Carisoprodol detailed information
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 60% |
| Metabolism | Hepatic (CYP2C19-mediated) |
| Elimination half-life | 8 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
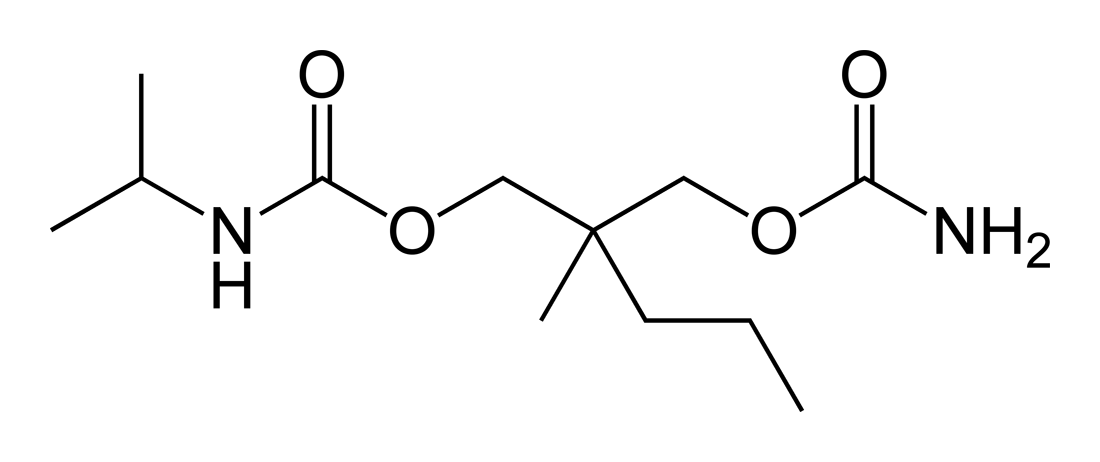
| Formula | C12H24N2O4 |
| Molar mass | 260.33 g/mol |
Carisoprodol is a centrally-acting skeletal muscle relaxant whose active metabolite is meprobamate. Although several case reports have shown that carisoprodol has abuse potential[1], it continues to be widely prescribed (except in the United Kingdom where use of benzodiazepines is preferred). Carisoprodol is a colourless, crystalline powder, having a mild, characteristic odor and a bitter taste. It is slightly soluble in water and freely soluble in alcohol, chloroform and acetone. Its solubility is practically independent of pH.
Carisoprodol is marketed in the United States under the brand name Soma, and in the United Kingdom and other countries under the brand names Sanoma and Carisoma. Carisoprodol is especially useful against various types of pain (whether or not related to muscle spasm) for its analgesic-sparing (potentiating) effect on opioid analgesics such as codeine, dihydrocodeine, hydrocodone &c. Carisoprodol is available by itself or mixed with aspirin and in one preparation (Soma Compound With Codeine®) along with codeine and caffeine as well.
History
Carisoprodol seems to be more effective as a muscle relaxant than meprobamate but it also has the effects of the latter drug (trade name Miltown®) which is a non-barbiturate and non-benzodiazepine sedative-hypnotic. Carisoprodol and meprobamate can produce a glutethemide-like euphoria in certain dose ranges; a euphoriant dose of either drug will rapidly produce somnolence and the patient can be in a deep sleep mere moments after euphoria, anxiolysis, and other side effects manifest. However, the usual dose of 350 mg will not do this or will only do so for the first one to three doses. At higher doses, in some patients, and/or early in therapy, carisoprodol can have the full spectrum of sedative-hypnotic side effects (and often to an extent to which the patient may not be fully aware) and can dangerously impair the patient's ability to operate an automobile, motorcycle, and other machinery of various types; slurred speech is also a side effect which manifests rather rapidly. The intensity of these side effects tends to lessen and/or become very predictable as therapy continues as is the case with many other drugs.
Meprobamate and other muscle relaxing drugs often were subjects of misuse and abuse in the 1950s and 1960s.[2][3] Overdose cases were reported as early as 1957 and have been reported on several occasions since then.[4][5][6][7][8][9][10]
On June 1, 1959 several American pharmacologists convened at Wayne State University in Detroit, Michigan to discuss a new drug. The drug, originally thought to have antiseptic properties, was found to have central muscle relaxing properties.[11] It had been developed by Dr. Frank M. Berger at Wallace laboratories and had been named carisoprodol (trade name Soma).
Carisoprodol was developed on the basis of meprobamate, in the hope that it would have better muscle relaxing properties, less potential for abuse, and less risk of overdose than meprobamate.[12] The substitution of one hydrogen atom with an isopropyl group on one of the carbamyl nitrogens was intended to yield a molecule with new pharmacological properties.
The brand name SOMA is shared with the fictional drug featured in Aldous Huxley's Brave New World.[13]
Chemistry
It is a carbamic acid ester. Carisoprodol is a racemic mixture of two stereoisomers.
Effects
- Analgesia
- Relief from hypertonia
Side effects
These are somewhat rare when used at normal doses.
Pharmacokinetics
Carisoprodol has a rapid, 30 minute onset of action, with the aforementioned effects lasting for approximately 2–6 hours. It is metabolized in the liver via the cytochrome P450 oxidase isozyme CYP2C19, excreted by the kidneys and has an approximate 8 hour half-life. A considerable proportion of carisoprodol is metabolized to meprobamate, which is a known drug of abuse and dependence; this could account for the abuse potential of carisoprodol.
Notes
- ↑ Bramness JG, Furu K, Engeland A, Skurtveit S. (2007). "Carisoprodol use and abuse in Norway. A pharmacoepidemiological study". Br J Clin Pharmacol (2): 210–218. Unknown parameter
|vol=ignored (|volume=suggested) (help) - ↑ Kamin I, Shaskan D. (1959). "Death due to massive overdose of meprobamate". Am J Psychiatry. 115 (12): 1123–1124.
- ↑ Hollister LE (1983). "The pre-benzodiazepine era". J Psychoactive Drugs. 15 (1–2): 9–13.
- ↑ Gaillard Y, Billault F, Pepin G (1997). "Meprobamate overdosage: a continuing problem. Sensitive GC-MS quantitation after solid phase extraction in 19 fatal cases". Forensic Sci.Int. 86 (3): 173–180.
- ↑ Allen MD, Greenblatt DJ, Noel BJ (1977). "Meprobamate overdosage: a continuing problem". Clin Toxicol. 11 (5): 501–515.
- ↑ Kintz P, Tracqui A, Mangin P, Lugnier AA (1988). "Fatal meprobamate self-poisoning". Am J Forensic Med Pathol. 9 (2): 139–140.
- ↑ Eeckhout E, Huyghens L, Loef B, Maes V, Sennesael J (1988). "Meprobamate poisoning, hypotension and the Swan-Ganz catheter". Intensive Care Med. 14 (4): 437–438.
- ↑ Lhoste F, Lemaire F, Rapin M (1977). "Treatment of hypotension in meprobamate poisoning". N Engl J Med. 296 (17): 1004.
- ↑ Bedson H (1959). "Coma due to meprobamate intoxication. Report of a case confirmed by chemical analysis". Lancet. 273 (1): 288–290.
- ↑ Blumberg A, Rosett H, Dobrow A (1959). "Severe hypotension reactions following meprobamate overdosage". Ann Intern Med. 51: 607–612.
- ↑ Miller JG, ed. The pharmacology and clinical usefulness of carisoprodol. Detroit:Wayne State University; 1959.
- ↑ Berger F, Kletzkin M, Ludwig B, Margolin S. The history, chemistry, and pharmacology of carisoprodol. Annals of the New York Academy of Sciences. 1959;86:90-107
- ↑ "Brave New Soma - TIME". Retrieved 2007-08-20.
References
- APhA Drug Information Handbook
External links
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Muscle relaxants
- Prodrugs