Cyclosporine (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cyclosporine (ophthalmic) is an anti-inflammatory, calcineurin inhibitor and immune suppressant agent that is FDA approved for the treatment of increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca. Common adverse reactions include burning sensation in eye.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
RESTASIS® ophthalmic emulsion is indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca. Increased tear production was not seen in patients currently taking topical anti-inflammatory drugs or using punctal plugs.
Dosing Information
- Invert the unit dose vial a few times to obtain a uniform, white, opaque emulsion before using. Instill one drop of RESTASIS® ophthalmic emulsion twice a day in each eye approximately 12 hours apart. RESTASIS® can be used concomitantly with artificial tears, allowing a 15 minute interval between products. Discard vial immediately after use.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cyclosporine (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cyclosporine (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Cyclosporine (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cyclosporine (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cyclosporine (ophthalmic) in pediatric patients.
Contraindications
- RESTASIS® is contraindicated in patients with known or suspected hypersensitivity to any of the ingredients in the formulation.
Warnings
Potential for Eye Injury and Contamination
- To avoid the potential for eye injury and contamination, be careful not to touch the vial tip to your eye or other surfaces.
Use with Contact Lenses=
- RESTASIS® should not be administered while wearing contact lenses. Patients with decreased tear production typically should not wear contact lenses. If contact lenses are worn, they should be removed prior to the administration of the emulsion. Lenses may be reinserted 15 minutes following administration of RESTASIS® ophthalmic emulsion.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In clinical trials, the most common adverse reaction following the use of RESTASIS® was ocular burning (17%).
- Other reactions reported in 1% to 5% of patients included conjunctival hyperemia, discharge, epiphora, eye pain, foreign body sensation, pruritus, stinging, and visual disturbance (most often blurring).
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of RESTASIS®. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Reported reactions have included: hypersensitivity (including eye swelling, urticaria, rare cases of severe angioedema, face swelling, tongue swelling, pharyngeal edema, and dyspnea); and superficial injury of the eye (from the vial tip touching the eye during administration).
Drug Interactions
There is limited information regarding Cyclosporine (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Adverse effects were seen in reproduction studies in rats and rabbits only at dose levels toxic to dams. At toxic doses (rats at 30 mg/kg/day and rabbits at 100 mg/kg/day), cyclosporine oral solution, USP, was embryo- and fetotoxic as indicated by increased pre- and postnatal mortality and reduced fetal weight together with related skeletal retardations. These doses are 5,000 and 32,000 times greater (normalized to body surface area), respectively, than the daily human dose of one drop (approximately 28 mcL) of 0.05% RESTASIS® twice daily into each eye of a 60 kg person (0.001 mg/kg/day), assuming that the entire dose is absorbed. No evidence of embryofetal toxicity was observed in rats or rabbits receiving cyclosporine at oral doses up to 17 mg/kg/day or 30 mg/kg/day, respectively, during organogenesis. These doses in rats and rabbits are approximately 3,000 and 10,000 times greater (normalized to body surface area), respectively, than the daily human dose.
- Offspring of rats receiving a 45 mg/kg/day oral dose of cyclosporine from Day 15 of pregnancy until Day 21 postpartum, a maternally toxic level, exhibited an increase in postnatal mortality; this dose is 7,000 times greater than the daily human topical dose (0.001 mg/kg/day) normalized to body surface area assuming that the entire dose is absorbed. No adverse events were observed at oral doses up to 15 mg/kg/day (2,000 times greater than the daily human dose).
- There are no adequate and well-controlled studies of RESTASIS® in pregnant women. RESTASIS® should be administered to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cyclosporine (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cyclosporine (ophthalmic) during labor and delivery.
Nursing Mothers
- Cyclosporine is known to be excreted in human milk following systemic administration, but excretion in human milk after topical treatment has not been investigated. Although blood concentrations are undetectable after topical administration of RESTASIS® ophthalmic emulsion, caution should be exercised when RESTASIS® is administered to a nursing woman.
Pediatric Use
- The safety and efficacy of RESTASIS® ophthalmic emulsion have not been established in pediatric patients below the age of 16.
Geriatic Use
- No overall difference in safety or effectiveness has been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Cyclosporine (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cyclosporine (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cyclosporine (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cyclosporine (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cyclosporine (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cyclosporine (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Cyclosporine (ophthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Cyclosporine (ophthalmic) and IV administrations.
Overdosage
There is limited information regarding Cyclosporine (ophthalmic) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Cyclosporine is an immunosuppressive agent when administered systemically.
- In patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca, cyclosporine emulsion is thought to act as a partial immunomodulator. The exact mechanism of action is not known.
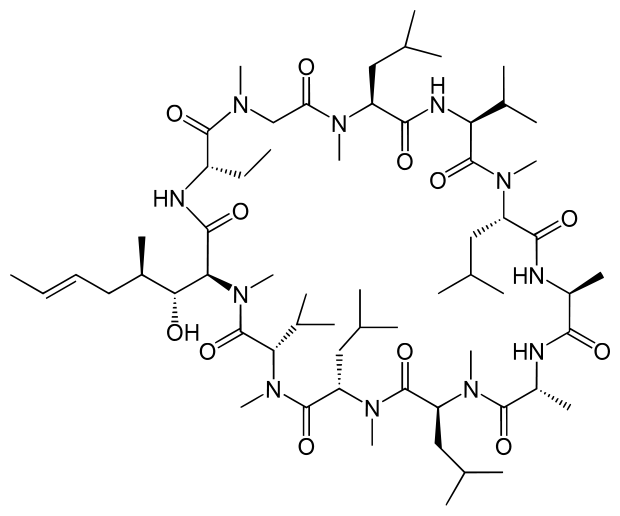
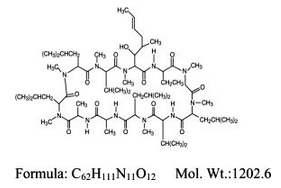
Structure
- RESTASIS® (cyclosporine ophthalmic emulsion) 0.05% contains a topical immunomodulator with anti-inflammatory effects. Cyclosporine's chemical name is Cyclo{{(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl}-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valy}}and it has the following structure:
- Structural Formula
Pharmacodynamics
There is limited information regarding Cyclosporine (ophthalmic) Pharmacodynamics in the drug label.
Pharmacokinetics
- Blood cyclosporine A concentrations were measured using a specific high pressure liquid chromatography-mass spectrometry assay. Blood concentrations of cyclosporine, in all the samples collected, after topical administration of RESTASIS® 0.05%, twice daily, in humans for up to 12 months, were below the quantitation limit of 0.1 ng/mL. There was no detectable drug accumulation in blood during 12 months of treatment with RESTASIS® ophthalmic emulsion.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:=
- Systemic carcinogenicity studies were carried out in male and female mice and rats. In the 78-week oral (diet) mouse study, at doses of 1, 4, and 16 mg/kg/day, evidence of a statistically significant trend was found for lymphocytic lymphomas in females, and the incidence of hepatocellular carcinomas in mid-dose males significantly exceeded the control value.
- In the 24-month oral (diet) rat study, conducted at 0.5, 2, and 8 mg/kg/day, pancreatic islet cell adenomas significantly exceeded the control rate in the low dose level. The hepatocellular carcinomas and pancreatic islet cell adenomas were not dose related. The low doses in mice and rats are approximately 80 times greater (normalized to body surface area) than the daily human dose of one drop (approximately 28 mcL) of 0.05% RESTASIS® twice daily into each eye of a 60 kg person (0.001 mg/kg/day), assuming that the entire dose is absorbed.
Mutagenesis:=
- Cyclosporine has not been found to be mutagenic/genotoxic in the Ames Test, the V79-HGPRT Test, the micronucleus test in mice and Chinese hamsters, the chromosome-aberration tests in Chinese hamster bone-marrow, the mouse dominant lethal assay, and the DNA-repair test in sperm from treated mice. A study analyzing sister chromatid exchange (SCE) induction by cyclosporine using human lymphocytes in vitro gave indication of a positive effect (i.e., induction of SCE).
Impairment of Fertility:
- No impairment in fertility was demonstrated in studies in male and female rats receiving oral doses of cyclosporine up to 15 mg/kg/day (approximately 2,000 times the human daily dose of 0.001 mg/kg/day normalized to body surface area) for 9 weeks (male) and 2 weeks (female) prior to mating.
Clinical Studies
- Four multicenter, randomized, adequate and well-controlled clinical studies were performed in approximately 1,200 patients with moderate to severe keratoconjunctivitis sicca. RESTASIS® demonstrated statistically significant increases in Schirmer wetting of 10 mm versus vehicle at six months in patients whose tear production was presumed to be suppressed due to ocular inflammation. This effect was seen in approximately 15% of RESTASIS® ophthalmic emulsion-treated patients versus approximately 5% of vehicle-treated patients. Increased tear production was not seen in patients currently taking topical anti-inflammatory drugs or using punctal plugs.
- No increase in bacterial or fungal ocular infections was reported following administration of RESTASIS®.
How Supplied
RESTASIS® ophthalmic emulsion is packaged in sterile, preservative-free single-use vials. Each vial contains 0.4 mL fill in a 0.9 mL LDPE vial; 30 or 60 vials are packaged in a polypropylene tray with an aluminum peelable lid. The entire contents of each tray (30 vials or 60 vials) must be dispensed intact.
30 Vials 0.4 mL each - NDC 0023-9163-30 60 Vials 0.4 mL each - NDC 0023-9163-60
Storage
- Storage: Store at 15°-25°C (59°-77°F).
Images
Drug Images
{{#ask: Page Name::Cyclosporine (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
NDC 0023-9163-60 No. 94529
Restasis® (Cyclosporine Ophthalmic Emulsion) 0.05%
60 Single-Use Vials (0.4 mL each) One Month Supply Sterile, Preservative-Free
Each mL contains: Active: cyclosporine 0.05% Inactives: glycerin; castor oil; polysorbate 80; carbomer copolymer type A; purified water; and sodium hydroxide to adjust pH. Usual Dosage: Twice daily approximately 12 hours apart. Invert the vial before using. Use immediately after opening and then discard. Storage: Store at 15°-25°C (59°-77°F). Store vials in the thermoformed tray until use. The entire contents of each package (60 vials) must be dispensed intact.
© 2013 Allergan, Inc. Irvine, CA 92612, U.S.A. ® marks owned by Allergan, Inc. Patented. See: www.allergan.com/products/patent_notices Made in the U.S.A.
ALLERGAN
Lot:
Exp.:
Rx only
55091US10

{{#ask: Label Page::Cyclosporine (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Handling the Container
- Advise patients to not allow the tip of the vial to touch the eye or any surface, as this may contaminate the emulsion. To avoid the potential for injury to the eye, advise patients to not touch the vial tip to their eye [see Warnings and Precautions (5.1)].
Use with Contact Lenses
- RESTASIS® should not be administered while wearing contact lenses. Patients with decreased tear production typically should not wear contact lenses. Advise patients that if contact lenses are worn, they should be removed prior to the administration of the emulsion. Lenses may be reinserted 15 minutes following administration of RESTASIS® ophthalmic emulsion [see Warnings and Precautions (5.2)].
Administration
- Advise patients that the emulsion from one individual single-use vial is to be used immediately after opening for administration to one or both eyes, and the remaining contents should be discarded immediately after administration.
© 2013 Allergan, Inc. Irvine, CA 92612, U.S.A. ® marks owned by Allergan, Inc. Patented. See: www.allergan.com/products/patent_notices Made in the U.S.A.
Precautions with Alcohol
Alcohol-Cyclosporine (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- RESTASIS[1]
Look-Alike Drug Names
There is limited information regarding Cyclosporine (ophthalmic) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.