Cefpodoxime dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
FILM-COATED TABLETS
VANTIN Tablets should be administered orally with food to enhance absorption. (See CLINICAL PHARMACOLOGY.)
The recommended dosages, durations of treatment, and applicable patient population are as described in the following chart:
Adults and Adolescents (age 12 years and older)
GRANULES FOR ORAL SUSPENSION
VANTIN Oral Suspension may be given without regard to food. The recommended dosages, durations of treatment, and applicable patient populations are as described in the following chart:
Adults and Adolescents (age 12 years and older)
Infants and Pediatric Patients (age 2 months through 12 years)
Patients with Renal Dysfunction
For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function.
Patients with Cirrhosis
Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population.
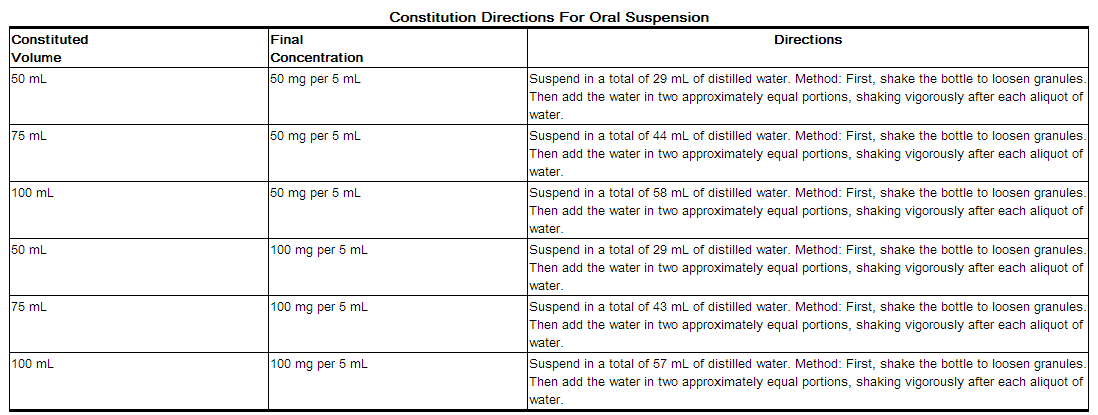
Preparation of Suspension
After mixing, the suspension should be stored in a refrigerator, 2° to 8°C (36° to 46°F). Shake well before using. Keep container tightly closed. The mixture may be used for 14 days. Discard unused portion after 14 days.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050674s015,050675s018lbl.pdf