Cromolyn (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cromolyn (ophthalmic) is a mast cell stabilizer that is FDA approved for the treatment of vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis. Common adverse reactions include dyspnea, rash, edema, itchy eyes, styes, and puffy eyes.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Indicated in the treatment of vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
Dosing
- The dose is 1 – 2 drops in each eye 4 – 6 times a day at regular intervals.
- One drop contains approximately 1.6 mg cromolyn sodium.
- Patients should be advised that the effect of cromolyn sodium ophthalmic solution therapy is dependent upon its administration at regular intervals, as directed.
- Symptomatic response to therapy (decreased itching, tearing, redness, and discharge) is usually evident within a few days, but longer treatment for up to six weeks is sometimes required. Once symptomatic improvement has been established, therapy should be continued for as long as needed to sustain improvement.
- If required, corticosteroids may be used concomitantly with cromolyn sodium ophthalmic solution.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cromolyn (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cromolyn (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Cromolyn (ophthalmic) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cromolyn (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cromolyn (ophthalmic) in pediatric patients.
Contraindications
- Cromolyn sodium ophthalmic solution is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium or to any of the other ingredients.
Warnings
There is limited information regarding Warning of Cromolyn (ophthalmic) in pediatric patients.
Adverse Reactions
Clinical Trials Experience
- The most frequently reported adverse reaction attributed to the use of cromolyn sodium ophthalmic solution, on the basis of reoccurrence following readministration, is transient ocular stinging or burning upon instillation.
- The following adverse reactions have been reported as infrequent events. It is unclear whether they are attributed to the drug:
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Cromolyn (ophthalmic) in the drug label.
Drug Interactions
There is limited information regarding Drug interactions of Cromolyn (ophthalmic) in pediatric patients.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category B
Reproduction studies with cromolyn sodium administered subcutaneously to pregnant mice and rats at maximum daily doses of 540 mg/kg (1620 mg/m2) and 164 mg/kg (984 mg/m2), respectively, and intravenously to rabbits at a maximum daily dose of 485 mg/kg (5820 mg/m2) produced no evidence of fetal malformation. These doses represent approximately 57, 35, and 205 times the maximum daily human dose, respectively, on a mg/m2 basis. Adverse fetal effects (increased resorption and decreased fetal weight) were noted only at the very high parenteral doses that produced maternal toxicity. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cromolyn (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cromolyn (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when cromolyn sodium ophthalmic solution is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 4 years have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Cromolyn (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cromolyn (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cromolyn (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cromolyn (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cromolyn (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cromolyn (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Monitoring of Cromolyn (ophthalmic) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Cromolyn (ophthalmic) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Cromolyn (ophthalmic) in the drug label.
Pharmacology
Mechanism of Action
- In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the degranulation of sensitized mast cells which occurs after exposure to specific antigens. Cromolyn sodium acts by inhibiting the release of histamine and SRS-A (slow-reacting substance of anaphylaxis) from the mast cell.
- Another activity demonstrated in vitro is the capacity of cromolyn sodium to inhibit the degranulation of non-sensitized rat mast cells by phospholipase A and the subsequent release of chemical mediators. Another study showed that cromolyn sodium did not inhibit the enzymatic activity of released phospholipase A on its specific substrate.
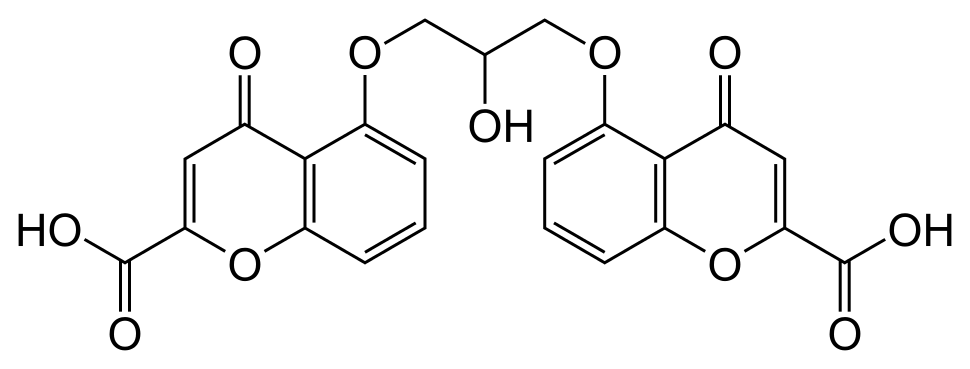
Structure
- Cromolyn sodium ophthalmic solution USP, 4% is a clear, colorless, sterile solution for topical ophthalmic use.
- Cromolyn sodium is represented by the following structural formula:
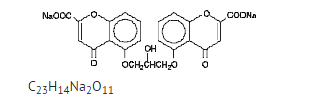
- Chemical Name: Disodium 5,5'- [(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]
- Pharmacologic Category: Mast cell stabilizer.
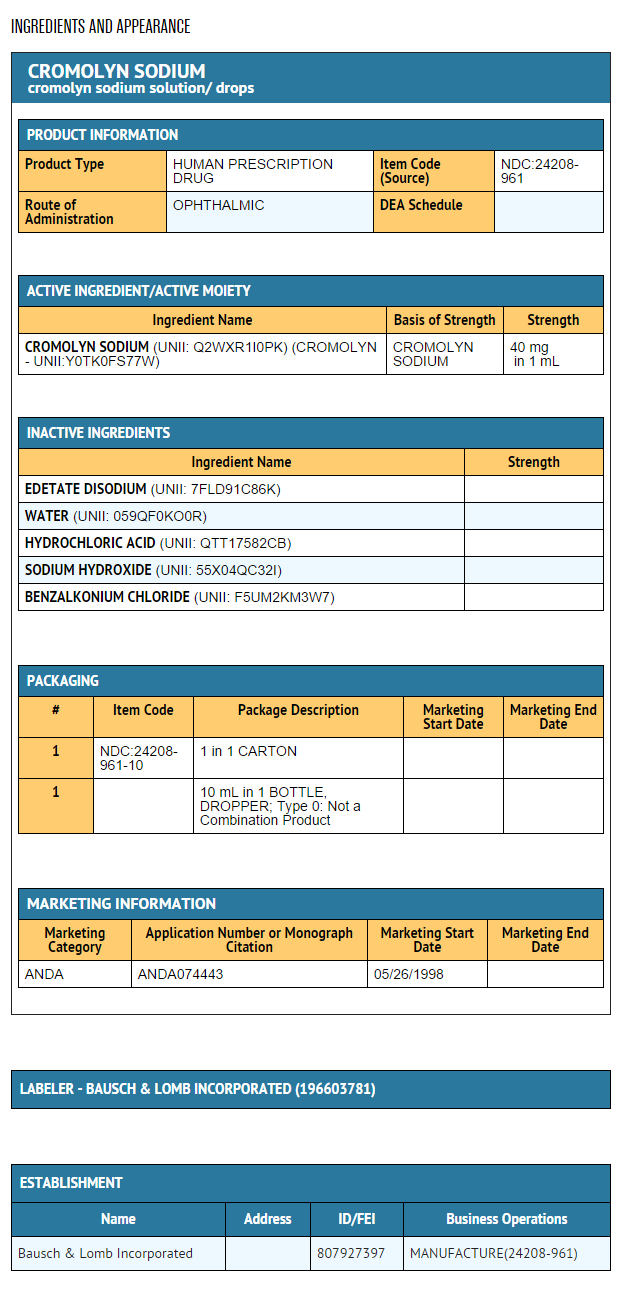
EACH mL CONTAINS: ACTIVE: Cromolyn Sodium 40 mg (4%); INACTIVES: Edetate Disodium 0.1% and Purified Water. Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (4.0 - 7.0). PRESERVATIVE ADDED: Benzalkonium Chloride 0.01%.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cromolyn (ophthalmic) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Cromolyn (ophthalmic) in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
- Long-term studies of cromolyn sodium in mice (12 months intraperitoneal administration at doses up to 150 mg/kg three days per week): hamsters (intraperitoneal administration at doses up to 52.6 mg/kg three days per week for 15 weeks followed by 17.5 mg/kg three days per week for 37 weeks), and rats (18 months subcutaneous administration at doses up to 75 mg/kg six days per week) showed no neoplastic effects. The average daily maximum dose levels administered in these studies were 192.9 mg/m2 for mice, 47.2 mg/m2 for hamsters and 385.8 mg/m2 for rats. These doses correspond to approximately 6.8, 1.7, and 14 times the maximum daily human dose of 28 mg/m2.
- Cromolyn sodium showed no mutagenic potential in the Ames Salmonella/microsome plate assays, mitotic gene conversion in Saccharomyces cerevisiae and in an in vitro cytogenetic study in human peripheral lymphocytes.
- No evidence of impaired fertility was shown in laboratory reproduction studies conducted subcutaneously in rats at the highest doses tested, 175 mg/kg/day (1050 mg/m2) in males and 100 mg/kg/day (600 mg/m2) in females. These doses are approximately 37 and 21 times the maximum daily human dose, respectively, based on mg/m2.
Clinical Studies
There is limited information regarding Clinical Studies of Cromolyn (ophthalmic) in the drug label.
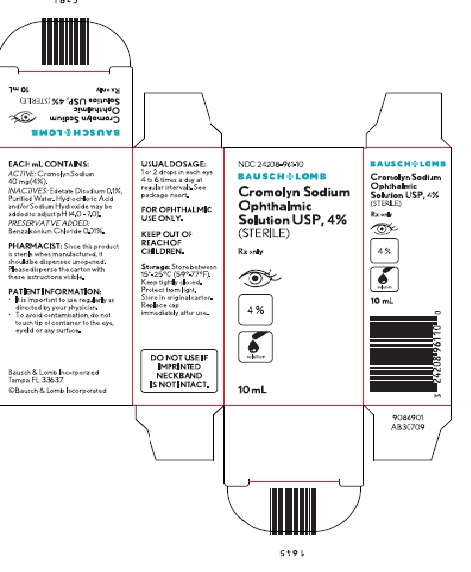
How Supplied
- Cromolyn sodium ophthalmic solution USP, 4% is supplied in a plastic bottle individually cartoned with a controlled drop tip in the following sizes:
- 10 mL bottle (NDC 24208-961-10)
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
Storage
Store between 15°-25°C (59°-77°F). Protect from light – store in original carton. Keep tightly closed. Replace cap immediately after use.
Images
Drug Images
{{#ask: Page Name::Cromolyn (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Cromolyn (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients:
- Patients should be advised to follow the patient instructions listed on the Information for Patients sheet.
- Users of contact lenses should refrain from wearing lenses while exhibiting the signs and symptoms of vernal keratoconjunctivitis, vernal conjunctivitis, or vernal keratitis. Do not wear contact lenses during treatment with cromolyn sodium ophthalmic solution.
Precautions with Alcohol
- Alcohol-Cromolyn (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CROMOLYN SODIUM®[1]
Look-Alike Drug Names
There is limited information regarding Cromolyn (ophthalmic) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Cromolyn (ophthalmic)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}