Carbon-nitrogen bond
A carbon-nitrogen bond is a covalent bond between carbon and nitrogen and one of the most abundant in organic chemistry and biochemistry [1].
Nitrogen has five valence electrons and in simple amines it is trivalent with two remaining electrons forming a lone pair. Through it, nitrogen can form an additional bond to hydrogen making it tetravalent and with a positive charge in ammonium salts. Many nitrogen compounds can thus be potentially basic but its degree depends on the configuration: the nitrogen atom in amides is not basic due to delocalization of the lone pair into a double bond and in pyrrole the lone pair is part of an aromatic sextet.
In common with carbon-carbon bonds, these bonds can be stable double bonds for instance imines and triple bonds such as nitriles. Bond lengths range from 147.9 pm for simple amines to 147.5 pm for C-N= compounds such as nitromethane to 135.2 pm for partial double bonds in pyridine to 115.8 pm for triple bonds as in nitriles [2]. The bond strength in a CN bond is higher (184 kcal/mol) than that of the CC bond (145 kcal/mol) [2]
A CN bond is strongly polarized towards nitrogen (Electronegativity C vs N = 2.55:3.04) and subsequently molecular dipole moments can be high: cyanamide 4.27D, diazomethane 1.5 D, methyl azide 2.17, pyridine 2.19. For this reason many compounds containing CN bonds are water-soluble.
Nitrogen functional groups
| Chemical class | Bond order | Formula | Structural Formula | Example |
|---|---|---|---|---|
| Amines | 1 | R2C-NH2 | 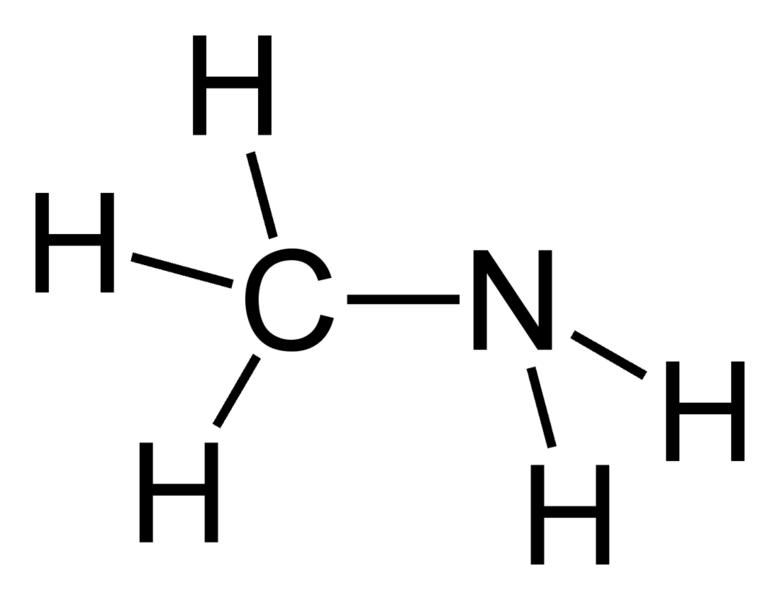 Methylamine | |
| Aziridines | 1 | CH2NHCH2 | Aziridine | Phenyl azide Mitomycin |
| Azides | 1 | R2C-N3 | 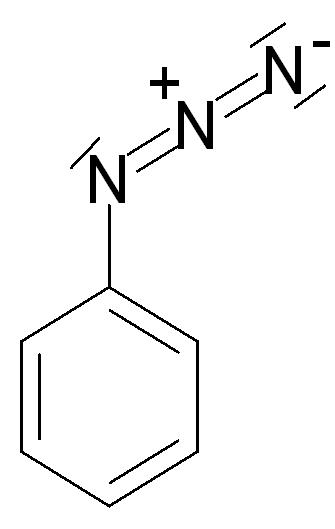 phenyl azide | |
| Anilines | 1 | Ph-NH2 | 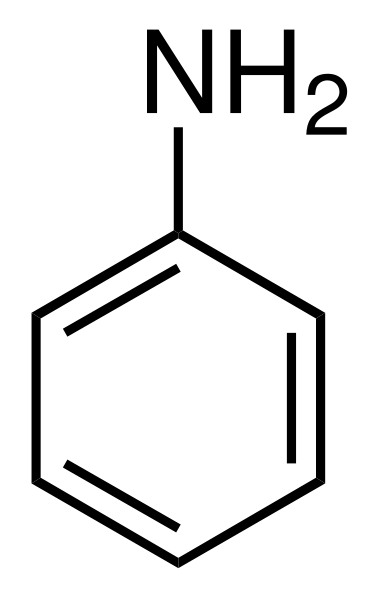
|
Ansidine Anisidine |
| Pyrroles | 1 | amide | 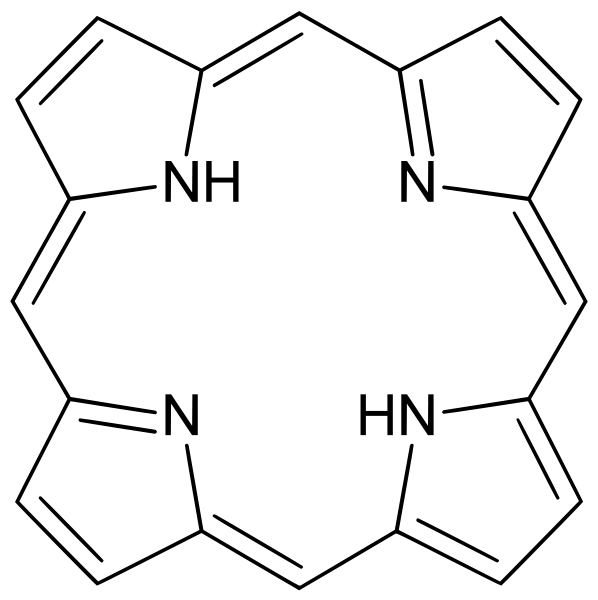 Porphyrin | |
| Amides | 1.2 | R-CO-NR2 | 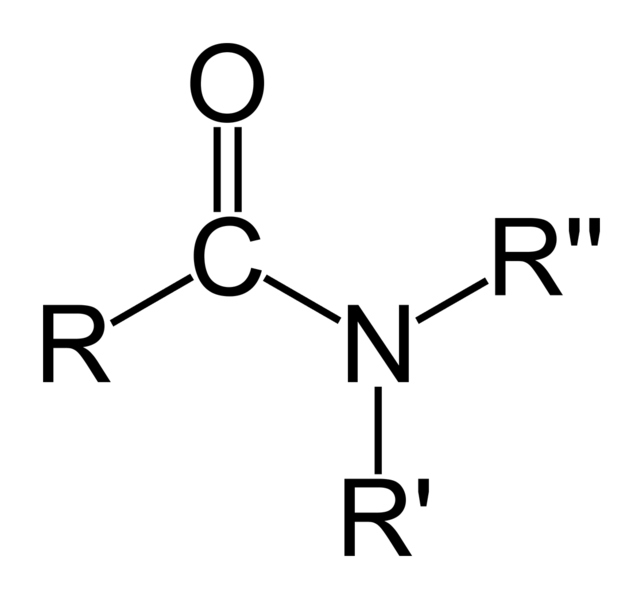
|
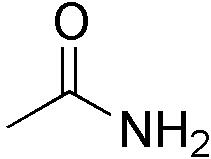 Acetamide |
| Pyridines | 1.5 | pyr | pyridine | Error creating thumbnail: File missing Nicotinamide |
| Imines | 2 | R2C=NR | imine | Ansidine DBN |
| Nitriles | 3 | R-CN | 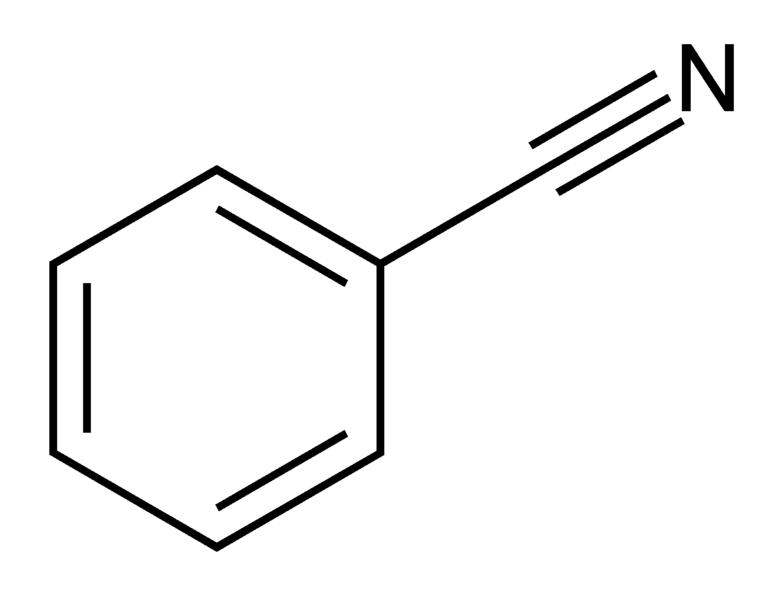 Benzonitrile | |
| Isonitriles | 3 | R-NC | TOSMIC TOSMIC |
See also
- Other carbon bonds with group 15 elements: carbon-nitrogen bonds, carbon-phosphorus bonds
- Other carbon bonds with period 2 elements: carbon-lithium bonds, carbon-beryllium bonds, carbon-boron bonds, carbon-carbon bonds, carbon-nitrogen bonds, carbon-oxygen bonds, carbon-fluorine bonds
Template:ChemicalBondsToCarbon
References
- ↑ Organic Chemistry John McMurry 2nd Ed.
- ↑ 2.0 2.1 CRC Handbook of Chemistry and Physics 65Th Ed.