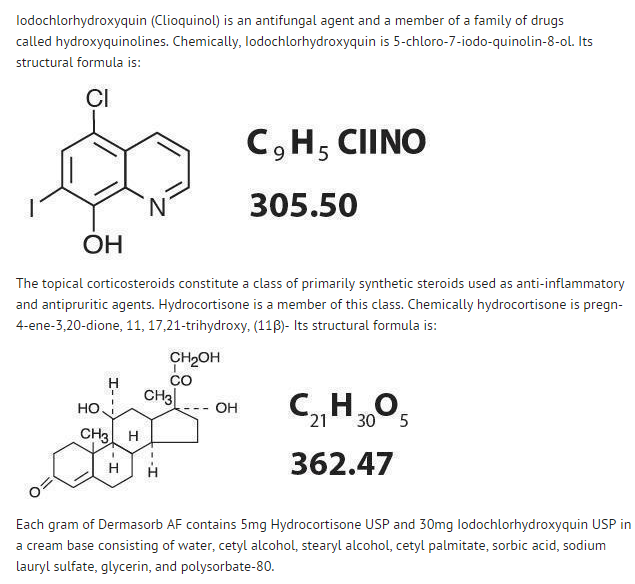
Clioquinol and hydrocortisone cream
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Clioquinol and hydrocortisone cream is an anti-allergic agents that is FDA approved for the treatment of dermatitis, stasis dermatitis, pyoderma, nuchal eczema, chronic eczematoid otitis externa, acne, urticata, localized or disseminated neurodermatitis, lichen simplex chronicus, anogenital pruritus (vulvae, scroti, ani), folliculitis and bacterial dermatoses. Common adverse reactions include rash and hypersensitivity.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Based on a review of this drug by the National Academy of Sciences-National Research Council and/or other information, FDA has classified the indications as follows: “Possibly” effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; infantile eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis; bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); moniliasis; intertrigo.
- Final classification of the less-than-effective indications requires further investigation.
- Apply a thin layer to the affected area 3 or 4 times daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clioquinol and hydrocortisone cream in adult patients.
Non–Guideline-Supported Use
- Acne urticata
- Candidiasis
- Dermatitis
- Disorder of skin, mycotic
- Eczema
- Folliculitis
- Intertrigo
- Lichen simplex chronicus
- Pruritus, Anogenital
- Pyoderma
- Superficial bacterial infection of skin, Minor
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Clioquinol and hydrocortisone cream FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clioquinol and hydrocortisone cream in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clioquinol and hydrocortisone cream in pediatric patients.
Contraindications
Risk-benefit should be considered when the following medical problems exist:
• Intolerance to iodochlorhydroxyquin, hydrocortisone, chloroxine, iodine, iodine-containing preparations, or related compounds
• Herpes simplex, vaccinia, eczema vaccinia, varicella, or other viral infections of the skin
Warnings
There is limited information regarding Clioquinol and hydrocortisone cream Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clioquinol and hydrocortisone cream Clinical Trials Experience in the drug label.
Postmarketing Experience
- There have been a few reports of rash and hypersensitivity as well as thinning of the skin with easy bruising. The following local adverse reactions have also been reported with topical corticosteroids and iodochlorhydroxyquin especially under occlusive dressings; burning; itching; irritation; dryness; folliculitis; blistering, peeling, redness, swelling; hypertrichosis; acneiform eruptions; hypopigmentation; perioral dermatitis; allergic contact dermatitis; maceration of the skin, secondary infection; skin atrophy; striae; miliaria or other signs of irritation not present before therapy.
- Discontinue therapy if any untoward reaction occurs.
Drug Interactions
There is limited information regarding Clioquinol and hydrocortisone cream Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Although topical steroids have not been reported to have an adverse effect on pregnancy, the safety of their use in pregnant women has not been absolutely established. Use of large amounts or for prolonged periods of time is not recommended since systemic absorption may occur. In laboratory animals, increases in incidence of fetal abnormalities have been associated with exposure of gestating females to topical corticosteroids, in some cases at rather low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Therefore, drugs of this class should not be used extensively on pregnant patients in large amounts or for prolonged periods of time.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clioquinol and hydrocortisone cream in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clioquinol and hydrocortisone cream during labor and delivery.
Nursing Mothers
- It is not known whether topical administration of this drug could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when this class of drug is administered to a nursing woman.
Pediatric Use
- Use is not recommended for infants or children up to 2 years of age. Iodochlorhydroxyquin may produce false-positive ferric chloride test results for phenylketonuria (PKU) if iodochlorhydroxyquin is present in the neonate’s diaper or urine.
Special care must be exercised in using this drug in a pediatric patient. It is recommended that only low-potency topical corticosteroids that are not fluorinated and that have a free 17-hydroxyl group be used in children unless there is a very specific indication for one of the other topical corticosteroids.
As a general rule, pediatric therapy continuing for longer than 2 weeks and consisting of doses in excess of 2 daily applications (with low-potency corticosteroids) should be carefully evaluated by the physician. This is especially important if medication is applied to more than 5-10% of the body surface or if an occlusive dressing is used. A tight-fitting diaper or one covered with plastic pants may constitute an occlusive dressing. Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
Geriatic Use
There is no FDA guidance on the use of Clioquinol and hydrocortisone cream in geriatric settings.
Gender
There is no FDA guidance on the use of Clioquinol and hydrocortisone cream with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clioquinol and hydrocortisone cream with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clioquinol and hydrocortisone cream in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clioquinol and hydrocortisone cream in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clioquinol and hydrocortisone cream in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clioquinol and hydrocortisone cream in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Clioquinol and hydrocortisone cream Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Clioquinol and hydrocortisone cream and IV administrations.
Overdosage
There is limited information regarding Clioquinol and hydrocortisone cream overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Clioquinol and hydrocortisone cream Pharmacology in the drug label.
Mechanism of Action
- Iodochlorhydroxyquin is a broad-spectrum antibacterial and antifungal. Its precise mechanism of action is unknown. Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and some evidence suggests a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Structure
Pharmacodynamics
There is limited information regarding Clioquinol and hydrocortisone cream Pharmacodynamics in the drug label.
Pharmacokinetics
- The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of the topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses.
- Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Nonclinical Toxicology
There is limited information regarding Clioquinol and hydrocortisone cream Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Clioquinol and hydrocortisone cream Clinical Studies in the drug label.
How Supplied
- DERMASORB™AF (Hydrocortisone USP, 0.5%, Iodochlorhydroxyquin USP, 3%) Cream is supplied in
- 1 ounce tube NDC 0316-1025-01
Storage
There is limited information regarding Clioquinol and hydrocortisone cream Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Clioquinol and hydrocortisone cream |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
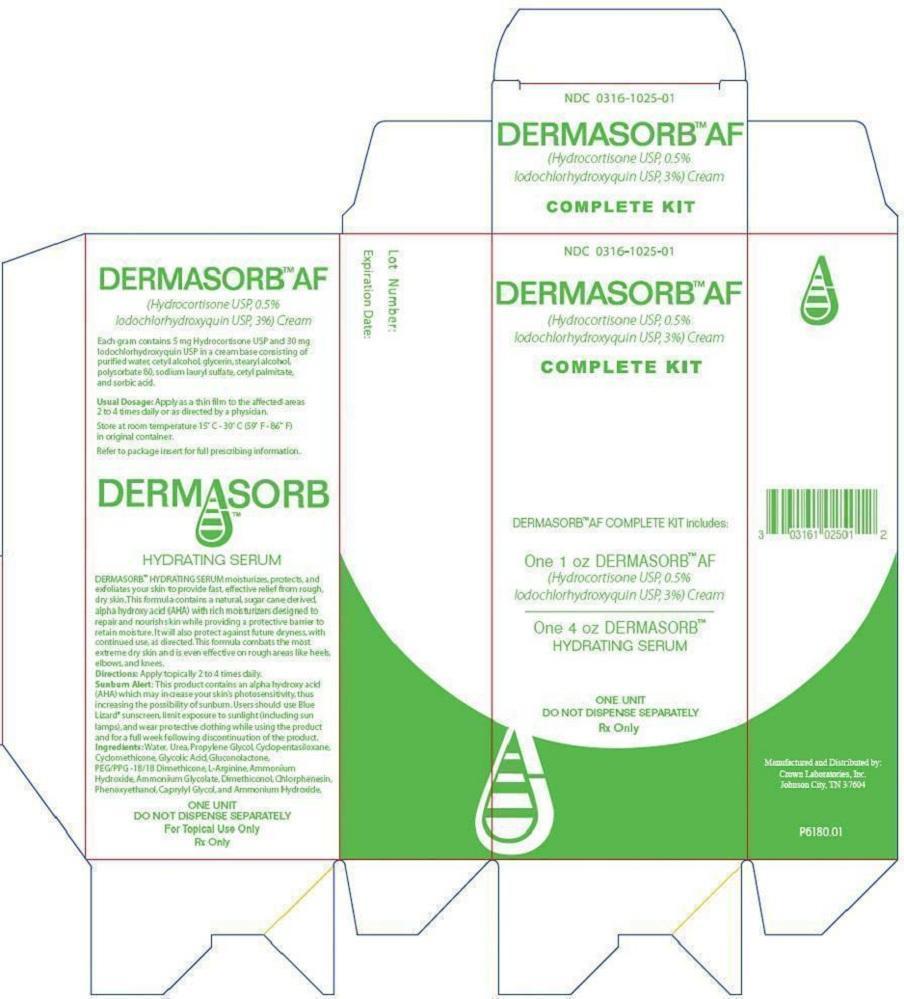
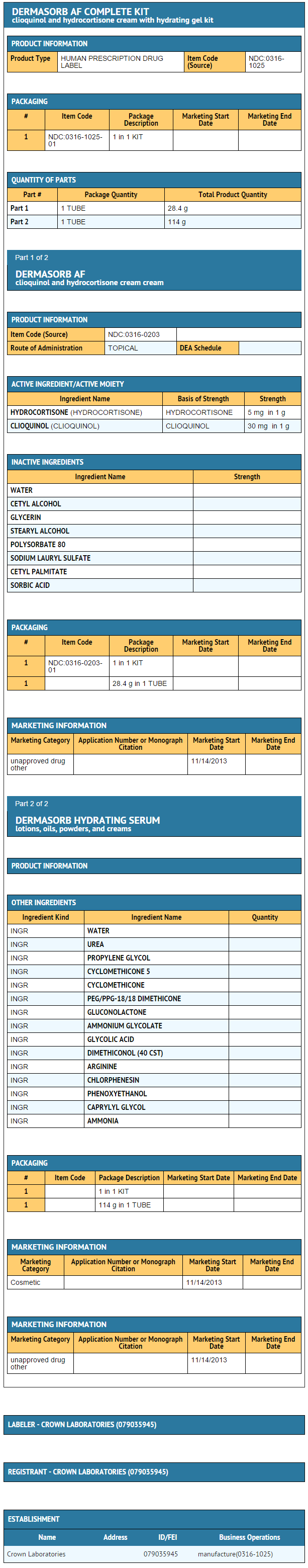
{{#ask: Label Page::Clioquinol and hydrocortisone cream |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Clioquinol and hydrocortisone cream Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Clioquinol and hydrocortisone cream interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DERMASORB AF COMPLETE KIT ®[1]
Look-Alike Drug Names
There is limited information regarding Clioquinol and hydrocortisone cream Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.