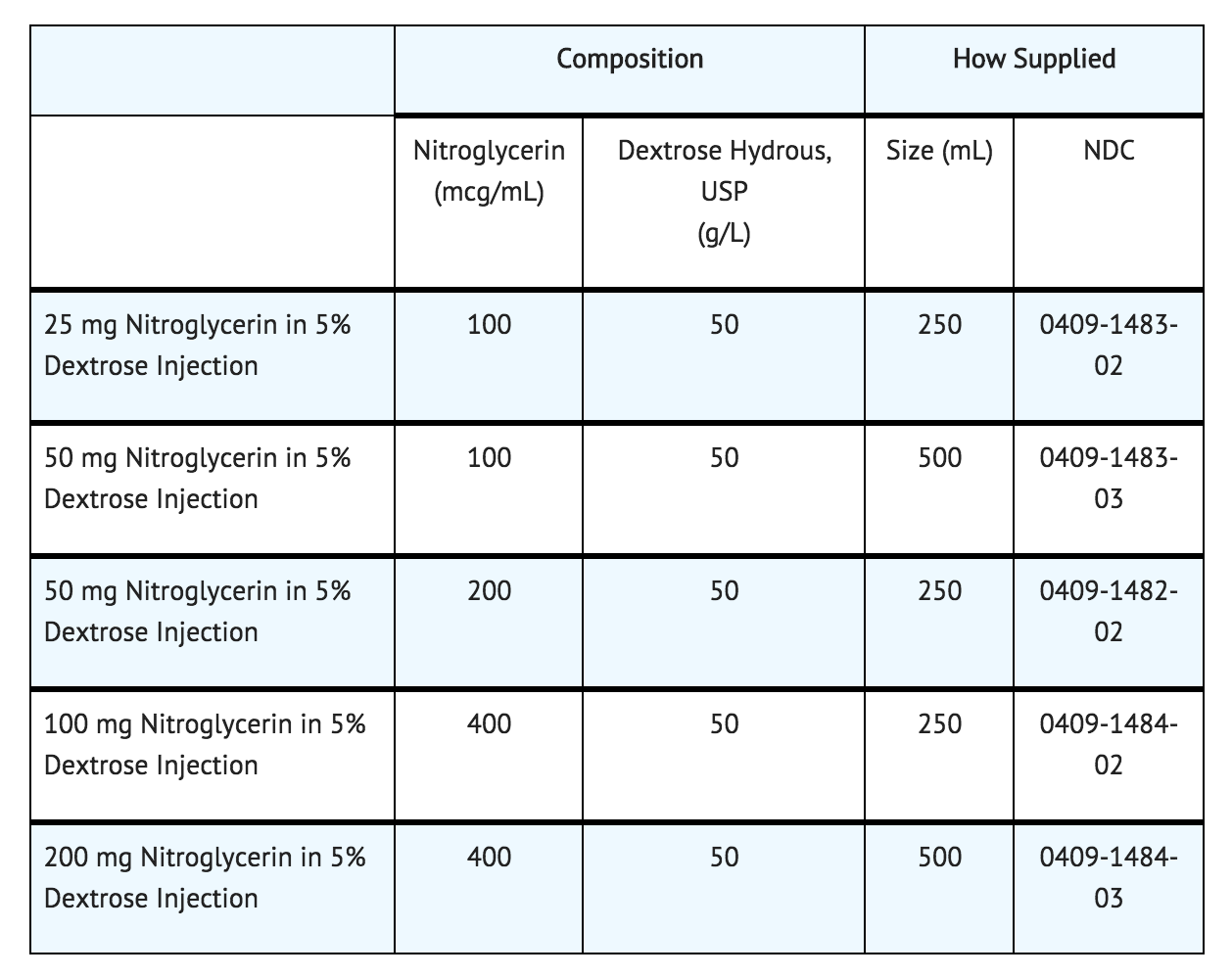
How Supplied
- Nitroglycerin in 5% Dextrose Injection is supplied in large volume glass containers as follows:
Storage
- Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Nitroglycerin (Injection solution in 5% dextrose) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
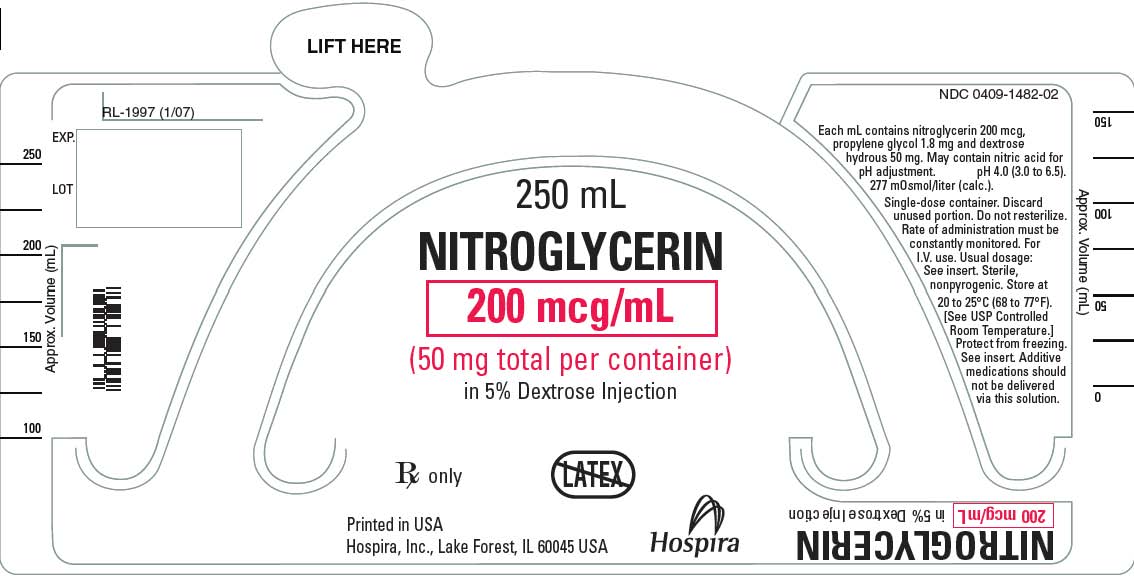
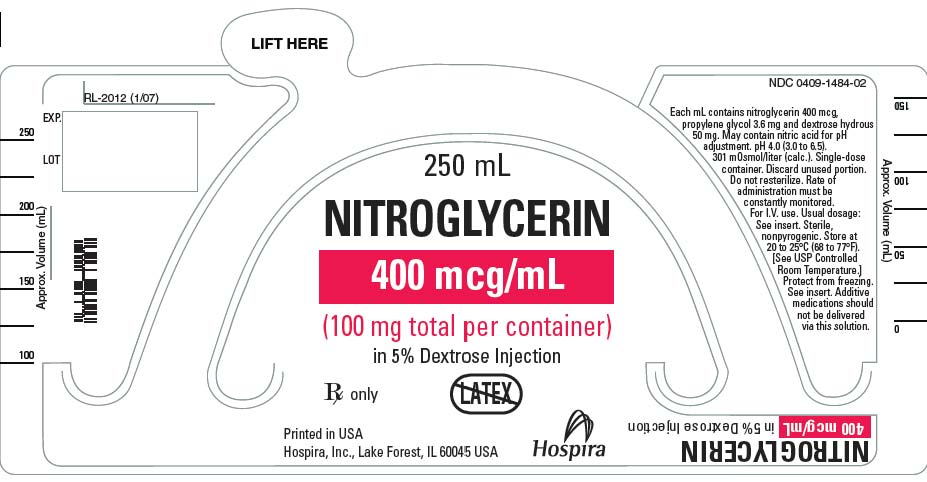
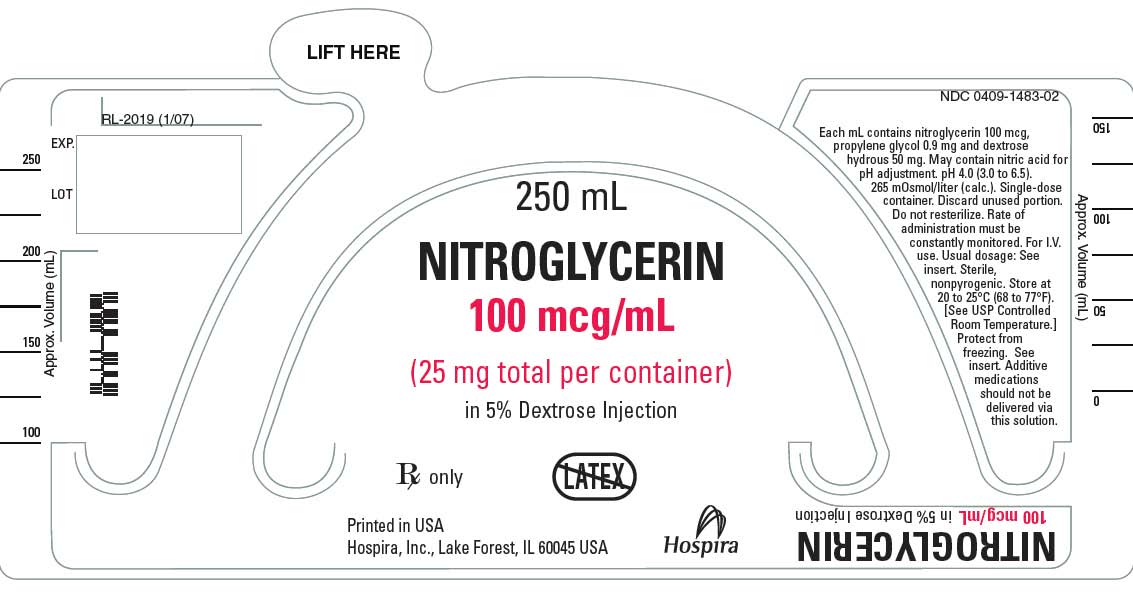

{{#ask: Label Page::Nitroglycerin (Injection solution in 5% dextrose) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- There is limited information regarding Patient Counseling Information of Nitroglycerin (Injection solution in 5% dextrose) in the drug label.
Precautions with Alcohol
- Alcohol-Nitroglycerin (Injection solution in 5% dextrose) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NITROGLYCERIN IN DEXTROSE®[1]
Look-Alike Drug Names
There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.