COL5A2
Jump to navigation
Jump to search
| Collagen, type V, alpha 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbols | COL5A2 ; MGC105115 | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 20119 | ||||||||||
| |||||||||||
| RNA expression pattern | |||||||||||
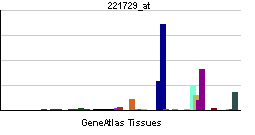 | |||||||||||
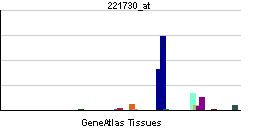 | |||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
Collagen, type V, alpha 2, also known as COL5A2, is a human gene.[1]
This gene encodes an alpha chain for one of the low abundance fibrillar collagens. Fibrillar collagen molecules are trimers that can be composed of one or more types of alpha chains. Type V collagen is found in tissues containing type I collagen and appears to regulate the assembly of heterotypic fibers composed of both type I and type V collagen. This gene product is closely related to type XI collagen and it is possible that the collagen chains of types V and XI constitute a single collagen type with tissue-specific chain combinations. Mutations in this gene are associated with Ehlers-Danlos syndrome, types I and II.[1]
See also
References
Further reading
- Mann K (1992). "Isolation of the alpha 3-chain of human type V collagen and characterization by partial sequencing". Biol. Chem. Hoppe-Seyler. 373 (2): 69–75. PMID 1571108.
- Greenspan DS, Lee ST, Lee BS, Hoffman GG (1992). "Homology between alpha 2(V) and alpha 1(III) collagen promoters and evidence for negatively acting elements in the alpha 2(V) first intron and 5' flanking sequences". Gene Expr. 1 (1): 29–39. PMID 1820205.
- Myers JC, Loidl HR, Seyer JM, Dion AS (1985). "Complete primary structure of the human alpha 2 type V procollagen COOH-terminal propeptide". J. Biol. Chem. 260 (20): 11216–22. PMID 2411731.
- Woodbury D, Benson-Chanda V, Ramirez F (1989). "Amino-terminal propeptide of human pro-alpha 2(V) collagen conforms to the structural criteria of a fibrillar procollagen molecule". J. Biol. Chem. 264 (5): 2735–8. PMID 2914927.
- Myers JC, Loidl HR, Stolle CA, Seyer JM (1985). "Partial covalent structure of the human alpha 2 type V collagen chain". J. Biol. Chem. 260 (9): 5533–41. PMID 2985598.
- Weil D, Bernard M, Gargano S, Ramirez F (1987). "The pro alpha 2(V) collagen gene is evolutionarily related to the major fibrillar-forming collagens". Nucleic Acids Res. 15 (1): 181–98. PMID 3029669.
- Tsipouras P, Schwartz RC, Liddell AC; et al. (1989). "Genetic distance of two fibrillar collagen loci, COL3A1 and COL5A2, located on the long arm of human chromosome 2". Genomics. 3 (3): 275–7. PMID 3224983.
- Emanuel BS, Cannizzaro LA, Seyer JM, Myers JC (1985). "Human alpha 1(III) and alpha 2(V) procollagen genes are located on the long arm of chromosome 2". Proc. Natl. Acad. Sci. U.S.A. 82 (10): 3385–9. PMID 3858826.
- Moradi-Améli M, Rousseau JC, Kleman JP; et al. (1994). "Diversity in the processing events at the N-terminus of type-V collagen". Eur. J. Biochem. 221 (3): 987–95. PMID 8181482.
- Wright GD, Hughes AE, Regan M, Doherty M (1996). "Association of two loci on chromosome 2q with nodal osteoarthritis". Ann. Rheum. Dis. 55 (5): 317–9. PMID 8660106.
- Michalickova K, Susic M, Willing MC; et al. (1998). "Mutations of the alpha2(V) chain of type V collagen impair matrix assembly and produce ehlers-danlos syndrome type I.". Hum. Mol. Genet. 7 (2): 249–55. PMID 9425231.
- Bertelli R, Valenti F, Oleggini R; et al. (1998). "Cell-specific regulation of alpha1(III) and alpha2(V) collagen by TGF-beta1 in tubulointerstitial cell models". Nephrol. Dial. Transplant. 13 (3): 573–9. PMID 9550630.
- Shrivastava A, Radziejewski C, Campbell E; et al. (1998). "An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors". Mol. Cell. 1 (1): 25–34. PMID 9659900.
- Richards AJ, Martin S, Nicholls AC; et al. (1999). "A single base mutation in COL5A2 causes Ehlers-Danlos syndrome type II". J. Med. Genet. 35 (10): 846–8. PMID 9783710.
- Wenstrup RJ, Florer JB, Willing MC; et al. (2001). "COL5A1 haploinsufficiency is a common molecular mechanism underlying the classical form of EDS". Am. J. Hum. Genet. 66 (6): 1766–76. PMID 10777716.
- Välkkilä M, Melkoniemi M, Kvist L; et al. (2002). "Genomic organization of the human COL3A1 and COL5A2 genes: COL5A2 has evolved differently than the other minor fibrillar collagen genes". Matrix Biol. 20 (5–6): 357–66. PMID 11566270.
- Unsöld C, Pappano WN, Imamura Y; et al. (2002). "Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases". J. Biol. Chem. 277 (7): 5596–602. doi:10.1074/jbc.M110003200. PMID 11741999.
- Grond-Ginsbach C, Wigger F, Morcher M; et al. (2002). "Sequence analysis of the COL5A2 gene in patients with spontaneous cervical artery dissections". Neurology. 58 (7): 1103–5. PMID 11940702.
- Takahara K, Schwarze U, Imamura Y; et al. (2002). "Order of intron removal influences multiple splice outcomes, including a two-exon skip, in a COL5A1 acceptor-site mutation that results in abnormal pro-alpha1(V) N-propeptides and Ehlers-Danlos syndrome type I.". Am. J. Hum. Genet. 71 (3): 451–65. PMID 12145749.
- Strausberg RL, Feingold EA, Grouse LH; et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMID 12477932.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |