Carglumic acid
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Carglumic acid is a hyperammonemia agent that is FDA approved for the treatment of acute hyperammonemia in patients with NAGS deficiency, maintenance therapy for chronic hyperammonemia in patients with NAGS deficiency. Common adverse reactions include abdominal pain, diarrhea, vomiting, anemia, infectious disease, headache, infection of ear, nasopharyngitis, tonsillitis, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute hyperammonemia in patients with NAGS deficiency
- Carbaglu® is indicated as an adjunctive therapy in pediatric and adult patients for the treatment of acute hyperammonemia due to the deficiency of the hepatic enzyme N-acetylglutamate synthase (NAGS). During acute hyperammonemic episodes concomitant administration of Carbaglu with other ammonia lowering therapies such as alternate pathway medications, hemodialysis, and dietary protein restriction are recommended.
Maintenance therapy for chronic hyperammonemia in patients with NAGS deficiency
- Carbaglu® is indicated for maintenance therapy in pediatric and adult patients for chronic hyperammonemia due to the deficiency of the hepatic enzyme N-acetylglutamate synthase (NAGS). During maintenance therapy, the concomitant use of other ammonia lowering therapies and protein restriction may be reduced or discontinued based on plasma ammonia levels.
- Carbaglu treatment should be initiated by a physician experienced in metabolic disorders.
Adult Dosage and Administration
- Recommended initial dose range for acute hyperammonemia is 100 mg/kg/day to 250 mg/kg/day.
- The recommended maintenance dose should be titrated to target normal plasma ammonia level for age.
- Divide the total daily dose into two to four doses to be given immediately before meals or feedings.
- Each divided dose should be rounded to the nearest 100 mg.
- Each 200 mg tablet should be dispersed in a minimum of 2.5 mL of water and taken immediately.
- Carbaglu can be administered orally or through a nasogastric tube.
- Carbaglu tablets should not be swallowed whole or crushed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carglumic acid in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carglumic acid in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Recommended initial dose range for acute hyperammonemia is 100 mg/kg/day to 250 mg/kg/day.
- The recommended maintenance dose should be titrated to target normal plasma ammonia levels for age.
- Divide the total daily dose into two to four doses to be given immediately before meals or feedings.
- Mix each 200 mg tablet in 2.5 mL of water to yield a concentration of 80 mg/mL.
- Carbaglu may be administered orally with an oral syringe or through a nasogastric tube.
- Carbaglu tablets should not be swallowed whole or crushed.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carglumic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carglumic acid in pediatric patients.
Contraindications
There is limited information regarding Carglumic acid Contraindications in the drug label.
Warnings
Hyperammonemia
- Any episode of acute symptomatic hyperammonemia should be treated as a life-threatening emergency. Treatment of hyperammonemia may require dialysis, preferably hemodialysis, to remove a large burden of ammonia. Uncontrolled hyperammonemia can rapidly result in brain injury/damage or death, and prompt use of all therapies necessary to reduce plasma ammonia levels is essential.
- Management of hyperammonemia due to NAGS deficiency should be done in coordination with medical personnel experienced in metabolic disorders. Ongoing monitoring of plasma ammonia levels, neurological status, laboratory tests and clinical responses in patients receiving Carbaglu is crucial to assess patient response to treatment.
Therapeutic Monitoring
- Plasma ammonia levels should be maintained within normal range for age via individual dose adjustment.
Nutritional Management
- Since hyperammonemia is the result of protein catabolism, complete protein restriction is recommended to be maintained for 24 to 48 hours and caloric supplementation should be maximized to reverse catabolism and nitrogen turnover.
Adverse Reactions
Clinical Trials Experience
Retrospective Case Series Experience
- The most common adverse reactions (occurring in ≥ 13% of patients), regardless of causality, are: Infections, vomiting, abdominal pain, pyrexia, tonsilitis, anemia, ear infection, diarrhea, nasopharyngitis, and headache.
- Table 1 summarizes adverse reactions occurring in 2 or more patients treated with Carbaglu in the retrospective case series. Because these reactions were reported retrospectively, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Table 1: Adverse Reactions Reported in > 2 Patients in the Retrospective Case Series treated with Carbaglu
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Carglumic acid in the drug label.
Drug Interactions
There is limited information regarding Carglumic acid Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- There are no adequate and well controlled studies or available human data with Carbaglu® in pregnant women. Decreased survival and growth occurred in offspring born to animals that received carglumic acid at doses similar to the maximum recommended starting human dose during pregnancy and lactation.
- Because untreated N-acetylglutamate synthase (NAGS) deficiency results in irreversible neurologic damage and death, women with NAGS must remain on treatment throughout pregnancy. In embryo-fetal developmental toxicity studies, pregnant rats and rabbits received oral carglumic acid during organogenesis at doses up to 1.3 times the maximum recommended human starting dose based on body surface area (mg/m2). Actual doses were 500 and 2000 mg/kg/day (rats) and 250 and 1000 mg/kg/day (rabbits). The high doses resulted in maternal toxicity in both rats and rabbits. No effects on embryo-fetal development were observed in either species.
- In a peri- and post-natal developmental study, female rats received oral carglumic acid from organogenesis through day 21 post-partum at doses up to 1.3 times the maximum recommended starting human dose based on body surface area (mg/m2). Actual doses were 500 and 2000 mg/kg/day. A reduction in offspring survival was seen at the high dose and a reduction in offspring growth was seen at both doses.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Carglumic acid in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Carglumic acid during labor and delivery.
Nursing Mothers
- It is not known whether Carbaglu® is excreted in human milk. Carglumic acid is excreted in rat milk, and an increase in mortality and impairment of body weight gain occurred in neonatal rats nursed by mothers receiving carglumic acid. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Carbaglu®, human milk-feeding is not recommended. * Treatment is continuous and life-long for NAGS deficiency patients.
Pediatric Use
- The efficacy of Carbaglu® for the treatment of hyperammonemia in patients with NAGS deficiency from birth to adulthood was evaluated in a retrospective review of the clinical course of 23 NAGS deficiency patients who all began Carbaglu treatment during infancy or childhood. There are no apparent differences in clinical response between adults and pediatric NAGS deficiency patients treated with Carbaglu, however, data are limited.
Geriatic Use
Carbaglu has not been studied in the geriatric population. Therefore, the safety and effectiveness in geriatric patients have not been established.
Gender
There is no FDA guidance on the use of Carglumic acid with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carglumic acid with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Carglumic acid in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Carglumic acid in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Carglumic acid in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Carglumic acid in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Monitor plasma ammonia levels during treatment. Prolonged exposure to elevated plasma ammonia levels can rapidly result in injury to the brain or death. Prompt use of all therapies necessary to reduce plasma ammonia levels is essential.
- Therapeutic Monitoring: Plasma ammonia levels should be maintained within normal range for age via individual dose adjustment.
IV Compatibility
There is limited information regarding IV Compatibility of Carglumic acid in the drug label.
Overdosage
- One patient treated with 650 mg/kg/day of carglumic acid developed symptoms characterized as a monosodium glutamate intoxication-like syndrome: tachycardia, profuse sweating, increased bronchial secretion, increased body temperature and restlessness. These symptoms resolved upon reduction of dose.
- Repeated oral dosing of carglumic acid at 2000 mg/kg/day was lethal to most neonatal rats within 2-3 days of treatment. In adult rats, a single oral administration of carglumic acid was not lethal at doses up to 2800 mg/kg (1.8 times the maximum recommended starting dose based on a body surface area comparison to adult humans).
Pharmacology
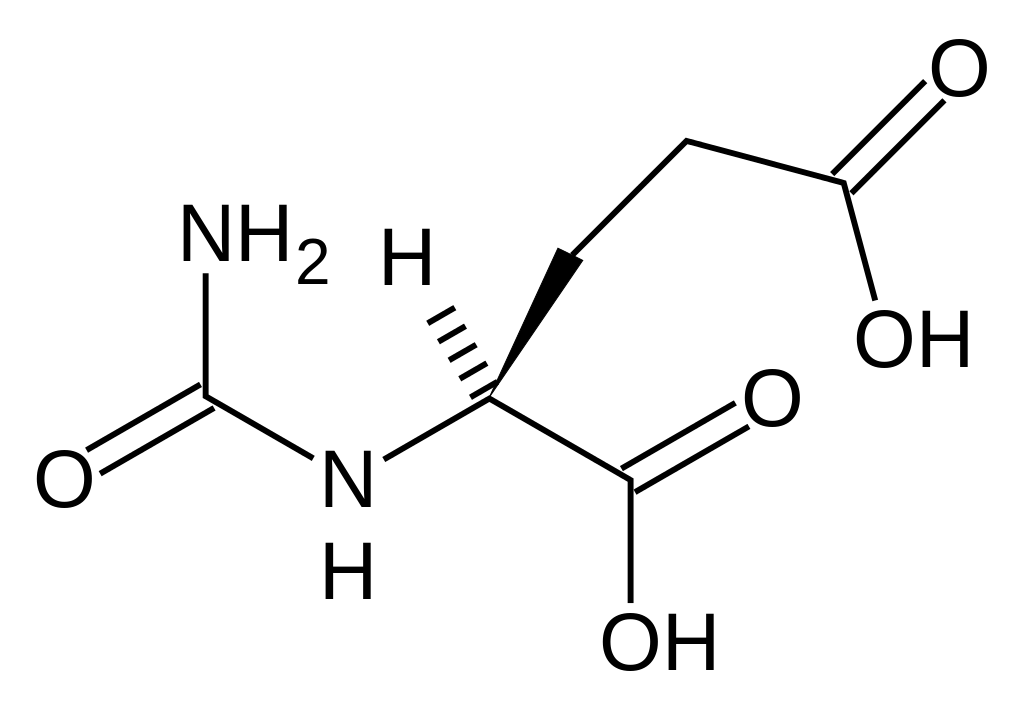
| |
Carglumic acid
| |
| Systematic (IUPAC) name | |
| (2S)-2-(carbamoylamino)pentanedioic acid | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 190.2 g/mol |
| SMILES | & |
| Synonyms | (S)-2-ureidopentanedioic acid |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | Undetermined |
| Metabolism | Partial |
| Half life | 4.3 to 9.5 hours |
| Excretion | Fecal (60%) and renal (9%, unchanged) |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
unknown |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Carglumic acid is a synthetic structural analogue of N-acetylglutamate (NAG), which is an essential allosteric activator of carbamoyl phosphate synthetase 1 (CPS 1) in liver mitochondria. CPS 1 is the first enzyme of the urea cycle, which converts ammonia into urea. NAG is the product of N-acetylglutamate synthase (NAGS), a mitochondrial enzyme. Carglumic acid acts as a replacement for NAG in NAGS deficiency patients by activating CPS 1.
Structure
- Carbaglu tablets for oral administration contain 200 mg of carglumic acid. Carglumic acid, the active substance, is a Carbamoyl Phosphate Synthetase 1 (CPS 1) activator and is soluble in boiling water, slightly soluble in cold water, practically insoluble in organic solvents.
- Chemically carglumic acid is, N-carbamoyl-L-glutamic acid or (2S)-2-(carbamoylamino) pentanedioic acid, with a molecular weight of 190.16.
The structural formula is:
Pharmacodynamics
- In a retrospective review of the clinical course in 23 patients with NAGS deficiency, carglumic acid reduced plasma ammonia levels within 24 hours when administered with and without concomitant ammonia lowering therapies. No dose response relationship has been identified.
Pharmacokinetics
- The pharmacokinetics of carglumic acid has been studied in healthy male volunteers using both radiolabeled and non-radiolabeled carglumic acid.
Absorption
- The median Tmax of Carbaglu was 3 hours (range: 2-4). Absolute bioavailability has not been determined.
Distribution
- The apparent volume of distribution was 2657 L (range: 1616-5797). Protein binding has not been determined.
Metabolism
- A proportion of carglumic acid may be metabolized by the intestinal bacterial flora. The likely end product of carglumic acid metabolism is carbon dioxide, eliminated through the lungs.
Elimination
- Median values for the terminal half-life was 5.6 hours (range 4.3-9.5), the apparent total clearance was 5.7 L/min (range 3.0-9.7), the renal clearance was 290 mL/min (range 204-445), and the 24-hour urinary excretion was 4.5% of the dose (range 3.5-7.5). Following administration of a single radiolabeled oral dose of 100 mg/kg of body weight, 9% of the dose was excreted unchanged in the urine and up to 60% of the dose was excreted unchanged in the feces.
Drug Interaction Studies
- No drug interaction studies have been performed. Based on in-vitro studies, Carbaglu is not an inducer of CYP1A1/2, CYP2B6, CYP2C, and CYP3A4/5 enzymes, and not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4/5 enzymes.
Nonclinical Toxicology
- Carcinogenicity studies have not been performed with carglumic acid.
- Carglumic acid was negative in the Ames test, chromosomal aberration assay in human lymphocytes, and the in vivo micronucleus assay in rats.
- There were no effects on fertility or reproductive performance in female rats at oral doses up to 2000 mg/kg/day (1.3 times the maximum recommended human starting dose based on body surface area). In a separate study, mating and fertility were unaffected in male rats at oral doses up to 1000 mg/kg/day (0.6 times the maximum recommended human starting dose based on body surface area).
Clinical Studies
Responses of Patients with NAGS Deficiency to Acute and Chronic Treatment
- The efficacy of Carbaglu in the treatment of hyperammonemia due to NAGS deficiency was evaluated in a retrospective review of the clinical course of 23 NAGS deficiency patients who received Carbaglu treatment for a median of 7.9 years (range 0.6 to 20.8 years).
- The demographics characteristics of the patient population are shown in Table 2.
- The clinical observations in the 23 patient case series were retrospective, unblinded and uncontrolled and preclude any meaningful formal statistical analyses of the data. However, short-term efficacy was evaluated using mean and median change in plasma ammonia levels from baseline to days 1 to 3. Persistence of efficacy was evaluated using long-term mean and median change in plasma ammonia level. Table 3 summarizes the plasma ammonia levels at baseline, days 1 to 3 post-Carbaglu treatment, and long-term Carbaglu treatment for 13 evaluable patients. Of the 23 NAGS deficiency patients who received treatment with Carbaglu, a subset of 13 patients who had both well documented plasma ammonia levels prior to Carbaglu treatment and after long-term treatment with Carbaglu were selected for analysis.
- All 13 patients had abnormal ammonia levels at baseline. The overall mean baseline plasma ammonia level was 271 µmol/L. By day 3, normal plasma ammonia levels were attained in patients for whom data were available. Long-term efficacy was measured using the last reported plasma ammonia level for each of the 13 patients analyzed (median length of treatment was 6 years; range 1 to 16 years). The mean and median ammonia levels were 23 µmol/L and 24 µmol/L, respectively, after a mean treatment duration of 8 years.
How Supplied
- Carbaglu is a white and elongated tablet, scored and coded “C” on one side.
- Each tablet contains 200 mg of carglumic acid. Carbaglu is available in 5 or 60 tablets in a polypropylene bottle with polyethylene cap and desiccant unit.
- NDC 52276-312-05 Bottle of 5 tablets.
- NDC 52276-312-60 Bottle of 60 tablets.
Storage
- Before opening, store refrigerated at 2-8 °C (36-46 °F).
- After first opening of the container:
- Do not refrigerate, do not store above 30 °C (86 °F).
- Keep the container tightly closed in order to protect from moisture.
- Write the date of opening on the tablet container.
- Do not use after the expiration date stated on the tablet container.
- Discard one month after first opening.
Images
Drug Images
{{#ask: Page Name::Carglumic acid |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Carglumic acid |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Physicians should inform patients and caregivers about the following instructions for safe use of Carbaglu:
- Carbaglu tablets should not be swallowed whole or crushed. Each tablet should be dispersed in a minimum of 2.5 mL of water. Carbaglu tablets do not dissolve completely in water and undissolved particles of the tablet may remain in the mixing container. The mixing container should be rinsed with additional volumes of water and the contents swallowed immediately.
- Before opening, store in a refrigerator 2-8 °C (36-46 °F).
- Keep the container tightly closed in order to protect from moisture.
- After first opening of the container: do not refrigerate, do not store above 30 °C (86 °F).
- Write the date of opening on the tablet container. Discard one month after first opening.
- Do not use after the expiration date stated on the tablet container.
- Physicians should also advise patients and caregivers that:
- When plasma ammonia levels have normalized, dietary protein intake can usually be increased with the goal of unrestricted protein intake.
- Human milk-feeding is not recommended.
- The most common adverse reactions are vomiting, abdominal pain, pyrexia, tonsilitis, anemia, ear infection, diarrhea, nasopharyngitis, and headache.
Precautions with Alcohol
- Alcohol-Carglumic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CARBAGLU®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "CARBAGLU - carglumic acid tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Carglumic acid |Label Name=Carbaglu 01.jpg
}}
{{#subobject:
|Label Page=Carglumic acid |Label Name=Carbaglu 02.jpg
}}
{{#subobject:
|Label Page=Carglumic acid |Label Name=Carbaglu 03.jpg
}}
{{#subobject:
|Label Page=Carglumic acid |Label Name=Carbaglu 04.jpg
}}
{{#subobject:
|Label Page=Carglumic acid |Label Name=DailyMed - CARBAGLU - carglumic acid tablet .png
}}
}}


