Belzutifan
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Embryo-Fetal Toxicity
See full prescribing information for complete Boxed Warning.
|
Overview
Belzutifan is a hypoxia-inducible factor inhibitor that is FDA approved for the treatment of von Hippel-Lindau disease under certain conditions. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, increased creatinine, nausea, anemia, decreased hemoglobin, dizziness, fatigue, and increased glucose.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- 120 mg is the recommended dosage of Belzutifan given to patients through oral administration once daily.
- Recommended dosage is given until disease progression or unacceptable toxicity.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Belzutifan in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Belzutifan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Belzutifan FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Belzutifan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Belzutifan in pediatric patients.
Contraindications
There are no contraindications associated with Belzutifan.
Warnings
|
Embryo-Fetal Toxicity
See full prescribing information for complete Boxed Warning.
|
Anemia
- Severe anemia can occur when taking Belzutifan.
- In Study 004, 90% of patients had experienced anemia in which 7% of these patients were experiencing Grade 3 Anemia when taking Belzutifan.
- In Study 004, median time to onset of anemia was 31 days.
- In Study 001, 76% of patients with advanced solid tumors displayed anemia. Of those patients with anemia, 28% displayed symptoms of Grade 3 anemia.
- Advise patients to be on the alert for anemia when treated with Belzutifan.
- Advise patients that severity of anemia can increase in patients who are dual CYP2C19 and UGT2B17 poor metabolizers.
- Withhold Belzutifan treatment in patients with hemoglobin <9g/dL or are experiencing life threatening anemia.
- Advise patients who's hemoglobin ≥9g/dL to either reduce their dosage or permanently stop Belzutifan use.
- Advise patients treated with Belzutifan that erythropoiesis stimulating agents is not recommended for anemia treatment.
- Advise patients that data from clinical studies show that the risk of serious cardiovascular reactions and death are increased when taking erythropoiesis stimulating agents.
- Advise patients that data from clinical studies show that there is a decrease in progression-free survival and/or overall survival when taking erythropoiesis stimulating agents.
Hypoxia
- Hypoxia may be caused by patients treated with Belzutifan that may lead to discontinuation of treatment, supplemental oxygen, or hospitalization.
- Study 004 showed that 1.6% of patients in the study that were treated with Belzutifan reported hypoxia.
- In Study 001, 29% of patients with advanced solid tumors displayed hypoxia. Of those patients with hypoxia, 16% displayed symptoms of Grade 3 hypoxia.
- Advise patients before start of, and throughout the treatment with Belzutifan to monitor oxygen saturation.
- Withhold treatment of Belzutifan in patients with decreased oxygen saturation (pulse oximeter <88% or PaO2 ≤55 mm Hg) until issue is resolved.
- Advise patients to permanently stop Belzutifan treatment if experiencing recurrent symptomatic hypoxia or life-threatening hypoxia.
Embryo-Fetal Toxicity
- Animal studies have shown that there is risk associated with the fetus in pregnant women who are taking Belzutifan.
- Studies done on pregnant rats have shown that reduced fetal body weight, embryo-fetal lethality, and fetal skeletal malformations when given maternal exposures ≥0.2 times the human exposures of Belzutifan during the period of organogenesis.
- Advise female patients with reproductive potential and pregnant women the risks associated to the fetus when treated with Belzutifan.
- Advise females of reproductive potential to use effective non-hormonal contraception during treatment with Belzutifan and for at least 1 week after the last dose.
- Advise males with female partners of reproductive potential to use effective contraception during treatment with Belzutifan and for at least 1 week after the last dose.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Study 004, an open-label clinical trial, looked into the safety of Belzutifan in 61 patients with VHL disease who had at least one measurable solid tumor localized to the kidney. These patients received the recommended dosage of 120 mg of Belzutifan once daily for a median duration of 68 weeks.
Study 004
- 15% of patients reported serious adverse reactions when treated with Belzutifan which included anaphylaxis reaction, central retinal vein occlusion, hypoxia, retinal detachment and anemia. Adverse reactions caused 3.3% of patients to permanently discontinue Belzutifan treatment. Of the 3.3% of patients who discontinued treatment, opioid overdose and dizziness were the most common adverse reactions that led to discontinuation. Adverse reactions caused 39% of taking Belzutifan to interrupt the dosage being taken. Headache, fatigue, influenza-like illness, decreased hemoglobin, nausea, anemia, and abdominal pain are some of the adverse reactions that caused dosage interruptions. Adverse reactions caused 13% of patients taking Belzutifan to reduce their dosage. Fatigue was the most common adverse reaction that caused a dosage reduction.
- Fatigue, increased glucose, decreased hemoglobin, headache, nausea, dizziness, and increased creatinine are the most reported adverse reactions when patients are treated with Belzutifan.
Table 2 summarizes the Adverse Reactions that occurred during Study 004 of Patients taking Belzutifan.

Table 3 summarizes the Laboratory abnormalities in Study 004 in Patients taking Belzutifan.

Other Clinical Trials Experience
- Study 001 is a clinical trial that contained 58 patients with advanced solid tumors. These patients had a median age of 62.5 years and a median number of prior therapies for cancer was 3. These patients received the recommended dosage of Belzutifan once daily. The most common adverse reactions reported in this clinical trial was cough, dehydration, vomiting, edema, diarrhea, and musculoskeletal pain.
Postmarketing Experience
There is limited information regarding Belzutifan Postmarketing Experience in the drug label.
Drug Interactions
Effects of Other Drugs on Belzutifan
UGT2B17 or CYP2C19 Inhibitors
- Plasma exposures of Belzutifan may increase with coadministration of UGT2B17 or CYP2C19 inhibitors and Belzutifan.
- Adverse reactions of Belzutifan may increase when the plasma exposures of Belzutifan are elevated.
- Reduce the dosage of Belzutifan if patients is experiencing hypoxia or anemia.
Effect of WELIREG on Other Drugs
Sensitive CYP3A4 Substrates
- CYP3A substrates concentrations decreased with coadministration of CYP3A substrates and Belzutifan.
- Patients with dual UGT2B17 and CYP2C19 poor metabolizers are more likely to experience a pronounced decrease in the *CYP3A substrates concentrations.
- Advise patients to avoid coadministration of sensitive CYP3A4 substrates and Belzutifan.
- Advise patients the dosage of sensitive CYP3A4 substrate should be increased if coadministration cannot be avoided.
Hormonal Contraceptives
- Increase in breakthrough bleeding or contraceptive failure may occur in patients with coadministration of hormonal contraceptives and Belzutifan.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Fetal harm may occur in pregnant women who are treated with Belzutifan based on data from animal studies. Pregnant rat studies have shown that there was evidence of reduced fetal body weight, embryo-fetal lethality, and fetal skeletal malformations during the period of organogenesis when given maternal exposures ≥0.2 times the human exposure. Embryo-fetal lethality was seen in pregnant rats that were given doses ≥60 mg/kg/day of Belzutifan during the period of organogenesis. Reduced skeletal ossification, reduced fetal body weights, and fetal rib malformations were seen in pregnant rats that were given doses of 6 and 60 mg/kg/day of Belzutifan. Advise pregnant women or females with reproductive potential about the harms associated with the fetus when treated with Belzutifan.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Belzutifan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Belzutifan during labor and delivery.
Nursing Mothers
No data is present on the effects done on the breastfed child and the effects on milk production when treated with Belzutifan. Advise women who are nursing to not nurse during or 1 week after the last of dose of Belzutifan treatment due to the potential serious adverse reactions associated with Belzutifan.
Pediatric Use
There is no FDA guidance on the use of Belzutifan in pediatric settings.
Geriatic Use
There were not enough patients in clinical studies to look into the differences of Belzutifan in younger patients to patients who are 65 years of age or older.
Gender
There is no FDA guidance on the use of Belzutifan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Belzutifan with respect to specific racial populations.
Renal Impairment
Patients with mild or moderate renal impairment required no dose modifications in Belzutifan treatment. The effects of Belzutifan treatment on patients with severe renal impairment have not been established.
Hepatic Impairment
Patients with mild hepatic impairment required no dose modifications in Belzutifan treatment. The effects of Belzutifan treatment on patients with severe hepatic impairment have not been established.
Females of Reproductive Potential and Males
Advise patients that fetal harm may occur in pregnant women treated with Belzutifan. Verify the pregnancy status of the female patient before starting Belzutifan treatment. Advise females of reproductive potential to use effective non-hormonal contraception during treatment with Belzutifan and for at least 1 week after the last dose. Advise male patients with females of reproductive potential to use effective contraception during treatment with Belzutifan and for at least 1 week after the last dose. Fertility may be impaired in both male and female patients who are treated with Belzutifan.
Immunocompromised Patients
There is no FDA guidance one the use of Belzutifan in patients who are immunocompromised.
Dual UGT2B17 and CYP2C19 Poor Metabolizers
Higher Belzutifan exposures in patients with dual CYP2C19 and UGT2B17 poor metabolizers. Serious adverse reactions susceptibility may increase when exposures of Belzutifan are high. Monitor patients with dual CYP2C19 and UGT2B17 poor metabolizers that are treated with Belzutifan for adverse reactions.
Administration and Monitoring
Administration
- Advise patients to swallow tablets whole.
- Prior to swallowing, advise patients to not crush, spit, or chew Belzutifan.
- If a dosage of Belzutifan is missed, then it can be taken the same day as soon as possible.
- Advise patients who missed a dose to not take extra tablets to make up for that dose which was missed.
Monitoring
Dose Reductions
- 80 mg orally once daily of Belzutifan would be the first dose reduction.
- 40 mg orally once daily of Belzutifan would be the second dose reduction.
- Permanently discontinue Belzutifan if a third dose reduction is needed.
Table 1 summarizes Dosage Modifications in Patients experiencing Adverse Reactions.

IV Compatibility
There is limited information regarding the compatibility of Belzutifan and IV administrations.
Overdosage
- No specific treatment is specified if a Belzutifan overdose occurs.
- Advise patients to withhold institute supportive care and Belzutifan if a suspected overdose has occurred.
- 120 mg twice a day of Belzutifan has resulted in Grade 3 hypoxia.
- 240 mg once daily of Belzutifan has resulted in Grade 4 Thrombocytopenia.
Pharmacology
| |
Belzutifan
| |
| Systematic (IUPAC) name | |
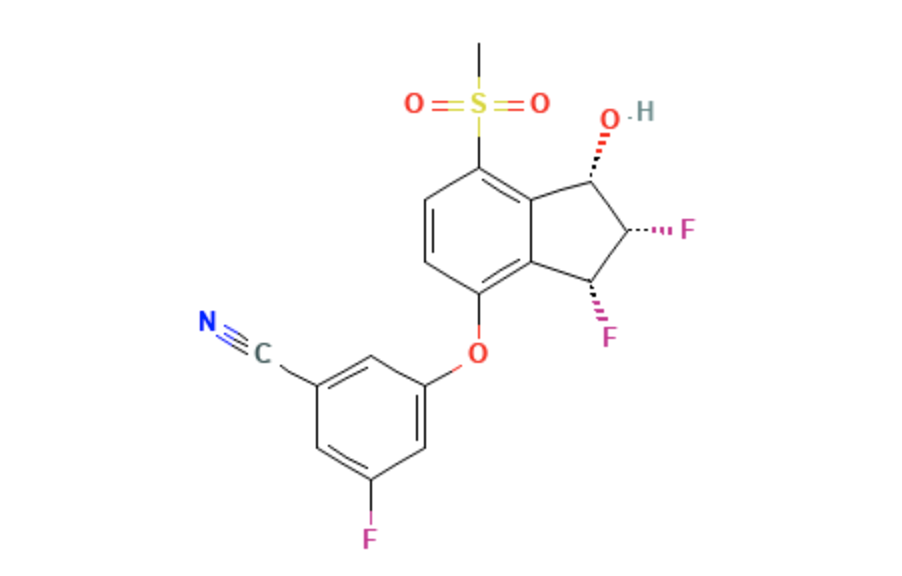
| 3-{[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1H-inden-4-yl]oxy}-5-fluorobenzonitrile | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | MK-6482, PT2977 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- Belzutifan is an inhibitor of hypoxia-inducible factor 2 alpha.
- Ubiquitin-proteasomal degradation by VHL protein targets HIF-2α when there are normal oxygen levels.
- Accumulation and stabilization of HIF-2α when there is a lack of VHL protein.
- A transcriptional complex is formed between HIF-2α and HIF-1β upon stabilization that induces expression of downstream genes associated with angiogenesis, cellular proliferation, and tumor growth.
- HIF-2α-HIF-1β interaction is blocked when a patient is treated with Belzutifan.
Structure
- Belzutifan is an inhibitor of hypoxia-inducible factor 2 alpha. It has an empirical formula of C17H12F3NO4S and a molecular weight of 383.34 Daltons.

Pharmacodynamics
- When given up to 120mg of Belzutifan once daily, there were reductions in the plasma levels of EPO.
- 2 weeks of Belzutifan led to maximum EPO suppression.
- After 12 weeks of Belzutifan treatment, the levels of mean EPO returned to the values found at baseline.
- Patients with baseline hemoglobin levels <12 g/dL were more likely to experience, when given higher doses of Belzutifan, Grade 3 Anemia.
Cardiac Electrophysiology
- Large mean increases in the QT interval is not caused by Belzutifan given at the recommended dosage in patients.
Pharmacokinetics
Mean steady-state
- In patients with VHL disease-associated RCC, the Cmax of Belzutifan is 1.3 μg/mL.
- In patients with VHL disease-associated RCC, the AUC0-24h of Belzutifan is 16.7 μg•hr/mL.
- It takes about 3 days to reach steady state of Belzutifan.
- Over a dose range of 20 mg to 120 mg of Belzutifan, the AUC and Cmax increase proportionally.
Absorption
- After 1 to 2 hours of Belzutifan administration, the Tmax median occurs.
Effect of Food
- Peak Belzutifan concentration was delayed by about 2 hours after a patient is given a high-fat, high-calorie meal.
- There was no clinically meaningful effect on Cmax when patients were given a high-fat, high-calorie meal.
- There was no effect done on AUC when patients were given a a high-fat, high-calorie meal.
Distribution
- 130 L is the mean steady-state volume of distribution for Belzutifan.
- 45% is the plasma protein binding percentage of Belzutifan.
- 0.88 is the Blood-to-plasma concentration ratio of Belzutifan.
Elimination
- 7.3 L/hr is the mean clearance of Belzutifan.
- 14 hrs is the mean elimination half-life of Belzutifan.
Metabolism
- CYP2C19 and UGT2B17 primarily metabolizes Belzutifan.
- CYP3A4 also shows signs of metabolizing Belzutifan.
Specific Populations
- A higher AUC of Belzutifan was detected in patients who are poor metabolizers of CYP2C19 and UGT2B17.
- Mild to moderate renal impairment, sex, age, ethnicity, mild hepatic impairment, or body weight did not cause any clinically significant differences in the pharmacokinetics of patients who are treated with Belzutifan.
- Differences in pharmacokinetics have not been studied in patients with moderate to severe hepatic impairment or severe renal impairment.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches: Effect of Belzutifan on CYP3A Substrates:
- The AUC of Midazolam decreased by 40% when there is coadministration of Midazolam and 120 mg of Belzutifan once daily.
- The Cmax of Midazolam decreased by 34% when there is coadministration of Midazolam and 120 mg of Belzutifan once daily.
- Higher Belzutifan concentrations may cause a decrease of up to 70% in the AUC of Midazolam.
In Vitro Studies: Cytochrome P450 (CYP) Enzymes:
- CYP2B6, CYP2C8, CYP3A4, CYP2C19, CYP1A2, or CYP2D6 are not inhibited by Belzutifan.
- CYP2B6 or CYP1A2 are not induced by Belzutifan.
Transporter Systems:
- Belzutifan is a substrate of OATP1B1, P-gp, and OATP1B3.
- Belzutifan is not a substrate of BCRP.
- MATE2K is inhibited by Belzutifan.
- OATP1B1, OATP1B3, MATE1, P-gp, OCT2, OAT1, BCRP, and OAT3 are not inhibited by Belzutifan.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Studies on carcinogenicity have not been conducted on Belzutifan.
- In vitro bacterial reverse mutation assay, Belzutifan was not mutagenic.
- In vivo rat bone marrow micronucleus assay or in a vitro micronucleus assay, Belzutifan was not clastogenic.
- Studies on animal fertility when given Belzutifan have not been conducted.
- Rat studies show cellular debris and hypospermia of the epididymis when given ≥2 mg/kg/day of Belzutifan in a repeat-dose toxicity study that lasted 3 months.
- Rat studies show degeneration/atrophy of testes when given ≥2 mg/kg/day of Belzutifan in a repeat-dose toxicity study that lasted 3 months.
- There was abnormal sperm morphology, decreased sperm motility, and decreased sperm count in rats who were administered ≥6 mg/kg/day of Belzutifan that did not return to normal levels during the recovery period.
- No adverse effects were seen on female reproductive organs of pregnant rats when given Belzutifan.
- During the period of organogenesis, pregnant rats experienced embryo-fetal lethality when given oral doses that were ≥60 mg/kg/day of Belzutifan.
Clinical Studies
Study 004
- A open-label clinical study was conducted on 61 patients with at least one measurable solid tumor localized to the kidney as defined by response evaluation criteria in solid tumors v1.1. These patients also had VHL-associated RCC diagnosed based on a VHL germline alteration. The patient population was largely Caucasian (90%), and included 53% males, and had a mean age of 41 years. 2.2 cm was the median diameter of RCC target lesions. 17.9 months is the median time from initial radiographic diagnosis of VHL-associated RCC tumors.
Table 4 shows the Efficacy Results of Belzutifan in VHL-associated RCC Patients found in Study 004.

Table 5 shows the Efficacy Results of Belzutifan in VHL-associated pNET or CNS Hemangioblastomas Patients found in Study 004.

How Supplied
How Supplied
- Belzutifan is given as blue tablets that are 40 mg.
- Bottles contain 90 tablets that also contain child resistant seals.
- Belzutifan bottles contains two desiccant canisters which should not be eaten.
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Belzutifan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
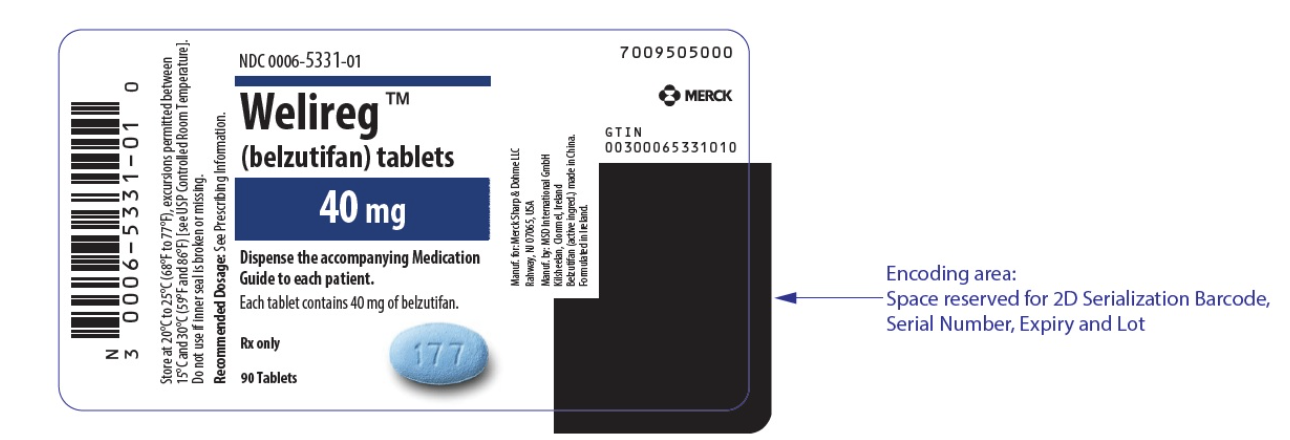

{{#ask: Label Page::Belzutifan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Anemia
- Advise patients that severe anemia may occur as a result of Belzutifan treatment.
- Advise patients that severe anemia may require blood transfusions.
- Monitor patients red blood cell levels during Belzutifan treatment.
- Advise patients to seek medical attention if they display signs of severe anemia.
Hypoxia
- Advise patients that severe hypoxia may occur when treated with Belzutifan.
- Advise patients that severe hypoxia may require supplemental oxygen, discontinuation of treatment, or hospitalization.
- Monitor patients oxygen levels during Belzutifan treatment.
- Advise patients to seek medical attention if they display signs of severe hypoxia.
Embryo-Fetal Toxicity
- Advise pregnant patients and females of reproductive potential to consider the risks associated to the fetus when treated with Belzutifan.
- Advise female patients that suspect they may be pregnant to inform their doctor.
- Advise females of reproductive potential to use effective non-hormonal contraception during treatment with Belzutifan and for at least 1 week after the last dose.
- Advise male patients with females of reproductive potential to use effective contraception during treatment with Belzutifan and for at least 1 week after the last dose.
Lactation
- Advise women who are nursing to not nurse during or 1 week after the last of dose of Belzutifan treatment.
Infertility
- Advise both male and female patients that fertility may be impaired when taking Belzutifan.
Dosage and Administration
- Advise patients to take Belzutifan once daily at the same time each day.
- Advise patients that Belzutifan may be take with or without food.
- Advise patients to swallow Belzutifan tablets whole.
Belzutifan Package Insert:


Precautions with Alcohol
Alcohol-Belzutifan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Welireg
Look-Alike Drug Names
There is limited information regarding Belzutifan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.