Beta secretase
β-Secretase — also called BACE1 (β-site of APP cleaving enzyme) or memapsin-2 — is an aspartic-acid protease important in the pathogenesis of Alzheimer's disease, and in the formation of myelin sheaths in peripheral nerve cells.[1] The transmembrane protein, contains two active site aspartate residues in its extracellular protein domain and may function as a dimer.
Function in Alzheimer's
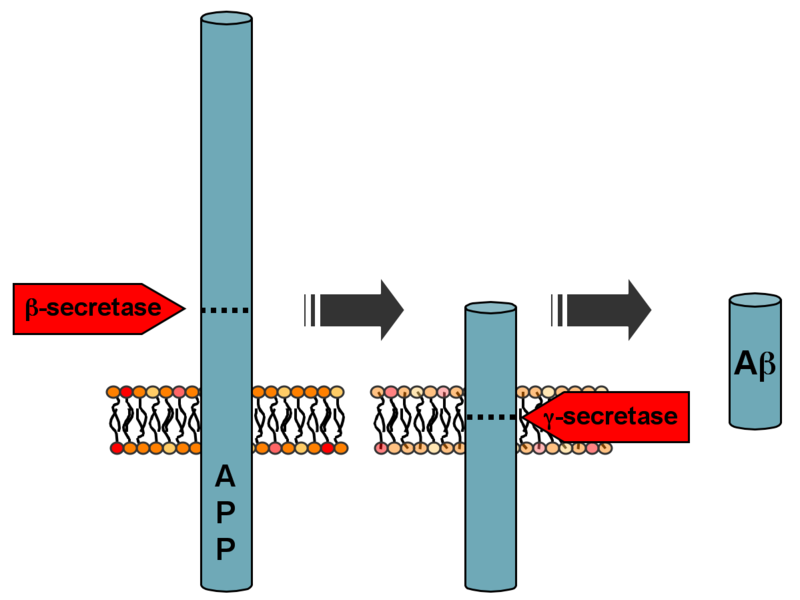
Generation of the 40 or 42 amino acid-long amyloid-β peptides that aggregate in the brain of Alzheimer's patients requires two sequential cleavages of the amyloid precursor protein (APP). Extracellular cleavage of APP by BACE releases a soluble extracellular fragment and is followed by APP cleavage within its transmembrane domain by γ-secretase. The second cleavage releases the intracellular domain of APP and amyloid-β. Since α-secretase cleaves APP closer to the cell membrane than BACE does, it removes a fragment of the amyloid-β peptide. Initial cleavage of APP by α-secretase rather than BACE prevents eventual generation of amyloid-β.
Unlike APP and the presenilin proteins important in γ-secretase, no known mutations in the gene encoding BACE cause the early-onset, familial Alzheimer's disease. However, levels of this enzyme have been shown to be elevated in Alzheimer's. The physiological purpose of BACE's cleavage of APP and other transmembrane proteins is unknown. BACE2 is a close homolog of BACE1.
BACE inhibitors
Drugs to block this enzyme (BACE inhibitors) in theory would prevent the build up of beta-amyloid and may help slow or stop the disease. Several companies are in the early stages of development and testing of this new potential class of treatment.[2][3]
References
- ↑ The Scientist, 22 September 2006 Alzheimer's enzyme important for myelin [1], reporting on a paper published in Science.
- ↑ LF Walker, RC Rosen. "Alzheimer therapeutics—what after the cholinesterase inhibitors?" Age and Ageing 2006 35(4): 332-335. PMID 16644763
- ↑ Baxter; et al. (2007). "2-Aminoquinazolines as Inhibitors of BACE-1 (β-Amyloid Cleaving Enzyme): Use of Structural Biology to Convert a Micromolar HTS Hit to a Nanomolar Lead". J. Med. Chem. 50 (18): 4261–4264.
Further reading
- Hong L, He X, Huang X; et al. (2005). "Structural features of human memapsin 2 (beta-secretase) and their biological and pathological implications". Acta Biochim. Biophys. Sin. (Shanghai). 36 (12): 787–92. PMID 15592644.
- Johnston JA, Liu WW, Todd SA; et al. (2006). "Expression and activity of beta-site amyloid precursor protein cleaving enzyme in Alzheimer's disease". Biochem. Soc. Trans. 33 (Pt 5): 1096–100. doi:10.1042/BST20051096. PMID 16246054.
- Dominguez DI, Hartmann D, De Strooper B (2006). "BACE1 and presenilin: two unusual aspartyl proteases involved in Alzheimer's disease". Neuro-degenerative diseases. 1 (4–5): 168–74. doi:10.1159/000080982. PMID 16908986.
- Zacchetti D, Chieregatti E, Bettegazzi B; et al. (2007). "BACE1 expression and activity: relevance in Alzheimer's disease". Neuro-degenerative diseases. 4 (2–3): 117–26. doi:10.1159/000101836. PMID 17596706.

