Deoxycholic acid
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Deoxycholic acid is a cytolytic drug that is FDA approved for the {{{indicationType}}} of improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
- Limitation of use: The safe and effective use of Deoxycholic acid for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.. Common adverse reactions include injection site edema/swelling, hematoma, pain, numbness, erythema and induration..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Deoxycholic acid is injected into subcutaneous fat tissue in the submental area using an area-adjusted dose of 2 mg/cm2.
- A single treatment consists of up to a maximum of 50 injections, 0.2 mL each (up to a total of 10 mL), spaced 1 cm apart.
- Up to 6 single treatments may be administered at intervals no less than 1 month apart.
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Deoxycholic acid FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
Deoxycholic acid is contraindicated in the presence of infection at the injection sites.
Warnings
Marginal mandibular nerve injury
- Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness (paresis), were reported during clinical trials.
- To avoid the potential for nerve injury, Deoxycholic acid should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve.
- All marginal mandibular nerve injuries reported from the trials resolved spontaneously (range 1-298 days, median 44 days).
Dysphagia
- Difficulty swallowing (dysphagia) occurred in clinical trials in the setting of administration site reactions, e.g., pain, swelling, and induration of the submental area.
- Cases of dysphagia spontaneously resolved (range 1-81 days, median 3 days).
- Subjects with current or prior history of dysphagia were excluded from clinical trials.
- Avoid use of Deoxycholic acid in these patients as current or prior history of dysphagia may exacerbate the condition.
Injection site hematoma/bruising
- In clinical trials, 72% of subjects treated with Deoxycholic acid experienced injection site hematoma/bruising.
- Deoxycholic acid should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur.
Risk of injecting in proximity to vulnerable anatomic structures
- To avoid potential tissue damage, Deoxycholic acid should not be injected into or in close proximity (1-1.5 cm) to salivary glands, lymph nodes and muscles.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In two double-blind, placebo-controlled clinical trials 513 subjects were treated with Deoxycholic acid and 506 subjects were treated with placebo.
- The population was 19-65 years old, 85% were women, 87% Caucasian, 8% African American.
- At baseline the population had a mean BMI of 29 kg/m2, moderate to severe submental convexity (graded as 2 or 3 on a 0 to 4 scale) and without excessive skin laxity.
- Subjects received up to 6 treatments at least 1 month apart and were followed for up to 6 months after the last received treatment.
- The most commonly reported adverse reactions are listed below
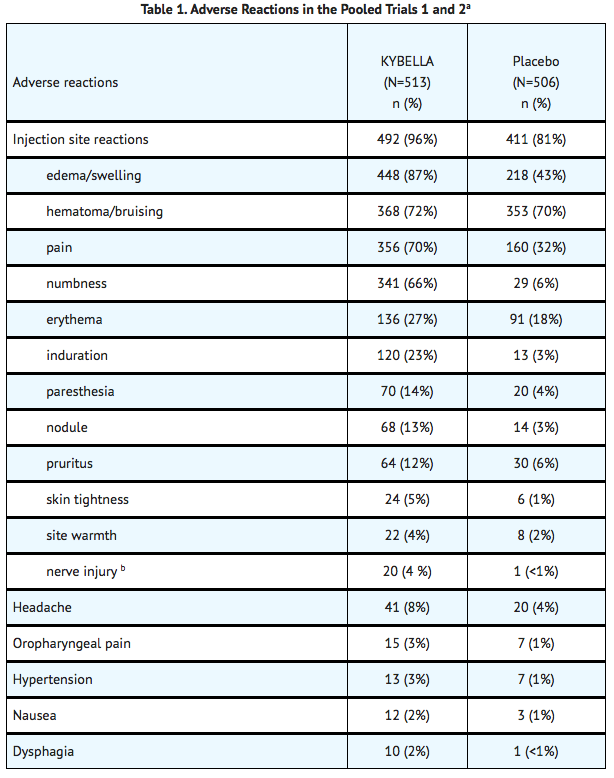
- Other adverse reactions associated with the use of Deoxycholic acid include: injection site hemorrhage, injection site discoloration, pre-syncope/syncope, lymphadenopathy, injection site urticaria and neck pain.
- Adverse reactions that lasted more than 30 days and occurred in more than 10% of subjects were injection site numbness (42%), injection site edema/swelling (20%), injection site pain (16%), and injection site induration (13%).
Postmarketing Experience
There is limited information regarding Deoxycholic acid Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Deoxycholic acid Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no adequate and well-controlled studies of Deoxycholic acid in pregnant women to inform the drug-associated risk.
- The background risk of major birth defects and miscarriage for the indicated population is unknown.
- However, the background risk of major birth defects in the U.S. general population is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
- In animal reproduction studies, no fetal harm was observed with the subcutaneous administration of deoxycholic acid to rats during organogenesis at doses up to 5 times the maximum recommended human dose (MRHD) of 100 mg.
Animal Data
- Embryofetal development studies have been performed in rats and rabbits using subcutaneous doses of deoxycholic acid administered during the period of organogenesis.
- For the basis of comparing animal to human doses, the MRHD is 1.7 mg/kg (100 mg/60 kg).
- No evidence of fetal harm was observed in rats at up to the highest dose tested (50 mg/kg) which is 5-fold higher than the MRHD of Deoxycholic acid based on a mg/m2 comparison.
- However, missing intermediate lung lobe was noted in rabbits at all dose levels tested including the lowest dose (10 mg/kg) which is 2-fold higher than the MRHD of Deoxycholic acid based on a mg/m2 comparison.
- These effects may be related to maternal toxicity, which was also seen at all dose levels tested.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Deoxycholic acid in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Deoxycholic acid during labor and delivery.
Nursing Mothers
- There is no information available on the presence of synthetic deoxycholic acid in human milk, the effects of the drug on the breastfed infant or the effects of the drug on milk production.
- The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Deoxycholic acid and any potential adverse effects on the breastfed child from Deoxycholic acid or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness in patients below the age of 18 years have not been established and Deoxycholic acid is not intended for use in children or adolescents
Geriatic Use
- The clinical trials of Deoxycholic acid did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
- Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
- In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Deoxycholic acid with respect to specific gender populations.
Race
There is no FDA guidance on the use of Deoxycholic acid with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Deoxycholic acid in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Deoxycholic acid in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Deoxycholic acid in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Deoxycholic acid in patients who are immunocompromised.
Administration and Monitoring
Administration
General Considerations for Administration
- Deoxycholic acid should be administered by a healthcare professional.
- Screen patients for other potential causes of submental convexity/fullness (e.g., thyromegaly and cervical lymphadenopathy).
- Give careful consideration to the use of Deoxycholic acid in patients with excessive skin laxity, prominent platysmal bands or other conditions for which reduction of submental fat may result in an aesthetically undesirable outcome.
- Use caution in patients who have had prior surgical or aesthetic treatment of the submental area.
- Changes in anatomy/landmarks or the presence of scar tissue may impact the ability to safely administer Deoxycholic acid or to obtain the desired aesthetic result.
- Deoxycholic acid is clear, colorless and free of particulate matter.
- Visually inspect Deoxycholic acid vials for particulate matter and/or discoloration, and discard the vial if the solution is discolored and/or contains particulate matter.
- After use, discard any remaining solution in the vial.
Injection Technique
- The safe and effective use of Deoxycholic acid depends on the use of the correct number and locations for injections, proper needle placement, and administration techniques.
- Health care professionals administering Deoxycholic acid must understand the relevant submental anatomy and associated neuromuscular structures in the area involved and any alterations to the anatomy due to prior surgical or aesthetic procedures.
Avoid injections near the area of the marginal mandibular nerve
- Needle placement with respect to the mandible is very important as it reduces the potential for injury to the marginal mandibular nerve, a motor branch of the facial nerve.
- Injury to the nerve presents as an asymmetrical smile due to paresis of lip depressor muscles.
- To avoid injury to the marginal mandibular nerve:
Avoid injection into the platysma
- Prior to each treatment session, palpate the submental area to ensure sufficient submental fat and to identify subcutaneous fat between the dermis and platysma (pre-platysmal fat) within the target treatment area.
- The number of injections and the number of treatments should be tailored to the individual patient's submental fat distribution and treatment goals.
Injecting into the treatment area
- Use of ice/cold packs, topical and/or injectable local anesthesia (e.g., lidocaine) may enhance patient comfort.
- Outline the planned treatment area with a surgical pen and apply a 1 cm injection grid to mark the injection sites.
Do not inject KYBELLA outside the defined parameters
- Using a large bore needle, draw 1 mL of Deoxycholic acid into a sterile 1 mL syringe and expel any air bubbles in the syringe barrel.
- Have the patient tense the platysma.
- Pinch the submental fat and, using a 30 gauge (or smaller) 0.5 inch needle, inject 0.2 mL of Deoxycholic acid into the pre-platysmal fat next to each of the marked injection sites by advancing the needle perpendicular to the skin.
- Injections that are too superficial (into the dermis) may result in skin ulceration.
- Do not withdraw the needle from the subcutaneous fat during injection as this could increase the risk of intradermal exposure and potential skin ulceration.
- Avoid injecting into the post-platysmal fat by injecting Deoxycholic acid into fat tissue at the depth of approximately mid-way into the subcutaneous fat layer.
- If at any time resistance is met as the needle is inserted, indicating the possibility of contact with fascial or nonfat tissue, the needle must be withdrawn to an appropriate depth before the injection is administered.
- Avoid injecting into other tissues such as the muscle, salivary glands and lymph nodes.
- Upon needle withdrawal, pressure may be applied to each injection site as necessary to minimize bleeding; an adhesive dressing may be applied.
Monitoring
There is limited information regarding Deoxycholic acid Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Deoxycholic acid and IV administrations.
Overdosage
- Injection of excessive doses/volumes of Deoxycholic acid may increase the risk of adverse reactions.
Pharmacology
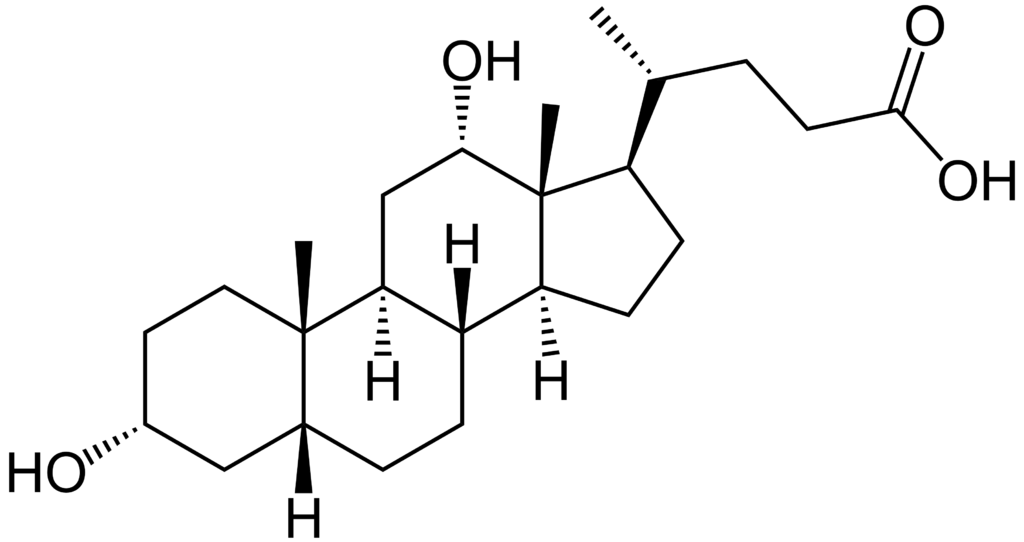
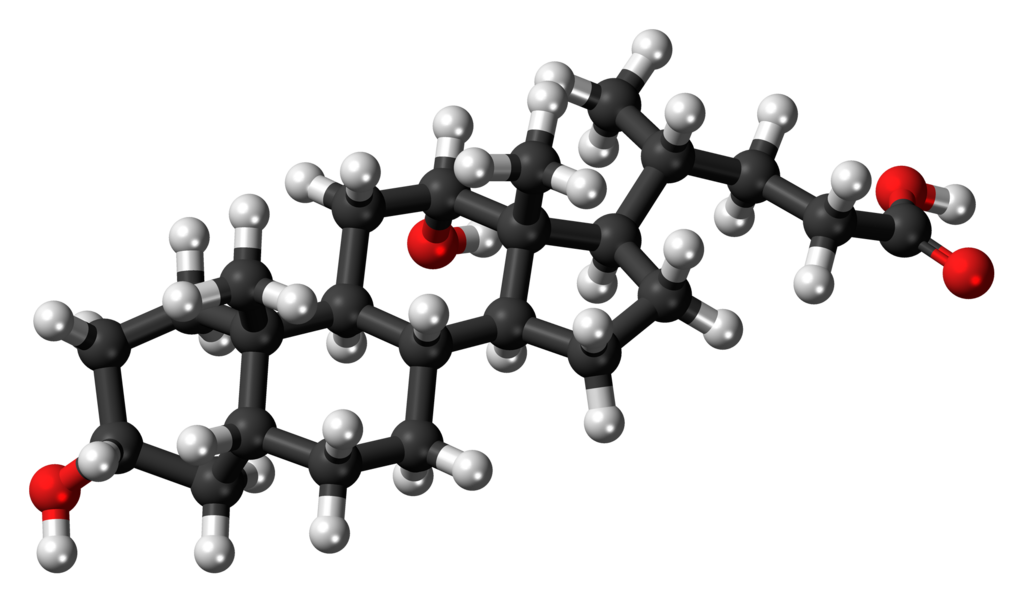
The chemical structure of deoxycholic acid is: Chemical Structure:


Mechanism of Action
- Deoxycholic acid is a cytolytic drug, which when injected into tissue physically destroys the cell membrane causing lysis.
Structure
- Deoxycholic acid injection, 10 mg/mL is a clear colorless, sterile solution for subcutaneous use.
- It contains a cytolytic agent, deoxycholic acid, as the active ingredient.
- The chemical name of deoxycholic acid is 3α,12α-dihydroxy-5β-cholan-24-oic acid, and its molecular formula is C24H40O4, and its molecular weight is 392.57 g/mol.
- Each 2 mL vial of Deoxycholic acid injection contains 20 mg synthetic deoxycholic acid as the active ingredient and the following inactive ingredients: benzyl alcohol (18 mg), dibasic sodium phosphate (2.84 mg), sodium chloride (8.76 mg), sodium hydroxide (2.86 mg) in water for injection, USP.
- Hydrochloric acid and additional sodium hydroxide are added as necessary to adjust the formulation to pH 8.3.
- Each vial is for single patient use.
Pharmacodynamics
Cardiac Electrophysiology
- At therapeutic doses, Deoxycholic acid does not prolong the QTc interval.
Pharmacokinetics
- Endogenous deoxycholic acid plasma levels are highly variable within and between individuals; most of this natural bile component is sequestered in the enterohepatic circulation loop.
Absorption and Distribution
- Deoxycholic acid from Deoxycholic acid is rapidly absorbed following subcutaneous injection.
- After dosing with the maximum recommended single treatment dose with Deoxycholic acid (100 mg), maximum plasma concentrations (mean Cmax) were observed with a median Tmax of 18 minutes after injection.
- The mean (±SD) Cmax value was 1024 ± 304 ng/mL and was 3.2-fold higher than average Cmax values observed during a 24-hour baseline endogenous period in the absence of Deoxycholic acid.
- After maximum recommended single treatment dose (100 mg), mean (±SD) deoxycholic acid exposure (AUC0-24) was 7896 ± 2269 ng.hr/mL and was 1.6-fold higher over endogenous exposure.
- Post-treatment deoxycholic acid plasma levels returned to the endogenous range within 24 hours.
- No accumulation is expected with the proposed treatment frequency.
- Deoxycholic acid is extensively bound to proteins in plasma (98%).
Metabolism and Excretion
- Endogenous deoxycholic acid is a product of cholesterol metabolism and is excreted intact in feces.
- Deoxycholic acid is not metabolized to any significant extent under normal conditions.
- Deoxycholic acid from Deoxycholic acid joins the endogenous bile acid pool in the enterohepatic circulation and is excreted along with the endogenous deoxycholic acid.
In Vitro Assessment of Drug Interactions
- Results from in vitro studies indicate that deoxycholic acid does not inhibit or induce human cytochrome P450 (CYP) enzymes at clinically relevant concentrations.
- Deoxycholic acid does not inhibit the following transporters: P-gp, BCRP, MRP4, MRP2, OATP1B1, OATP2B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, NTCP, and ASBT.
Specific Populations
Hepatic Impairment
- Deoxycholic acid has not been studied in subjects with hepatic impairment.
- Considering the intermittent dose frequency, the small dose administered that represents approximately 3% of the total bile acid pool, and the highly variable endogenous deoxycholic acid levels, the pharmacokinetics of deoxycholic acid following Deoxycholic acid injection is unlikely to be influenced by hepatic impairment.
Pharmacokinetic Effects of Gender
- Deoxycholic acid pharmacokinetics were not influenced by gender.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Deoxycholic acid.
- Deoxycholic acid was negative in a battery of in vitro (Ames test and chromosomal aberration assay in human lymphocytes) and in vivo (rat erythrocyte micronucleus assay) genetic toxicology assays.
- No effects on fertility were observed in male and female rats administered deoxycholic acid at subcutaneous doses up to 50 mg/kg (5 times the MRHD based on a mg/m2 comparison) once weekly prior to and during the mating period and through gestation day 7 in female rats.
Clinical Studies
- Two randomized, multi-center, double-blind, placebo-controlled trials of identical design were conducted to evaluate Deoxycholic acid for use in improvement in the appearance of convexity or fullness associated with submental fat.
- The trials enrolled healthy adults (ages 19 to 65, BMI ≤ 40 kg/m2) with moderate or severe convexity or fullness associated with submental fat (i.e., grade 2 or 3 on 5-point grading scales, where 0 = none and 4 = extreme), as judged by both clinician and subject ratings.
- Subjects received up to 6 treatments with Deoxycholic acid (N=514, combined trials) or placebo (N=508, combined trials) at no less than 1 month intervals.
- Use of ice/cold packs, topical and/or injectable local anesthesia was allowed during the clinical trials.
- Injection volume was 0.2 mL per injection site, spaced 1 cm apart into the submental fat tissue, which is also expressed in dose per area as 2 mg/cm2.
- For each treatment session a maximum of 100 mg (10 mL) was permitted over the entire treatment area.
- Subjects were administered an average of 6.4 mL at the first treatment session, and subjects who received all six treatments were administered an average of 4.4 mL at the sixth treatment session.
- Fifty-nine percent of subjects received all six treatments.
- In these trials, the mean age was 49 years and the mean BMI was 29 kg/m2.
- Most of the subjects were women (85%) and Caucasian (87%).
- At baseline, 51% of the subjects had a clinician-rated submental fat severity rating of moderate and 49% had a severe submental fat rating.
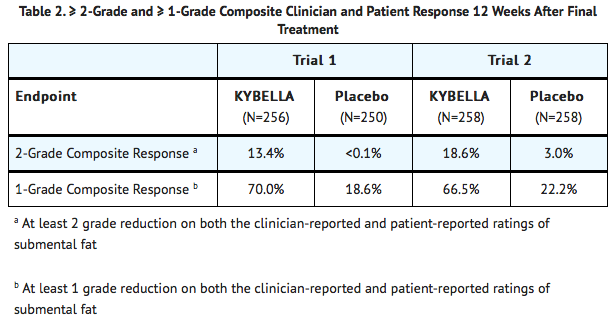
- The co-primary efficacy assessments were based on at least 2-grade and at least 1-grade improvements in submental convexity or fullness on the composite of clinician-reported and patient-reported ratings of submental fat 12 weeks after final treatment.
- Additionally, changes in submental fat volume were evaluated in a subset of subjects (N=449, combined trials) using magnetic resonance imaging (MRI).
- Visual and emotional impacts of submental fat (happy, bothered, self-conscious, embarrassed, looking older or overweight) were also evaluated using a 6-question survey, with each question rated from 0 (not at all) to 10 (extremely/very much).
- Reductions in submental fat volume were observed more frequently in the Deoxycholic acid group compared to the placebo group as measured by the composite clinician and patient ratings (TABLE 2).
- The composite response rates by visit are presented in FIGURE 4.
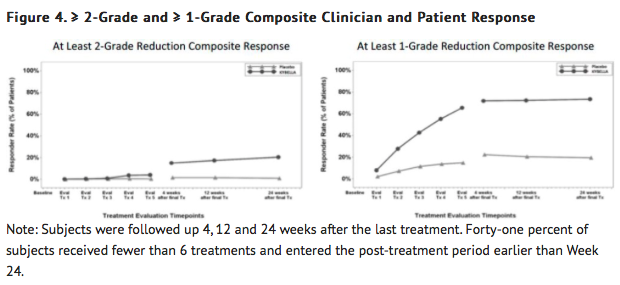
- A greater proportion of Deoxycholic acid-treated subjects had at least a 10% reduction in submental fat volume as compared to placebo-treated subjects when evaluated by MRI (43% vs 5%, respectively).
- The overall patient-reported satisfaction and self-perceived visual attributes showed greater improvement in the Deoxycholic acid group than in the placebo group.
How Supplied
- Deoxycholic acid injection, 10 mg/mL is a clear, colorless, sterile solution supplied in 2 mL, single patient use vials in the following dispensing pack:
- 4 vials, NDC 61168-101-04
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F).
- KYBELLA has a unique hologram on the vial label.
- If you do not see a hologram, do not use the product and call 1-844-KYTHERA (1-844-598-4372).
- Each vial is for a single patient use.
- Do not dilute.
- Discard unused portion.
Images
Drug Images
{{#ask: Page Name::Deoxycholic acid |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Deoxycholic acid |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Advise patients to inform their healthcare professional if they develop signs of marginal mandibular nerve paresis (e.g., asymmetric smile, facial muscle weakness), difficulty swallowing, or if any existing symptom worsens.
Precautions with Alcohol
Alcohol-Deoxycholic acid interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
KYBELLA (deoxycholic acid) injection.
Look-Alike Drug Names
There is limited information regarding Deoxycholic acid Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.