Coagulation factor IX
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Coagulation factor IX is an antihemophilic factor that is FDA approved for the prevention of bleeding episodes in adult and pediatric patients with hemophilia B and peri-operative management in adult and pediatric patients with hemophilia B. Common adverse reactions include nausea, injection site reaction, injection site pain, headache, dizziness and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Control and Prevention of Bleeding Episodes in Hemophilia B
- Coagulation Factor IX (Recombinant), is indicated for the control and prevention of bleeding episodes in adult and pediatric patients with hemophilia B (congenital factor IX deficiency or Christmas disease).
Peri-operative Management in Patients with Hemophilia B
- Coagulation Factor IX (Recombinant), is indicated for peri-operative management in adult and pediatric patients with hemophilia B.
- Coagulation Factor IX (Recombinant), isNOT indicated for:
- A.treatment of other factor deficiencies (e.g., factors II, VII, VIII, and X),
- B.treatment of hemophilia A patients with inhibitors to factor VIII,
- C.reversal of coumarin-induced anticoagulation,
- D.treatment of bleeding due to low levels of liver-dependent coagulation factors.
Dosage
General Considerations for Administration
- For Intravenous Use after Reconstitution
- Treatment with Coagulation Factor IX (Recombinant), should be initiated under the supervision of a physician experienced in the treatment of hemophilia B.
- Each vial of coagulation factor IX has the rFIX potency in the International Units (IU) stated on the vial.
- Dosage and duration of treatment for all factor IX products depend on the severity of the factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of factor IX.
- To ensure that the desired factor IX activity level has been achieved, precise monitoring using the factor IX activity assay is advised. Doses should be titrated using the factor IX activity, pharmacokinetic parameters, such as half-life and recovery, as well as taking the clinical situation into consideration in order to adjust the dose as appropriate.
- Dosing of coagulation factor IX may differ from that of plasma-derived factor IX products. Subjects at the low end of the observed factor IX recovery may require upward dosage adjustment of coagulation factor IX to as much as two times (2X) the initial empirically calculated dose in order to achieve the intended rise in circulating factor IX activity.
- The safety and efficacy of coagulation factor IX administration by continuous infusion have not been established.
Method of Calculating Initial Estimated Dose
- The method of calculating the factor IX dose is shown in TABLE 1.

Average Recovery Adult Patients in Clinical Trial
- In adult PTPs, on average, one International Unit (IU) of coagulation factor IX per kilogram of body weight increased the circulating activity of factor IX by 0.8 ® 0.2 IU/dL (range 0.4 to 1.2 IU/dL). The method of dose estimation is illustrated in TABLE 2. If you use 0.8 IU/dL average increase of factor IX per IU/kg body weight administered, then:

Dosing Guide for Control and Prevention of Bleeding Episodes and Peri-operative Management

Instructions for Use
- Coagulation factor IX is administered by intravenous (IV) infusion after reconstitution of the lyophilized powder with the supplied pre-filled diluent (0.234% sodium chloride solution) syringe.
- Patients should follow the specific reconstitution and administration procedures provided by their physicians.
- For instructions, patients should follow the recommendations in the FDA-Approved Patient Labeling.
- Reconstitution, product administration, and handling of the administration set must be done with caution. Discard all equipment, including any reconstituted coagulation factor IX product, in an appropriate container. Place needles used for venipuncture in a sharps container after single use. Percutaneous puncture with a needle contaminated with blood from an infected patient can transmit infectious viruses including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs.
Preparation and Reconstitution

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Coagulation factor IX in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Coagulation factor IX in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
Control and Prevention of Bleeding Episodes in Hemophilia B
- Coagulation Factor IX (Recombinant), is indicated for the control and prevention of bleeding episodes in adult and pediatric patients with hemophilia B (congenital factor IX deficiency or Christmas disease).
Peri-operative Management in Patients with Hemophilia B
- Coagulation Factor IX (Recombinant), is indicated for peri-operative management in adult and pediatric patients with hemophilia B.
Dosage
Average Recovery Pediatric Patients (<15 years) in Clinical Trial
- In pediatric patients, on average, one international unit of coagulation factor IX per kilogram of body weight increased the circulating activity of factor IX by 0.7 ® 0.3 IU/dL (range 0.2 to 2.1 IU/dL; median of 0.6 IU/dL per IU/kg). The method of dose estimation is illustrated in TABLE 3. If you use 0.7 IU/dL average increase of factor IX per IU/kg body weight administered, then:
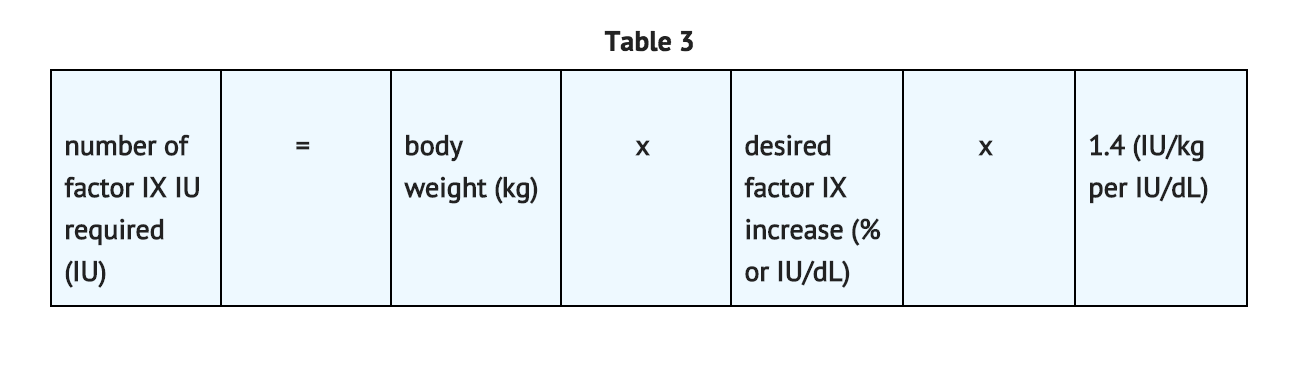
- Doses administered should be titrated to the patient's clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses to coagulation factor IX. Although the dose can be estimated by the calculations above, it is highly recommended that, whenever possible, appropriate laboratory tests, including serial factor IX activity assays, be performed.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Coagulation factor IX in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Coagulation factor IX in pediatric patients.
Contraindications
- Coagulation factor IX is contraindicated in patients who have manifested life-threatening, immediate hypersensitivity reactions, including anaphylaxis, to the product or its components, including hamster protein.
Warnings
General
- The clinical response to coagulation factor IX may vary. If bleeding is not controlled with the recommended dose, the plasma level of factor IX should be determined, and a sufficient dose of coagulation factor IX should be administered to achieve a satisfactory clinical response. If the patient's plasma factor IX level fails to increase as expected or if bleeding is not controlled after the expected dose, the presence of an inhibitor (neutralizing antibodies) should be suspected, and appropriate testing performed.
Anaphylaxis and Severe Hypersensitivity Reactions
- Allergic type hypersensitivity reactions, including anaphylaxis, have been reported with coagulation factor IX and have manifested as pruritus, rash, urticaria, hives, facial swelling, dizziness, hypotension, nausea, chest discomfort, cough, dyspnea, wheezing, flushing, discomfort (generalized) and fatigue. Frequently, these events have occurred in close temporal association with the development of factor IX inhibitors. Advise patients to discontinue use of the product and contact their physician and/or seek immediate emergency care.
- Coagulation factor IX contains trace amounts of hamster (CHO) proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Thromboembolic Complications
- The safety and efficacy of coagulation factor IX administration by continuous infusion have not been established. There have been post-marketing reports of thrombotic events in patients receiving continuous-infusion coagulation factor IX through a central venous catheter, including life-threatening superior vena cava (SVC) syndrome in critically ill neonates.
Nephrotic Syndrome
- Nephrotic syndrome has been reported following immune tolerance induction with factor IX products in hemophilia B patients with factor IX inhibitors and a history of allergic reactions to factor IX. The safety and efficacy of using coagulation factor IX for immune tolerance induction have not been established.
Neutralizing Antibodies (Immunogenicity)
- Patients using coagulation factor IX should be monitored for the development of factor IX inhibitors by appropriate clinical observations and laboratory tests. Inhibitors have been reported following administration of coagulation factor IX. If expected plasma factor IX activity levels are not attained, or if bleeding is not controlled with an expected dose, an assay that measures factor IX inhibitor concentration should be performed.
- Patients with factor IX inhibitors may be at an increased risk of anaphylaxis upon subsequent challenge with factor IX.2 Patients experiencing allergic reactions should be evaluated for the presence of an inhibitor. Patients should be observed closely for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of initial exposure to product. Because of the potential for allergic reactions with factor IX concentrates, the initial (approximately 10 - 20) administrations of factor IX should be performed under medical supervision where proper medical care for allergic reactions could be provided.
Monitoring Laboratory Tests
- Patients should be monitored for factor IX activity levels by the one-stage clotting assay to confirm that adequate factor IX levels have been achieved and maintained, when clinically indicated.
Patients should be monitored for the development of inhibitors if expected factor IX activity plasma levels are not attained, or if bleeding is not controlled with the recommended dose of coagulation factor IX. Assays used to determine if factor IX inhibitor is present should be titered in Bethesda Units (BUs).
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
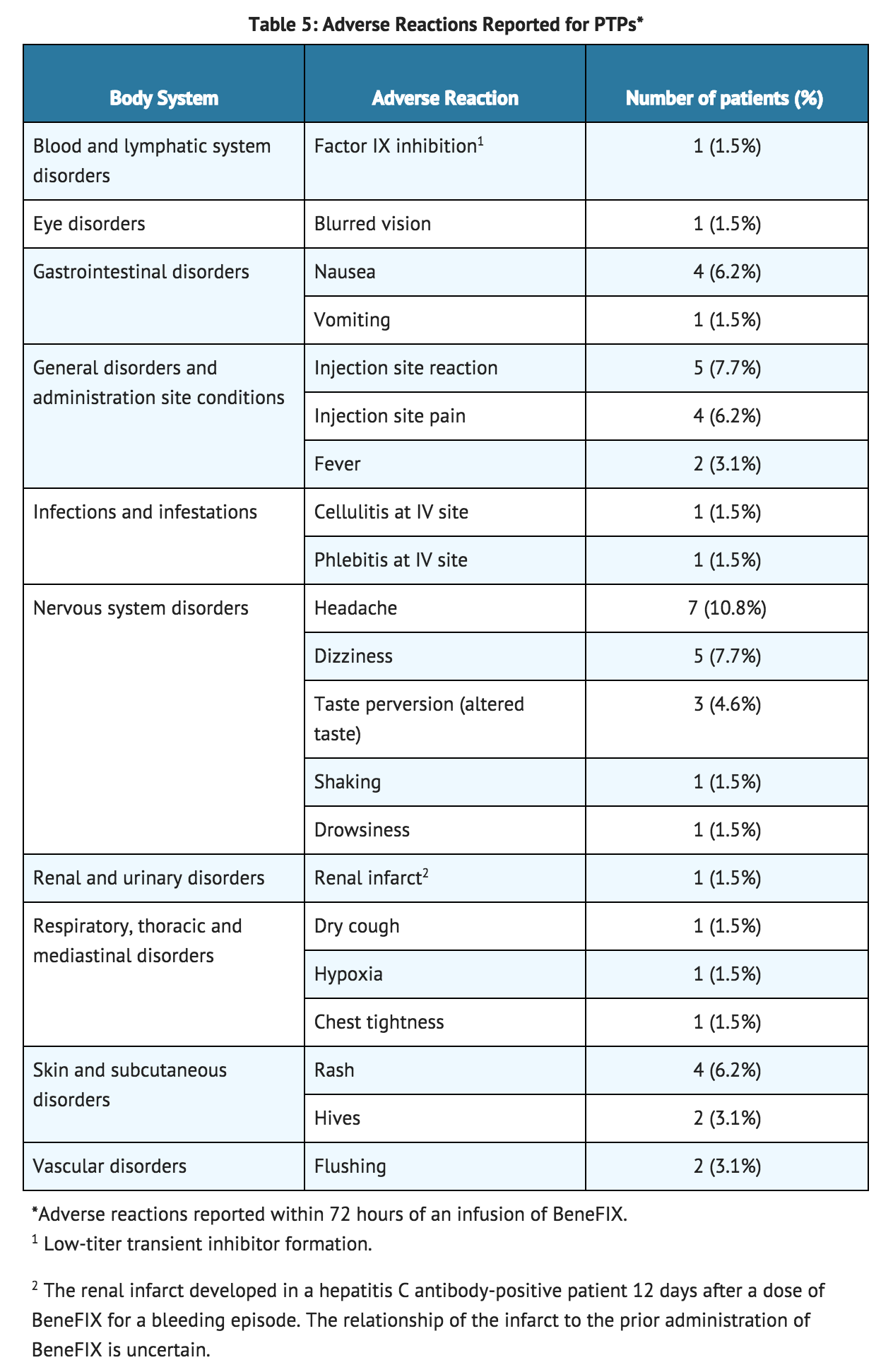
- During uncontrolled open-label clinical studies with coagulation factor IX (Recombinant), conducted in previously treated patients (PTPs), 113 adverse reactions with known or unknown relation to coagulation factor IX therapy were reported among 38.5% (25 of 65) of subjects (with some subjects reporting more than one event) who received a total of 7,573 infusions. These adverse reactions are summarized in TABLE 5.
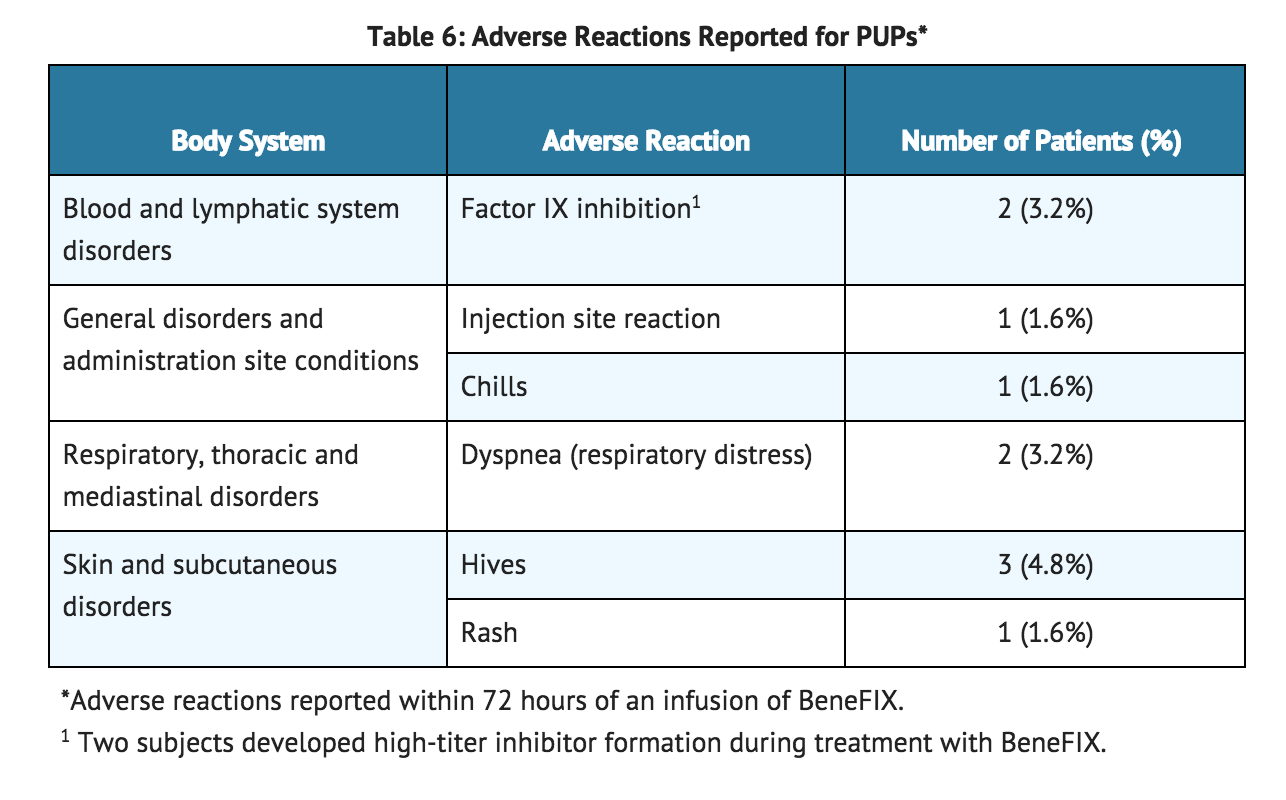
- In the 63 previously untreated patients (PUPs), who received a total of 5,538 infusions, 10 adverse reactions were reported among 9.5% of the patients (6 out of 63) having known or unknown relationship to coagulation factor IX. These events are summarized in TABLE 6.
- For adverse reactions thought to be related to the administration of coagulation factor IX, the rate of infusion should be decreased or the infusion stopped.
Immunogenicity
- In clinical studies with 65 PTPs (defined as having more than 50 exposure days), a low-titer inhibitor was observed in one patient. The inhibitor was transient, the patient continued on study and had normal factor IX recovery pharmacokinetics at study completion (approximately 15 months after inhibitor detection).
- In clinical studies with pediatric PUPs, inhibitor development was observed in 2 out of 63 patients (3.2%), both were high-titer (> 5 BU) inhibitors detected after 7 and 15 exposure days, respectively. Both patients were withdrawn from the study.
Postmarketing Experience
- The following post-marketing adverse reactions have been reported for coagulation factor IX: inadequate factor IX recovery, inadequate therapeutic response, inhibitor development, anaphylaxis, angioedema, dyspnea, hypotension, and thrombosis.
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The safety and efficacy of BeneFIX administration by continuous infusion have not been established. There have been post-marketing reports of thrombotic events, including life-threatening SVC syndrome in critically ill neonates, while receiving continuous-infusion coagulation factor IX through a central venous catheter. Cases of peripheral thrombophlebitis and DVT have also been reported. In some, coagulation factor IX was administered via continuous infusion, which is not an approved method of administration.
Drug Interactions
- None known.
Use in Specific Populations
Pregnancy
- Animal reproduction and lactation studies have not been conducted with coagulation factor IX (Recombinant). It is not known whether coagulation factor IX can affect reproductive capacity or cause fetal harm when given to pregnant women. Coagulation factor IX should be administered to pregnant women only if needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Coagulation factor IX in women who are pregnant.
Labor and Delivery
- There is no information available on the effect of factor IX replacement therapy on labor and delivery. Use only if needed.
Nursing Mothers
- It is not known whether this drug is excreted into human milk. Because many drugs are excreted into human milk, caution should be exercised if coagulation factor IX is administered to nursing mothers.
- Use only if needed.
Pediatric Use
- Safety, efficacy, and pharmacokinetics of coagulation factor IX have been evaluated in previously treated (PTP) and previously untreated pediatric patients (PUP). On average, lower recovery has been observed in pediatric patients (<15 years). A dose adjustment may be needed.
Geriatic Use
- Clinical studies of coagulation factor IX did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Dose selection for an elderly patient should be individualized.
Gender
There is no FDA guidance on the use of Coagulation factor IX with respect to specific gender populations.
Race
There is no FDA guidance on the use of Coagulation factor IX with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Coagulation factor IX in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Coagulation factor IX in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Coagulation factor IX in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Coagulation factor IX in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous.
- Coagulation factor IX is administered by intravenous (IV) infusion after reconstitution with the pre-filled diluent (0.234% sodium chloride solution) syringe.

Monitoring
- Patients using coagulation factor IX should be monitored for the development of factor IX inhibitors by appropriate clinical observations and laboratory tests.
- Patients should be monitored for factor IX activity levels by the one-stage clotting assay to confirm that adequate factor IX levels have been achieved and maintained, when clinically indicated
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- No symptoms of overdose have been reported.
Pharmacology
There is limited information regarding Coagulation factor IX Pharmacology in the drug label.
Mechanism of Action
- Coagulation factor IX temporarily replaces the missing clotting factor IX that is needed for effective hemostasis.
Structure
- Coagulation factor IX (Recombinant), is a purified protein produced by recombinant DNA. It has a primary amino acid sequence that is identical to the Ala148 allelic form of plasma-derived factor IX, and has structural and functional characteristics similar to those of endogenous factor IX. Coagulation factor IX is produced by a genetically engineered Chinese hamster ovary (CHO) cell line that is extensively characterized. No human or animal proteins are added during the purification and formulation processes of coagulation factor IX.
- Coagulation factor IX is not derived from human blood and contains no preservatives, and the manufacture of coagulation factor IX includes no added animal or human components. The stored cell banks are free of human blood or plasma products. The CHO cell line secretes recombinant factor IX into a defined cell culture medium that does not contain any proteins derived from animal or human sources, and the recombinant factor IX is purified by a chromatography purification process that does not require a monoclonal antibody step. The process also includes a membrane nanofiltration step that has the ability to retain molecules with apparent molecular weights >70,000 Da (such as large proteins and viral particles). Coagulation factor IX is a single component by SDS-polyacrylamide gel electrophoresis evaluation. The potency (in International Units, IU) is determined using an in vitro one-stage clotting assay against the World Health Organization (WHO) International Standard for Factor IX concentrate. One International Unit is the amount of factor IX activity present in 1 mL of pooled, normal human plasma. The specific activity of coagulation factor IX is greater than or equal to 200 IU per milligram of protein.
- Coagulation factor IX is formulated as a sterile, nonpyrogenic, lyophilized powder preparation. Coagulation factor IX is intended for intravenous (IV) injection. It is available in single-use vials containing the labeled amount of factor IX activity, expressed in IU. Each vial contains nominally 250, 500, 1000, 2000, or 3000 IU of Coagulation Factor IX (Recombinant). After reconstitution of the lyophilized drug product, the concentrations of excipients are 0.234% sodium chloride, 8 mM L-histidine, 0.8% sucrose, 208 mM glycine, 0.004% polysorbate 80. All dosage strengths yield a clear, colorless solution upon reconstitution.
Pharmacodynamics
- The activated partial thromboplastin time (aPTT) is prolonged in people with hemophilia B. Treatment with factor IX concentrate may normalize the aPTT by temporarily replacing the factor IX. The administration of coagulation factor IX, Coagulation Factor IX (Recombinant), increases plasma levels of factor IX, and can temporarily correct the coagulation defect in these patients.
Pharmacokinetics
- After single intravenous (IV) doses of 50 IU/kg of previously marketed coagulation factor IX, Coagulation Factor IX (Recombinant) [reconstituted with Sterile Water for Injection], in 37 previously treated adult patients (>15 years), each given as a 10-minute infusion, the mean increase from pre-infusion level in circulating factor IX activity was 0.8 ® 0.2 IU/dL per IU/kg infused (range 0.4 to 1.4 IU/dL per IU/kg) and the mean biologic half-life was 18.8 ® 5.4 hours (range 11 to 36 hours). In the original randomized, cross-over pharmacokinetic study in previously treated patients (PTPs), the in vivo recovery using previously marketed coagulation factor IX was statistically significantly less (28% lower, p<0.05) than the recovery using a highly purified plasma-derived factor IX product (pdFIX). A summary of pharmacokinetic data for coagulation factor IX and pdFIX are presented in TABLE 7.

- There was no significant difference in biological half-life. Structural differences of the coagulation factor IX molecule compared with pdFIX were shown to contribute to the lower recovery. In subsequent evaluations for up to 24 months, the pharmacokinetic parameters were similar to the initial results.
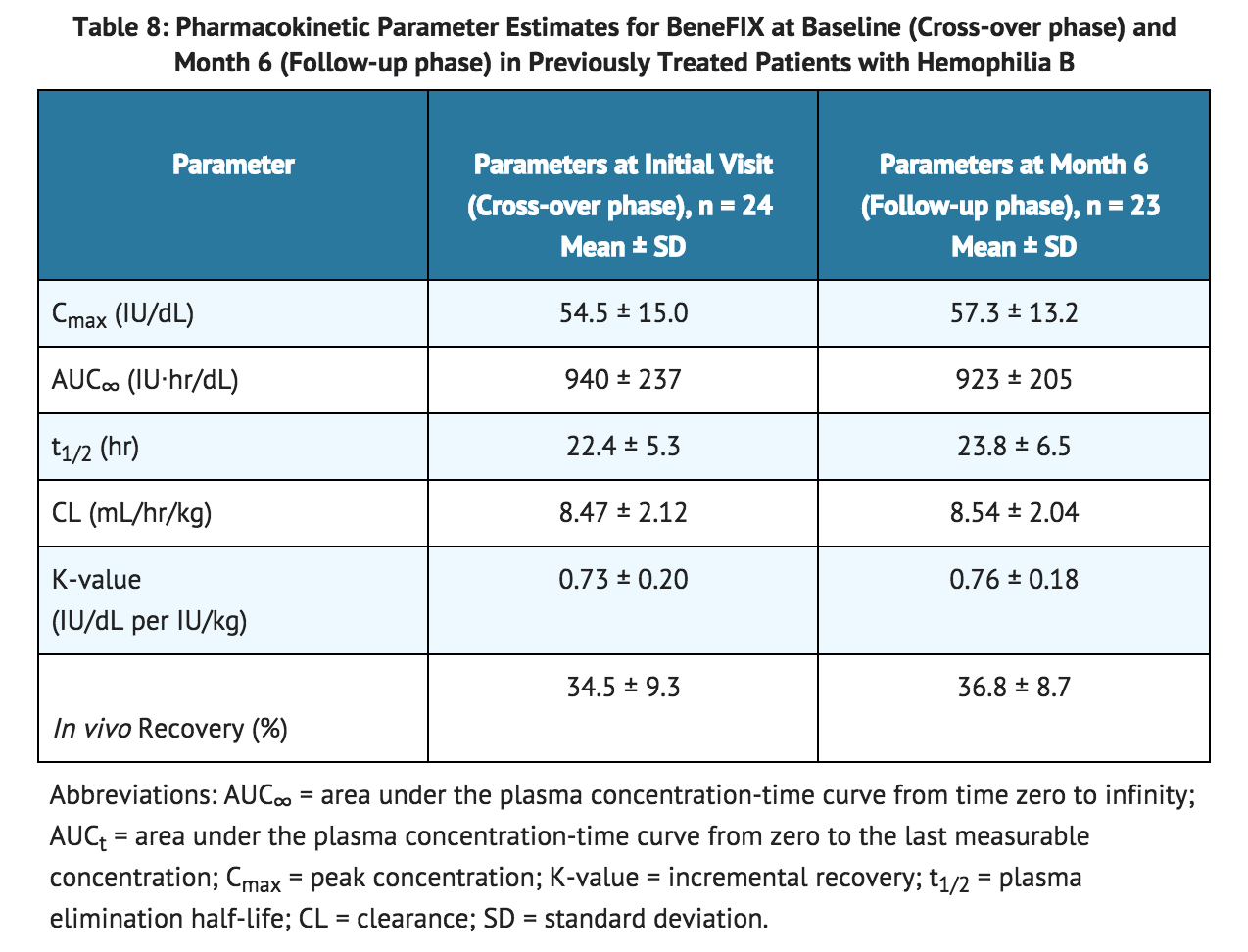
- In a subsequent randomized, cross-over pharmacokinetic study, coagulation factor IX reconstituted in 0.234% sodium chloride diluent was shown to be pharmacokinetically equivalent to the previously marketed coagulation factor IX (reconstituted with Sterile Water for Injection) in 24 previously treated patients (≥12 years) at a dose of 75 IU/kg. In addition, pharmacokinetic parameters were followed up in 23 previously treated patients after repeated administration of coagulation factor IX for six months and found to be unchanged compared with those obtained at the initial evaluation. A summary of pharmacokinetic data are presented in TABLE 8:
Pediatric Patients (≤15 years)
- Nineteen (19) previously treated pediatric patients (range 4 to ≤15 years) underwent pharmacokinetic evaluations for up to 24 months. Fifty-eight previously untreated patients [PUPs] less than 15 years of age at baseline underwent at least one recovery assessment within 30 minutes post-infusion in the presence or absence of hemorrhage during the study. A total of 202 recovery assessments collected during the 60-month period from these 58 PUPs are combined with 19 recovery assessments from PTPs and were summarized by age group in TABLE 9. There was one recovery assessment in a neonate, which had a value of 0.46 IU/dL per IU/kg. The overall mean recovery and FIX elimination half-life values were 0.7 ® 0.3 IU/dL per IU/kg and 20.2 ® 4.0 hours, respectively.
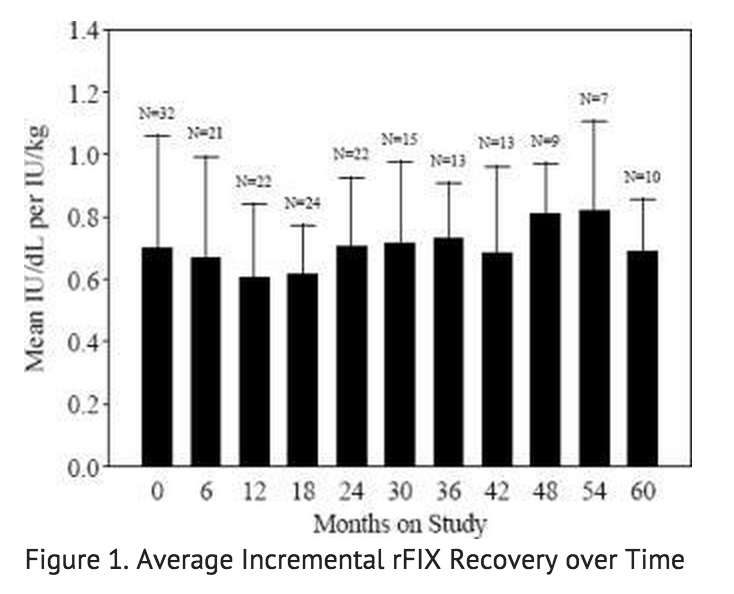
- Data from 57 PUP subjects who underwent repeat recovery testing for up to 60 months demonstrated that the average incremental FIX recovery was consistent over time, as shown in FIGURE 1.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Coagulation factor IX (Recombinant), has been shown to be nonmutagenic in the Ames assay and non-clastogenic in a chromosomal aberrations assay. No investigations on carcinogenesis or impairment of fertility have been conducted.
Clinical Studies
- Efficacy of coagulation factor IX has been evaluated in clinical studies in which a total of 128 subjects received coagulation factor IX either for the treatment of bleeding episodes on an on-demand basis, for the prevention of bleeds (prophylaxis) or for management of hemostasis in the surgical setting (surgical prophylaxis).
- Fifty-six PTPs and sixty-three PUPs were treated for bleeding episodes on an on-demand basis or for the prevention of bleeds (see Tables 9 and 10). The PTPs were followed over a median interval of 24 months (mean 23.4 ® 5.3 months) and for a median of 83.5. The PUPs were followed over a median interval of 37 months (mean 38.1 ® 16.4 months) and for a median of 89 exposure days.
- Fifty-five PTPs and fifty-four PUPs received coagulation factor IX for the treatment of bleeding episodes (see TABLE 10). Bleeding episodes that were managed successfully included hemarthrosis and bleeding in soft tissue and muscle. Data concerning the severity of bleeding episodes were not reported. In the PTPs, 88% of total infusions administrated for on-demand treatment were rated as an "excellent" or "good" response.

- A total of 20 PTPs were treated with coagulation factor IX for secondary prophylaxis (the regular administration of FIX replacement therapy to prevent bleeding in patients who may have already demonstrated clinical evidence of hemophilic arthropathy or joint disease) at some regular interval during the study with a mean of 2.0 infusions per week (see Table 11). Thirty-two PUPs were administered coagulation factor IX for routine (primary and secondary) prophylaxis (see TABLE 11). Twenty-four PUPs were administered coagulation factor IX at least twice weekly, and eight PUPs were administered coagulation factor IX once weekly. Seven PTPs experienced a total of 26 spontaneous bleeding episodes within 48 hours after an infusion. Six spontaneous bleeds within 48 hours after an infusion were reported in 5 PUPs. Prophylaxis therapy was rated as "excellent" or "effective" in 93% of PTPs receiving prophylaxis one to two times per week.

- Management of hemostasis was evaluated in the surgical setting in both PTPs and PUPs (see TABLE 12). Thirty-six surgical procedures have been performed in 28 PTPs with 23 major surgical procedures performed (including 6 complicated dental extractions). Thirty surgical procedures have been performed in 23 PUPs. Twenty-eight of these procedures were considered minor. Hemostasis was maintained throughout the surgical period; however, one PTP subject required evacuation of a surgical wound-site hematoma, and another PTP subject who received coagulation factor IX after a tooth extraction required further surgical intervention due to oozing at the extraction site. There was no clinical evidence of thrombotic complications in any of the subjects.
- Among the PTP surgery subjects, the median increase in circulating factor IX activity was 0.7 IU/dL per IU/kg infused (range 0.3 – 1.2 IU/dL; mean 0.8 ® 0.2 IU/dL per IU/kg). The median elimination half-life for the PTP surgery subjects was 19.4 hours (range 10 – 37 hours; mean 21.3 ® 8.1 hours).

- Nine of the major surgical procedures were performed in 8 PUPs using a continuous-infusion regimen. Five of the surgical procedures were performed in PUPs using a continuous-infusion regimen over 3 to 5 days. Although circulating factor IX levels targeted to restore and maintain hemostasis were achieved with both pulse replacement and continuous infusion regimens, clinical trial experience with continuous infusion of coagulation factor IX for surgical prophylaxis in hemophilia B has been too limited to establish the safety and clinical efficacy of administration of the product by continuous infusion.
- All subjects participating in the PTP, PUP and surgery studies were monitored for clinical evidence of thrombosis [see Warnings and Precautions (5.3)]. No thrombotic complications were reported in PUPs or surgery subjects. One PTP subject experienced a renal infarct 12 days after a dose of coagulation factor IX for a bleeding episode; the relationship of the infarct to the prior administration of coagulation factor IX is uncertain. Laboratory studies of thrombogenecity (fibrinopeptide A and prothrombin fragment 1 + 2) were obtained in 41 PTPs and 7 surgery subjects prior to infusion and up to 24 hours following infusion. The results of these studies were inconclusive. Out of 29 PTP subjects noted to have elevated fibrinopeptide A levels post-infusion of coagulation factor IX, 22 also had elevated levels at baseline. Surgery subjects showed no evidence of significant increase in coagulation activation.
How Supplied
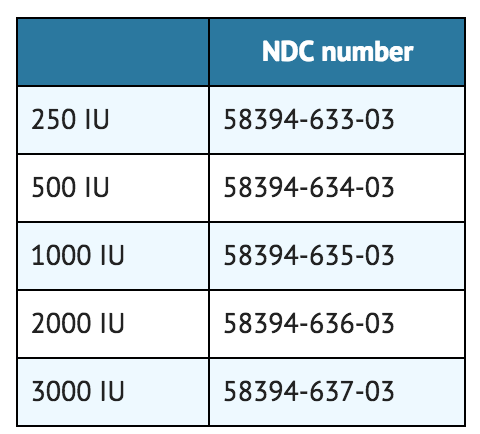
- Coagulation Factor IX (Recombinant), is supplied in kits that include single-use vials which contain nominally 250, 500, 1000, 2000, or 3000 IU per vial with sterile pre-filled diluent syringe, vial adapter reconstitution device, sterile infusion set, and two (2) alcohol swabs, one bandage, and one gauze pad. *Actual factor IX activity in IU is stated on the label of each vial.
- Product labeled "Room Temperature Storage". Store at 2 to 30°C (36 to 86°F).
- Product labeled for refrigeration. Store at 2 to 8°C (36 to 46°F).

Storage
- Store at 2 to 30°C (36 to 86°F).
- If the product kit is labeled for room temperature storage, it can be stored at room temperature (not to exceed 30°C or 86°F) or under refrigeration (2 to 8°C or 36 to 46°F).
Images
Drug Images
{{#ask: Page Name::Coagulation factor IX |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



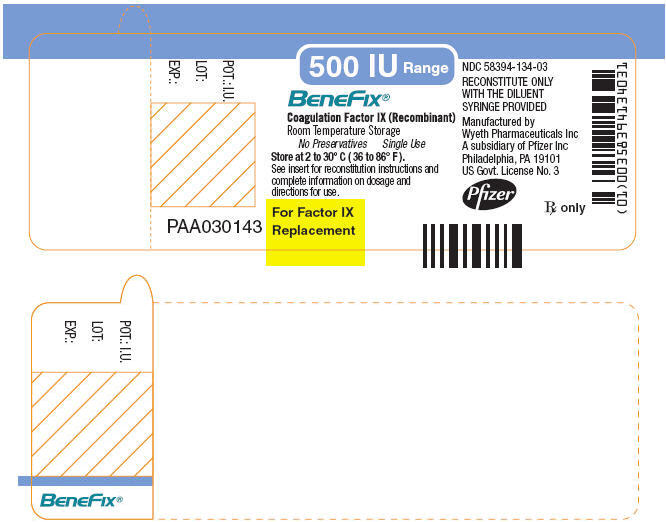


{{#ask: Label Page::Coagulation factor IX |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients to report any adverse reactions or problems following BeneFIX administration to their physician or healthcare provider.
- Allergic-type hypersensitivity reactions are possible. Inform patients of the early signs of hypersensitivity reactions [including hives (rash with itching), generalized urticaria, tightness of the chest, wheezing, hypotension] and anaphylaxis. Advise patients to discontinue use of the product and contact their physicians if these symptoms occur.
- Advise patients to contact their physician or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to factor IX replacement therapy, as in some cases this may be a manifestation of an inhibitor.
Precautions with Alcohol
- Alcohol-Coagulation factor IX interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BENEFIX ®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.