Carmustine (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS
See full prescribing information for complete Boxed Warning.
|
Overview
Carmustine (injection) is an alkylating agent that is FDA approved for the treatment of brain tumors (glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors), multiple myeloma, Hodgkin’s disease, and non-Hodgkin's lymphoma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, nausea, and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Carmustine for injection is indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following:
- Brain tumors-glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors.
- Multiple myeloma-in combination with prednisone.
- Hodgkin’s disease-as secondary therapy in combination with other approved drugs in patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy.
- Non-Hodgkin's lymphoma-as secondary therapy in combination with other approved drugs for patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy.
Dosage
- The recommended dose of carmustine as a single agent in previously untreated patients is 150 to 200 mg/m2 intravenously every 6 weeks.
- This may be given as a single dose or divided into daily injections such as 75 to 100 mg/m2 on 2 successive days.
- When carmustine is used in combination with other myelosuppressive drugs or in patients in whom bone marrow reserve is depleted, the doses should be adjusted accordingly.
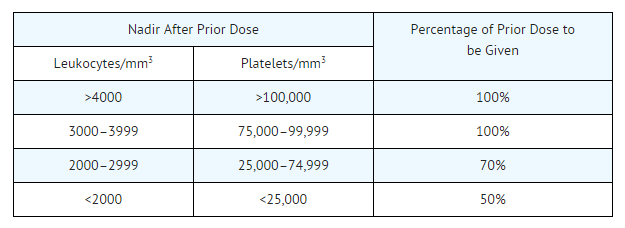
- Doses subsequent to the initial dose should be adjusted according to the hematologic response of the patient to the preceding dose. The following schedule is suggested as a guide to dosage adjustment:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carmustine in adult patients.
Non–Guideline-Supported Use
- Preparative regimens for bone marrow transplant
- Carcinoma of stomach
- Colorectal cancer
- Malignant melanoma
- Mycosis fungoides
- Waldenström macroglobulinemia
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness of carmustine have not been established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carmustine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carmustine in pediatric patients.
Contraindications
Carmustine should not be given to individuals who have demonstrated a previous hypersensitivity to it.
Warnings
|
WARNINGS
See full prescribing information for complete Boxed Warning.
|
- Since the major toxicity is delayed bone marrow suppression, blood counts should be monitored weekly for at least 6 weeks after a dose. At the recommended dosage, courses of carmustine should not be given more frequently than every 6 weeks.
- The bone marrow toxicity of carmustine is cumulative; therefore, dosage adjustment must be considered on the basis of nadir blood counts from prior dose.
- Pulmonary toxicity from carmustine appears to be dose related. Patients receiving greater than 1400 mg/m2 cumulative dose are at significantly higher risk than those receiving less. Additionally delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in patients who received carmustine in childhood and early adolescence.
- Long-term use of nitrosoureas has been reported to be associated with the development of secondary malignancies.
- Liver and renal function tests should be monitored periodically.
- carmustine has been administered through an intraarterial intracarotid route; this procedure is investigational and has been associated with ocular toxicity.
- Injection site reactions may occur during the administration of carmustine. Given the possibility of extravasation, close monitoring of the infusion site for possible infiltration during drug administration is recommended. A specific treatment for extravasation reactions is unknown at this time.
Adverse Reactions
Clinical Trials Experience
Pulmonary Toxicity
Pulmonary toxicity characterized by pulmonary infiltrates and/or fibrosis has been reported to occur from 9 days to 43 months after treatment with carmustine and related nitrosoureas. Most of these patients were receiving prolonged therapy with total doses of carmustine greater than 1400 mg/m2. However, there have been reports of pulmonary fibrosis in patients receiving lower total doses. Other risk factors include past history of lung disease and duration of treatment. Cases of fatal pulmonary toxicity with carmustine have been reported.
Additionally, delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in a long-term study with 17 patients who received carmustine in childhood and early adolescence (1–16 years) in cumulative doses ranging from 770 to 1800 mg/m2 combined with cranial radiotherapy for intracranial tumors. Chest x-rays demonstrated pulmonary hypoplasia with upper zone contraction. Gallium scans were normal in all cases. Thoracic CT scans have demonstrated an unusual pattern of upper zone fibrosis. There was some late reduction of pulmonary function in all long-term survivors. This form of lung fibrosis may be slowly progressive and has resulted in death in some cases. In this long-term study, 8 of 17 died of delayed pulmonary lung fibrosis, including all those initially treated (5 of 17) at less than 5 years of age.
Hematologic Toxicity
A frequent and serious toxicity of carmustine is delayed myelosuppression. It usually occurs 4 to 6 weeks after drug administration and is dose related. Thrombocytopenia occurs at about 4 weeks postadministration and persists for 1 to 2 weeks. Leukopenia occurs at 5 to 6 weeks after a dose of carmustine and persists for 1 to 2 weeks. Thrombocytopenia is generally more severe than leukopenia. However, both may be dose-limiting toxicities.
Carmustine may produce cumulative myelosuppression, manifested by more depressed indices or longer duration of suppression after repeated doses.
The occurrence of acute leukemia and bone marrow dysplasias has been reported in patients following long-term nitrosourea therapy.
Anemia also occurs, but is less frequent and less severe than thrombocytopenia or leukopenia.
Greater myelotoxicity (e.g., leukopenia and neutropenia) has been reported when carmustine was combined with cimetidine.
Gastrointestinal Toxicity
Nausea and vomiting after intravenous administration of carmustine are noted frequently. This toxicity appears within 2 hours of dosing, usually lasting 4 to 6 hours, and is dose related. Prior administration of antiemetics is effective in diminishing and sometimes preventing this side effect.
Hepatotoxicity
A reversible type of hepatic toxicity, manifested by increased transaminase, alkaline phosphatase and bilirubin levels, has been reported in a small percentage of patients receiving carmustine.
Nephrotoxicity
Renal abnormalities consisting of progressive azotemia, decrease in kidney size, and renal failure have been reported in patients who received large cumulative doses after prolonged therapy with carmustine and related nitrosoureas. Kidney damage has also been reported occasionally in patients receiving lower total doses.
Other Toxicities
Accidental contact of reconstituted carmustine with skin has caused burning and hyperpigmentation of the affected areas.
Rapid intravenous infusion of carmustine may produce intensive flushing of the skin and suffusion of the conjunctiva within 2 hours, lasting about 4 hours. It is also associated with burning at the site of injection although true thrombosis is rare.
Local soft tissue toxicity has been reported following extravasation of carmustine. Infiltration of carmustine may result in swelling, pain, erythema, burning sensation, and skin necrosis.
Neuroretinitis, chest pain, headache, allergic reaction, hypotension, and tachycardia have been reported as part of ongoing surveillance.
Postmarketing Experience
There is limited information regarding Carmustine (injection) Postmarketing Experience in the drug label.
Drug Interactions
Greater myelotoxicity (e.g., leukopenia and neutropenia) has been reported when carmustine was combined with cimetidine.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
Carmustine for injection may cause fetal harm when administered to a pregnant woman. carmustine has been shown to be embryotoxic in rats and rabbits and teratogenic in rats when given in doses equivalent to the human dose. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Carmustine (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Carmustine (injection) during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because of the potential for serious adverse events in nursing infants, nursing should be discontinued while taking carmustine.
Pediatric Use
Safety and effectiveness in children have not been established. Delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in a long-term study of patients who received carmustine in childhood and early adolescence (1–16 years). Eight out of the 17 patients (47%) who survived childhood brain tumors, including all the 5 patients initially treated at less than 5 years of age, died of pulmonary fibrosis. Therefore, the risks and benefits of carmustine therapy must be carefully considered, due to the extremely high risk of pulmonary toxicity.
Geriatic Use
No data from clinical studies of carmustine are available for patients 65 years of age and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dose range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
carmustine and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
Gender
There is no FDA guidance on the use of Carmustine (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carmustine (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Carmustine (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Carmustine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
Carmustine also affects fertility in male rats at doses somewhat higher than the human dose.
Immunocompromised Patients
There is no FDA guidance one the use of Carmustine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Carmustine (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Carmustine (injection) and IV administrations.
Overdosage
No proven antidotes have been established for carmustine overdosage.
Pharmacology
There is limited information regarding Carmustine (injection) Pharmacology in the drug label.
Mechanism of Action
Although it is generally agreed that carmustine alkylates DNA and RNA, it is not cross-resistant with other alkylators. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins.
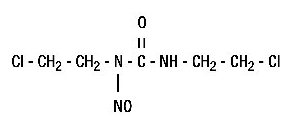
Structure
The structural formula is:
Pharmacodynamics
There is limited information regarding Carmustine (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
Intravenously administered carmustine is rapidly degraded, with no intact drug detectable after 15 minutes. However, in studies with 14C-labeled drug, prolonged levels of the isotope were detected in the plasma and tissue, probably representing radioactive fragments of the parent compound.
It is thought that the antineoplastic and toxic activities of carmustine may be due to metabolites. Approximately 60% to 70% of a total dose is excreted in the urine in 96 hours and about 10% as respiratory CO2. The fate of the remainder is undetermined.
Because of the high lipid solubility and the relative lack of ionization at physiological pH, carmustine crosses the blood-brain barrier quite effectively. Levels of radioactivity in the CSF are ≥50% of those measured concurrently in plasma.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
carmustine is carcinogenic in rats and mice, producing a marked increase in tumor incidence in doses approximating those employed clinically. Nitrosourea therapy does have carcinogenic potential in humans
Clinical Studies
There is limited information regarding Carmustine (injection) Clinical Studies in the drug label.
How Supplied
Carmustine is available in 100-mg single dose vials of lyophilized material
- NDC 0015-3012-60
Storage
- Store in a refrigerator (2°-8°C, 36°-46°F).
- Store diluent at controlled room temperature (15°-30°C, 59°-86°F) or in a refrigerator (2°-8°C, 36°-46°F).
Images
Drug Images
{{#ask: Page Name::Carmustine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Carmustine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Carmustine (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Carmustine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Bicnu[1]
- Gliadel
Look-Alike Drug Names
There is limited information regarding Carmustine (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Carmustine (injection) |Label Name=Carmustine 100 mg.png
}}