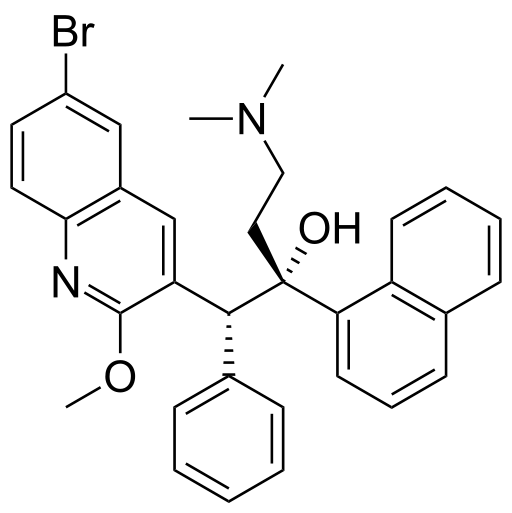
Bedaquiline fumarate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS:
See full prescribing information for complete Boxed Warning.
* An increased risk of death was seen in the bedaquiline fumarate® treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial. Only use bedaquiline fumarate when an effective treatment regimen cannot otherwise be provided.
|
Overview
Bedaquiline fumarate is an antitubercular drug that is FDA approved for the treatment of multidrug resistant tuberculosis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include chest pain, nausea, arthralgia, headache, hemoptysis, increased liver enzymes.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Bedaquiline fumarate is a diarylquinoline antimycobacterial drug indicated as part of combination therapy in adults (≥ 18 years) with pulmonary multi-drug resistant tuberculosis (MDR-TB). Reserve bedaquiline fumarate for use when an effective treatment regimen cannot otherwise be provided. bedaquiline fumarate should be administered by directly observed therapy (DOT).
- This indication is based on analysis of time to sputum culture conversion from two controlled Phase 2 trials in patients with pulmonary MDR-TB.
Limitations of Use:
- The safety and efficacy of bedaquiline fumarate for the treatment of latent infection due to Mycobacterium tuberculosis have not been established. The safety and efficacy of bedaquiline fumarate for the treatment of drug-sensitive TB have not been established. In addition, there are no data on the treatment with bedaquiline fumarate of extra-pulmonary TB (e.g., central nervous system). The safety and efficacy of bedaquiline fumarate for the treatment of infections caused by non-tuberculous mycobacteria (NTM) have not been established. Therefore, use of bedaquiline fumarate in these settings is not recommended.
Dosage
- Bedaquiline fumarate should only be used in combination with at least 3 other drugs to which the patient's MDR-TB isolate has been shown to be susceptible in vitro. If in vitro testing results are unavailable, treatment may be initiated with bedaquiline fumarate in combination with at least 4 other drugs to which the patient's MDR-TB isolate is likely to be susceptible.
- Throughout treatment with, and following the last intake of bedaquiline fumarate, patients should continue to take their companion drugs as directed.
The recommended dosage of Bedaquiline fumarate is:
- Weeks 1–2: 400 mg (4 tablets of 100 mg) once daily with food
- Weeks 3–24: 200 mg (2 tablets of 100 mg) 3 times per week with food (with at least 48 hours between doses) for a total dose of 600 mg per week.
- The total duration of treatment with bedaquiline fumarate is 24 weeks. The bedaquiline fumarate tablet should be swallowed whole with water. Patients should avoid alcohol use while on treatment.
Missed doses
- Patients should be advised of the need to take bedaquiline fumarate as prescribed. Compliance with the full course of therapy must be emphasized.
- If a dose is missed during the first 2 weeks of treatment, patients should not make up the missed dose but should continue the usual dosing schedule. From Week 3 onwards, if a 200 mg dose is missed, patients should take the missed dose as soon as possible, and then resume the 3 times a week regimen.
DOSAGE FORMS AND STRENGTHS
- 100 mg Tablet
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Bedaquiline fumarate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Bedaquiline fumarate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Bedaquiline fumarate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Bedaquiline fumarate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Bedaquiline fumarate in pediatric patients.
Contraindications
- None
Warnings
|
WARNINGS:
See full prescribing information for complete Boxed Warning.
* An increased risk of death was seen in the bedaquiline fumarate® treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial. Only use bedaquiline fumarate when an effective treatment regimen cannot otherwise be provided.
|
Increased Mortality
- An increased risk of death was seen in the bedaquiline fumarate treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial (based on the 120-week visit window). One death occurred during the 24 weeks of administration of bedaquiline fumarate. The imbalance in deaths is unexplained. No discernible pattern between death and sputum culture conversion, relapse, sensitivity to other drugs used to treat TB, HIV status, or severity of disease could be observed. Only use bedaquiline fumarate when an effective treatment regimen cannot otherwise be provided.
QT Prolongation
- Bedaquiline fumarate prolongs the QT interval. An ECG should be obtained before initiation of treatment, and at least 2, 12, and 24 weeks after starting treatment with bedaquiline fumarate. Serum potassium, calcium, and magnesium should be obtained at baseline and corrected if abnormal. Follow-up monitoring of electrolytes should be performed if QT prolongation is detected.
- The following may increase the risk for QT prolongation when patients are receiving bedaquiline fumarate and therefore ECGs should be monitored closely:
- Use with other QT prolonging drugs including fluoroquinolones and macrolide antibacterial drugs and the antimycobacterial drug, clofazimine
- A history of Torsade de Pointes
- A history of congenital long QT syndrome
- A history of hypothyroidism and bradyarrhythmias
- A history of uncompensated heart failure
- Serum calcium, magnesium, or potassium levels below the lower limits of normal
- Discontinue bedaquiline fumarate and all other QT prolonging drugs if the patient develops:
- Clinically significant ventricular arrhythmia
- A QTcF interval of > 500 ms (confirmed by repeat ECG)
- Monitor ECGs frequently to confirm that the QTc interval has returned to baseline.
- If syncope occurs, obtain an ECG to detect QT prolongation.
- Bedaquiline fumarate has not been studied in patients with ventricular arrhythmias or recent myocardial infarction.
Hepatic-Related Adverse Drug Reactions (ADRs)
- More hepatic-related adverse drug reactions were reported with the use of bedaquiline fumarate plus other drugs used to treat TB compared to other drugs used to treat TB without the addition of bedaquiline fumarate. Alcohol and other hepatotoxic drugs should be avoided while on bedaquiline fumarate, especially in patients with diminished hepatic reserve.
- Monitor symptoms and laboratory tests (ALT, AST, alkaline phosphatase, and bilirubin) at baseline, monthly while on treatment, and as needed.
- An increase of serum aminotransferases to > 3×ULN should be followed by repeat testing within 48 hours. Testing for viral hepatitis should be performed and other hepatotoxic medications discontinued.
- Evidence of new or worsening liver dysfunction (including clinically significant elevation of aminotransferases and/or bilirubin and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) in patients on bedaquiline fumarate should prompt additional evaluation by the prescriber.
- Discontinue bedaquiline fumarate if:
- Aminotransferase elevations are accompanied by total bilirubin elevation > 2×ULN
- Aminotransferase elevations are > 8×ULN
- Aminotransferase elevations persist beyond 2 weeks
Drug Interactions
'CYP3A4 inducers/inhibitors'
- Bedaquiline is metabolized by CYP3A4 and its systemic exposure and therapeutic effect may therefore be reduced during co-administration with inducers of CYP3A4. Co-administration of rifamycins (e.g., rifampin, rifapentine and rifabutin) or other strong CYP3A4 inducers used systemically should therefore be avoided while on treatment with bedaquiline fumarate.
- Co-administration of bedaquiline fumarate with strong CYP3A4 inhibitors may increase the systemic exposure to bedaquiline, which could potentially increase the risk of adverse reactions. Therefore, the use of strong CYP3A4 inhibitors used systemically for more than 14 consecutive days should be avoided while on bedaquiline fumarate, unless the benefit of treatment with the drug combination outweighs the risk. Appropriate clinical monitoring for bedaquiline fumarate-related adverse reactions is recommended.
HIV-TB Co-Infected patients
- There are no clinical data on the combined use of antiretroviral agents and bedaquiline fumarate in HIV/MDR-TB co-infected patients and only limited clinical data on the use of bedaquiline fumarate in HIV/MDR-TB co-infected patients (n=22) who were not receiving antiretroviral (ARV) therapy.
Treatment Failure
- Bedaquiline fumarate should be administered by directly observed therapy (DOT). Bedaquiline fumarate should only be administered in combination with at least 3 drugs active against the patient's TB isolate. Isolates from patients who fail to convert or relapse following treatment should be tested for bedaquiline minimum inhibitory concentrations.
Adverse Reactions
Clinical Trials Experience
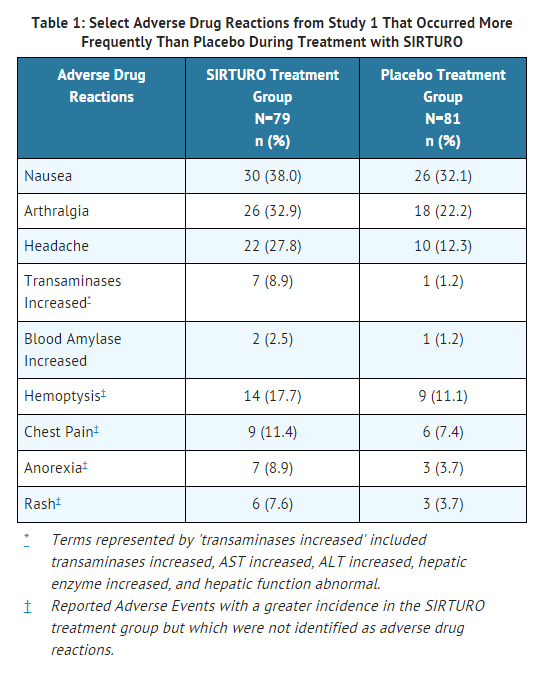
The most frequent adverse drug reactions (> 10.0% of patients) during treatment with bedaquiline fumarate in the controlled trials were nausea, arthralgia, and headache. Additional adverse events reported in ≥10% of patients treated with bedaquiline fumarate and with a higher frequency than the placebo treatment group were hemoptysis and chest pain.
Clinical Studies Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
- Adverse drug reactions for bedaquiline fumarate were identified from the pooled safety data from 335 bedaquiline-exposed patients who received 8 weeks (Study 2) and 24 weeks (Studies 1 and 3) at the proposed dose. Studies 1 and 2 are randomized, double-blind, placebo-controlled trial in newly diagnosed patients with pulmonary MDR-TB. In both treatment arms, patients received bedaquiline fumarate or placebo in combination with other drugs used to treat MDR-TB. Study 3 is an ongoing, open-label, noncomparative study with bedaquiline fumarate administered as part of an individualized pulmonary MDR-TB treatment regimen in previously treated patients.
- In Study 1 overall, 35.0% were Black, 17.5% were Hispanic, 12.5% were White, 9.4% were Asian, and 25.6% were of another race. Eight of 79 (10.1%) patients in the bedaquiline fumarate group and 16 of 81 (19.8%) patients in the placebo treatment group were HIV-infected. Seven (8.9%) bedaquiline fumarate-treated patients and six (7.4%) placebo-treated patients discontinued Study 1 because of an adverse event.
- No additional unique ADRs were identified from the uncontrolled Study 3.
Deaths:
- In Study 1, there was a statistically significant increased mortality risk by Week 120 in the bedaquiline fumarate treatment group compared to the placebo treatment group (9/79 (11.4%) versus 2/81 (2.5%), p-value=0.03, an exact 95% confidence interval of the difference [1.1%, 18.2%]). Five of the 9 bedaquiline fumarate deaths and the 2 placebo deaths were TB-related. One death occurred during the 24-week bedaquiline fumarate treatment period. The median time to death for the remaining eight subjects in the bedaquiline fumarate treatment group was 329 days after last intake of bedaquiline fumarate. The imbalance in deaths is unexplained; no discernible pattern between death and sputum conversion, relapse, sensitivity to other drugs used to treat TB, HIV status, and severity of disease was observed.
QT Prolongation:
- In Study 1, the mean increases in QTc, corrected using the Fridericia method, were greater in the bedaquiline fumarate treatment group compared to the placebo treatment group from the first week of treatment (9.9 ms at Week 1 for bedaquiline fumarate and 3.5 ms for placebo). The largest mean increase in QTc during the 24 weeks of bedaquiline fumarate treatment was 15.7 ms compared to 6.2 ms with placebo treatment (at Week 18). QT increases from baseline in the bedaquiline fumarate group persisted even after bedaquiline fumarate treatment was stopped. During the trial, there was no clear correlation of antecedent significant QT prolongation or clinically significant dysrhythmia in any of the subjects that died.
- In Study 3, where patients with no treatment options received other QT-prolonging drugs used to treat TB, including clofazimine, concurrent use with bedaquiline fumarate resulted in additive QT prolongation, proportional to the number of QT prolonging drugs in the treatment regimen. Patients receiving bedaquiline fumarate alone with no other QT prolonging drug developed a mean QTcF increase over baseline of 23.7 ms with no QT segment duration in excess of 480 ms, whereas patients with at least 2 other QT prolonging drugs developed a mean QTcF prolongation of 30.7 ms over baseline, resulting in QTcF segment durations in excess of 500 ms in one patient.
- There were no documented cases of Torsade de Pointes in the safety database
Hepatic-Related ADRs (including abnormalities in serum transaminases):
- Hepatic ADRs developed in more bedaquiline fumarate-treated patients than those treated with other drugs used to treat TB.
Laboratory tests: In both Studies 1 and 2, reversible aminotransferase elevations of at least 3×ULN developed more frequently in the bedaquiline fumarate treatment group (11/102 [10.8%] vs 6/105 [5.7%] in the placebo treatment group.
Reported adverse reactions: In Study 1, increased aminotransferases were reported in 7/79 (8.9%) patients in the bedaquiline fumarate treatment group compared to 1/81 (1.2%) patients in the placebo treatment group.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Bedaquiline fumarate in the drug label.
Drug Interactions
- CYP3A4 is the major CYP isoenzyme involved in the metabolism of bedaquiline and the formation of the major N-monodesmethyl metabolite (M2), which is 4 to 6-times less active in terms of antimycobacterial potency.
- In vitro, bedaquiline does not significantly inhibit the activity of the following CYP450 enzymes that were tested. CYP1A2, CYP2A6, CYP2C8/9/10, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A4/5 and CYP4A, and it does not induce CYP1A2, CYP2C9, CYP2C19, or CYP3A4 activities.
CYP3A4 Inducers/Inhibitors
Bedaquiline exposure may be reduced during co-administration with inducers of CYP3A4 and increased during co-administration with inhibitors of CYP3A4.
'Rifampin(strong CYP3A4 inducer)'
- In a drug interaction study of 300 mg bedaquiline and rifampin 600 mg once daily for 21 days in healthy subjects, the exposure (AUC) to bedaquiline was reduced by 52%. Due to the possibility of a reduction of the therapeutic effect of bedaquiline because of the decrease in systemic exposure, co-administration of bedaquiline and rifamycins (e.g., rifampin, rifapentine and rifabutin) or other strong CYP3A4 inducers used systemically should be avoided.
'Ketoconazole (strong CYP3A4 inhibitor)'
- Co-administration of 400 mg bedaquiline once daily for 14 days and ketoconazole 400 mg once daily for 4 days in healthy subjects increased the exposure (AUC) to bedaquiline by 22%. Due to the potential risk of adverse reactions to bedaquiline because of the increase in systemic exposure, prolonged co-administration of bedaquiline and strong CYP3A4 inhibitors used systemically for more than 14 consecutive days should be avoided unless the benefit outweighs the risk . Appropriate clinical monitoring for bedaquiline fumarate-related adverse reactions is recommended.
Other Antimicrobial Medications
- The combination of 400 mg bedaquiline once daily for 14 days with isoniazid (300 mg once daily for 5 days)/pyrazinamide (1000 mg once daily for 5 days) in healthy subjects did not result in clinically relevant changes in the exposure (AUC) to bedaquiline, isoniazid or pyrazinamide. No dose-adjustment of isoniazid or pyrazinamide is required during co-administration with bedaquiline fumarate. In a placebo-controlled clinical trial in patients with MDR-TB, no major impact of co-administration of bedaquiline fumarate on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine was observed.
Antiretroviral Medications
- Clinical data in HIV/MDR-TB co-infected patients on the combined use of Kaletra or nevirapine, as well as other antiretroviral agents, with bedaquiline fumarate are not available .
'Kaletra (400 mg lopinavir/100 mg ritonavir)'
- In a healthy volunteer drug interaction study of 400 mg single dose bedaquiline and Kaletra twice daily for 24 days, exposure (AUC) to bedaquiline was increased by 22%. Bedaquiline fumarate must be used with caution when co-administered with Kaletra and only if the benefit outweighs the risk.
'Nevirapine'
- Co-administration of bedaquiline 400 mg single dose and nevirapine 200 mg twice daily for 4 weeks with bedaquiline did not result in clinically relevant changes in the exposure to bedaquiline in HIV-infected patients. No dosage adjustment of bedaquiline is required when co-administered with nevirapine.
QT Interval Prolonging Drugs
- In a drug interaction study of bedaquiline and ketoconazole, a greater effect on QTc was observed after repeated dosing with bedaquiline and ketoconazole in combination than after repeated dosing with the individual drugs. Additive or synergistic QT prolongation was observed when bedaquiline was co-administered with other drugs that prolong the QT interval.
- In Study 3, mean increases in QTc were larger in the 17 subjects who were using clofazimine with bedaquiline at Week 24 (mean change from reference of 31.9 ms) than in subjects who were not using clofazimine with bedaquiline at Week 24 (mean change from reference of 12.3 ms)
Use in Specific Populations
Pregnancy
- Pregnancy Category B. Reproduction studies performed in rats and rabbits have revealed no evidence of harm to the fetus due to bedaquiline. In these studies, the corresponding plasma exposure (AUC) was 2-fold higher in rats compared to humans. There are, however, no adequate and well-controlled studies of bedaquiline fumarate in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bedaquiline fumarate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bedaquiline fumarate during labor and delivery.
Nursing Mothers
- It is not known whether bedaquiline or its metabolites are excreted in human milk, but rat studies have shown that drug is concentrated in breast milk.
- In rats, treated with bedaquiline at doses 1 to 2 times the clinical dose (based on AUC comparisons), concentrations in milk were 6- to 12-fold higher than the maximum concentration observed in maternal plasma. Pups from these dams showed reduced body weights compared to control animals throughout the lactation period.
- Because of the potential for adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of bedaquiline fumarate in children and adolescents less than 18 years of age have not been established.
Geriatic Use
- Clinical studies of bedaquiline fumarate did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Bedaquiline fumarate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Bedaquiline fumarate with respect to specific racial populations.
Renal Impairment
- Bedaquiline fumarate has mainly been studied in patients with normal renal function. Renal excretion of unchanged bedaquiline is not substantial (< 0.001%). No dose adjustment is required in patients with mild or moderate renal impairment. In patients with severe renal impairment or end stage renal disease requiring hemodialysis or peritoneal dialysis, bedaquiline fumarate should be used with caution.
Hepatic Impairment
- The pharmacokinetics of bedaquiline were assessed after single-dose administration to subjects with moderate hepatic impairment (Child-Pugh B). Based on these results, no dose adjustment is necessary for bedaquiline fumarate in patients with mild or moderate hepatic impairment. Bedaquiline fumarate has not been studied in patients with severe hepatic impairment and should be used with caution in these patients only when the benefits outweigh the risks. Clinical monitoring for bedaquiline fumarate-related adverse reactions is recommended.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bedaquiline fumarate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bedaquiline fumarate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Bedaquiline fumarate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Bedaquiline fumarate in the drug label.
Overdosage
- There is no experience with the treatment of acute overdose with bedaquiline fumarate. General measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) should be taken in case of deliberate or accidental overdose. Removal of unabsorbed bedaquiline may be achieved by gastric lavage or aided by the administration of activated charcoal. Since bedaquiline is highly protein-bound, dialysis is not likely to significantly remove bedaquiline from plasma.
Pharmacology
Mechanism of Action
- Bedaquiline is a diarylquinoline antimycobacterial drug.
Structure
- SIRTURO (bedaquiline) for oral administration is available as 100 mg strength tablets. Each tablet contains 120.89 mg of bedaquiline fumarate drug substance, which is equivalent to 100 mg of bedaquiline. Bedaquiline is a diarylquinoline antimycobacterial drug.
- Bedaquiline fumarate is a white to almost white powder and is practically insoluble in aqueous media. The chemical name of bedaquiline fumarate is (1R, 2S)-1-(6-bromo-2-methoxy-3-quinolinyl)-4-(dimethylamino)-2-(1-naphthalenyl)-1-phenyl-2-butanol compound with fumaric acid (1:1). It has a molecular formula of C32H31BrN2O2•C4H4O4 and a molecular weight of 671.58 (555.50 + 116.07). The molecular structure of bedaquiline fumarate is the following:
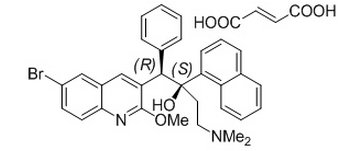
- SIRTURO (bedaquiline) contains the following inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose 2910 15 mPa.s, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, purified water (removed during processing).
Pharmacodynamics
- Bedaquiline is primarily subjected to oxidative metabolism leading to the formation of N-monodesmethyl metabolite (M2). M2 is not thought to contribute significantly to clinical efficacy given its lower average exposure (23% to 31%) in humans and lower antimycobacterial activity (4 to 6-fold lower) compared to the parent compound. M2 concentrations appeared to correlate with QT prolongation.
Pharmacokinetics
Absorption
After oral administration bedaquiline maximum plasma concentrations (Cmax) are typically achieved at approximately 5 hours post-dose. Cmax and the area under the plasma concentration-time curve (AUC) increased proportionally up to the highest doses studied in healthy volunteers (700 mg single-dose and once daily 400 multiple doses). Administration of bedaquiline with a standard meal containing approximately 22 grams of fat (558 total Kcal) increased the relative bioavailability by about 2-fold compared to administration under fasted conditions. Therefore, bedaquiline should be taken with food to enhance its oral bioavailability.
Distribution
The plasma protein binding of bedaquiline is > 99.9%. The volume of distribution in the central compartment is estimated to be approximately 164 L.
Metabolism/Excretion
CYP3A4 was the major CYP isoenzyme involved in vitro in the metabolism of bedaquiline and the formation of the N-monodesmethyl metabolite (M2), which is 4 to 6-times less active in terms of antimycobacterial potency. Based on preclinical studies, bedaquiline is mainly eliminated in feces. The urinary excretion of unchanged bedaquiline was < 0.001% of the dose in clinical studies, indicating that renal clearance of unchanged drug is insignificant. After reaching Cmax, bedaquiline concentrations decline tri-exponentially. The mean terminal elimination half-life of bedaquiline and the N-monodesmethyl metabolite (M2) is approximately 5.5 months. This long terminal elimination phase likely reflects slow release of bedaquiline and M2 from peripheral tissues.
Specific Populations
'Hepatic Impairment'
- After single-dose administration of 400 mg SIRTURO to 8 patients with moderate hepatic impairment (Child-Pugh B), mean exposure to bedaquiline and M2 (AUC672h) was approximately 20% lower compared to healthy subjects. No dose adjustment is deemed necessary in patients with mild or moderate hepatic impairment. SIRTURO has not been studied in patients with severe hepatic impairment and should be used with caution in these patients only when the benefits outweigh the risks. Clinical monitoring for SIRTURO-related adverse reactions is recommended.
'Renal Impairment'
- Bedaquiline fumarate has mainly been studied in patients with normal renal function. Renal excretion of unchanged bedaquiline is not substantial (< 0.001%).
- In a population pharmacokinetic analysis of MDR-TB patients treated with SIRTURO 200 mg three times per week, creatinine clearance was not found to influence the pharmacokinetic parameters of bedaquiline. It is therefore not expected that mild or moderate renal impairment will have a clinically relevant effect on the exposure to bedaquiline, and no adjustment of the bedaquiline dose is needed in patients with mild or moderate renal impairment. However, in patients with severe renal impairment or end-stage renal disease requiring hemodialysis or peritoneal dialysis, bedaquiline should be used with caution and with increased monitoring for adverse effects, as bedaquiline concentrations may be increased due to alteration of drug absorption, distribution, and metabolism secondary to renal dysfunction. As bedaquiline is highly bound to plasma proteins, it is unlikely that it will be significantly removed from plasma by hemodialysis or peritoneal dialysis.
'Gender'
- In a population pharmacokinetic analysis of MDR-TB patients treated with SIRTURO no clinically relevant difference in exposure between men and women were observed.
'Race'
- In a population pharmacokinetic analysis of MDR-TB patients treated with SIRTURO, systemic exposure (AUC) to bedaquiline was found to be 34% lower in Black patients than in patients from other race categories. This lower exposure was not considered to be clinically relevant as no clear relationship between exposure to bedaquiline and response has been observed in clinical trials of MDR-TB. Furthermore, response rates were comparable in patients of different race categories that completed 24 weeks of bedaquiline treatment.
'HIV Co-infection'
- There are limited data on the use of bedaquiline fumarate in HIV co-infected patients.
'Geriatric Patients'
- There is limited data on the use of bedaquiline fumarate in TB patients 65 years and older.
- In a population pharmacokinetic analysis of MDR-TB patients treated with bedaquiline fumarate, age was not found to influence the pharmacokinetics of bedaquiline.
'Pediatric Patients'
- The pharmacokinetics of bedaquiline fumarate in pediatric patients have not been evaluated.
Drug-Drug Interactions
'Ketoconazole'
- Co-administration of multiple-dose bedaquiline (400 mg once daily for 14 days) and multiple-dose ketoconazole (once daily 400 mg for 4 days) in healthy subjects increased the AUC24h, Cmax and Cmin of bedaquiline by 22% [90% CI (12; 32)], 9% [90% CI (-2, 21)] and 33% [90% CI (24, 43)] respectively. Co-administration of bedaquiline and ketoconazole or other strong CYP3A4 inhibitors used systemically for more than 14 consecutive days should be avoided, unless the benefit of the combination of these drugs outweighs the risk. Appropriate clinical monitoring for SIRTURO-related adverse reactions is recommended.
'Rifampin'
- In a drug interaction study of single-dose 300 mg bedaquiline and multiple-dose rifampin (once daily 600 mg for 21 days) in healthy subjects, the exposure (AUC) to bedaquiline was reduced by 52% [90% CI (-57; -46)]. The combination of bedaquiline and rifamycins (e.g., rifampin, rifapentine and rifabutin) or other strong CYP3A4 inducers used systemically should be avoided.
'Antimicrobial agents'
- The combination of multiple-dose bedaquiline 400 mg once daily with multiple-dose isoniazid/pyrazinamide (300 mg/2000 mg once daily) in healthy subjects did not result in clinically relevant changes in the exposure (AUC) to bedaquiline, isoniazid or pyrazinamide. No dose adjustment of isoniazid or pyrazinamide during co-administration with SIRTURO is required.
- In a placebo-controlled study in patients with MDR-TB, no major impact of co-administration of bedaquiline on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine was observed.
'Kaletra (400 mg lopinavir/100 mg ritonavir)'
- In a drug interaction study in healthy volunteers of single-dose bedaquiline (400 mg) and multiple-dose Kaletra given twice daily for 24 days, the mean AUC of bedaquiline was increased by 22% [90% CI (11; 34)] while the mean Cmax was not substantially affected.
'Nevirapine'
- Co-administration of multiple-dose nevirapine 200 mg twice daily for 4 weeks in HIV-infected patients with a single 400 mg dose of bedaquiline did not result in clinically relevant changes in the exposure to bedaquiline.
Microbiology
Mechanism of Action
Bedaquiline is a diarylquinoline antimycobacterial drug that inhibits mycobacterial ATP (adenosine 5'-triphosphate) synthase, an enzyme that is essential for the generation of energy in Mycobacterium tuberculosis.
Mechanisms of Resistance
Mycobacterial resistance mechanisms that affect bedaquiline include modification of the atpE target gene. Not all isolates with increased minimum inhibitory concentrations (MICs) have atpE mutations, suggesting the existence of at least one other mechanism of resistance.
Spectrum of Activity
Bedaquiline has been shown to be active against most isolates of Mycobacterium tuberculosis.
Susceptibility Test Methods
In vitro susceptibility tests should be performed according to published methods1,2. Based on the available information, susceptibility test interpretive criteria for bedaquiline cannot be established at this time. When susceptibility testing is performed by the 7H10 or 7H11 agar method, a range of concentrations from 0.008 microgram per mL to 1.0 microgram per mL should be assessed. The minimum inhibitory concentration (MIC) should be determined as the lowest concentration of bedaquiline that results in growth of less than or equal to 1% of the residual subpopulation. When susceptibility testing is performed by the resazurin microtiter assay (REMA) method, a range of concentrations from 0.008 microgram per mL to 1.0 microgram per mL should be assessed. The MIC should be determined as the lowest concentration of bedaquiline that prevents a visible change of resazurin color from blue to pink. All assays should be performed in polystyrene plates or tubes. Löwenstein-Jensen (LJ) medium should not be used.
The actual MIC should be reported. A specialist in drug-resistant TB should be consulted in evaluating therapeutic options.
The bedaquiline agar (left) and REMA (right) MIC distributions against clinical isolates resistant to isoniazid and rifampin from Studies 1, 2, and 3 are provided below.
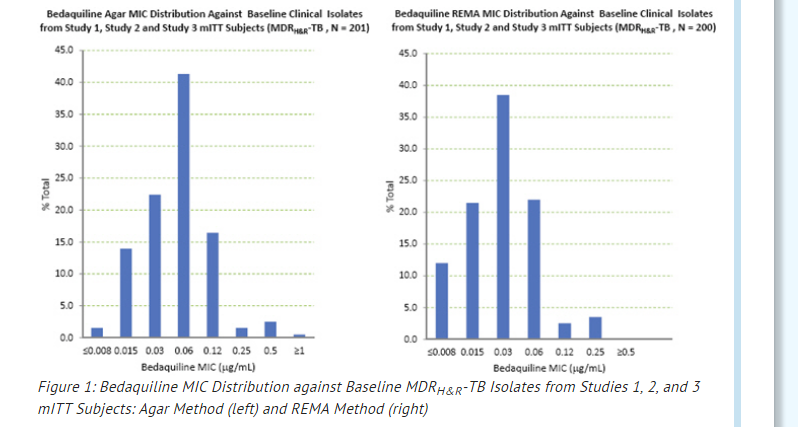
MICs for baseline M. tuberculosis isolates from subjects in Studies 1 and 3 and their sputum culture conversion rates at Week 24 are shown in Table 2 below. No correlation was seen between the culture conversion rates at Week 24 and baseline MICs.
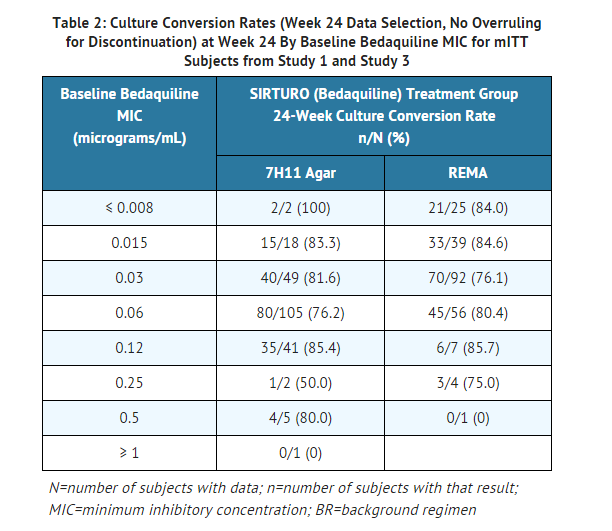
In bedaquiline fumarate-treated patients from Studies 1, 2, and 3, with at least a four-fold increase in bedaquiline MIC from baseline and with atp operon sequencing results, no coding variation in the atp operon was seen, suggesting a mechanism of resistance other than mutations in the atpE gene. Of these subjects for the agar method, patients who experienced failure to convert their sputum or relapsed (n=9) had post-baseline isolates with 4-fold to greater than 8-fold increases in MIC (corresponding to post-baseline MICs of 0.24 to greater than 0.48 microgram/mL). For the REMA method, patients who experienced failure or relapsed had post-baseline isolates with 4-fold to greater than 16-fold increases in MIC (corresponding to post-baseline MICs of 0.015 to 1.0 microgram/mL). All 9 subjects with increased MICs and failure or relapse were infected with MDR-TB isolates that were resistant to additional antimycobacterial drugs in their treatment regimen.
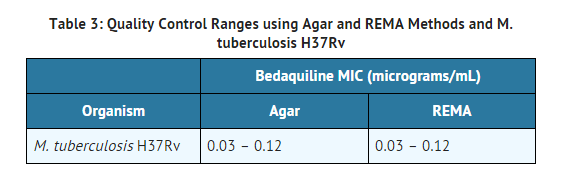
Quality Control
Susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of testing. Assays using standard bedaquiline powder should provide the following range of MIC values shown in Table 3.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Bedaquiline was not carcinogenic in rats up to the maximum tolerated dose of 10 mg/kg/day. Exposures at this dose in rats (AUCs) were within 1 to 2-fold of those observed in subjects in the Phase 2 clinical trials.
- No mutagenic or clastogenic effects were detected in the in vitro non-mammalian reverse mutation (Ames) test, in vitro mammalian (mouse lymphoma) forward mutation assay and an in vivo mouse bone marrow micronucleus assay.
- Bedaquiline fumarate had no effects on fertility when evaluated in male and female rats. No relevant drug-related effects on developmental toxicity parameters were observed in rats and rabbits. The corresponding plasma exposure (AUC) was 2-fold higher in rats and lower for rabbits compared to humans. There was no effect of maternal treatment with bedaquiline at any dose level on sexual maturation, behavioral development, mating performance, fertility or reproductive capacity of the F1 generation animals. Body weight decreases in pups were noted in high dose groups during the lactation period after exposure to bedaquiline via milk and were not a consequence of in utero exposure. Concentrations of bedaquiline in milk were 6- to 12-fold higher that the maximum concentration observed in maternal plasma.
Animal Toxicology and/or Pharmacology
Bedaquiline is a cationic, amphiphilic drug that induced phospholipidosis (at almost all doses, even after very short exposures) in drug-treated animals, mainly in cells of the monocytic phagocytic system (MPS). All species tested showed drug-related increases in pigment-laden and/or foamy macrophages, mostly in the lymph nodes, spleen, lungs, liver, stomach, skeletal muscle, pancreas and/or uterus. After treatment ended, these findings were slowly reversible. Muscle degeneration was observed in several species at the highest doses tested. For example the diaphragm, esophagus, quadriceps and tongue of rats were affected after 26 weeks of treatment at doses similar to clinical exposures based on AUC comparisons. These findings were not seen after a 12-week, treatment-free, recovery period and were not present in rats given the same dose biweekly. Degeneration of the fundic mucosa of the stomach, hepatocellular hypertrophy and pancreatitis were also seen.
Clinical Studies
- A placebo-controlled, double-blind, randomized trial (Study 1) was conducted in newly diagnosed patients with multi-drug resistant pulmonary Mycobacterium tuberculosis. Patients were randomized to receive treatment with either bedaquiline fumarate and other drugs used to treat MDR-TB (bedaquiline fumarate treatment group) (n=79) or placebo plus other drugs used to treat MDR-TB (placebo treatment group) (n=81); the other drugs used to treat MDR-TB consisted of a combination of 5 other antimycobacterial drugs (ethionamide, kanamycin, pyrazinamide, ofloxacin, and cycloserine/terizidone or available alternative). Sixty-three percent of the population was male, with a median age of 34 years, 35% were Black, and 15% were HIV-positive. Most patients had cavitation in one lung (62%); cavitation in both lungs was observed in 18% of patients.
- Bedaquiline fumarate was administered as 400 mg once daily for the first 2 weeks and as 200 mg 3 times per week for the following 22 weeks. After the 24-week study drug (bedaquiline fumarate or placebo) treatment phase, patients continued to receive their other drugs used to treat MDR-TB until a total treatment duration of 18 to 24 months was achieved, or at least 12 months after the first confirmed negative culture.
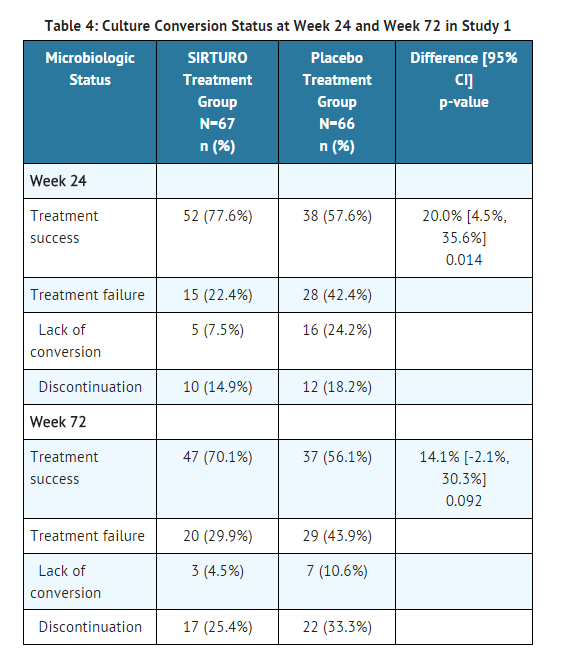
- Time to sputum culture conversion was defined as the interval in days between the first dose of study drug and the date of the first of two consecutive negative sputum cultures collected at least 25 days apart during treatment. In this ongoing trial, the bedaquiline fumarate treatment group had a decreased time to culture conversion and improved culture conversion rates compared to the placebo treatment group at Week 24. Median time to culture conversion was 83 days for the bedaquiline fumarate treatment group compared to 125 days for the placebo treatment group. Table 4 shows the proportion of patients with sputum culture conversion after 24 weeks and 72 weeks of treatment with bedaquiline fumarate or placebo in combination with other drugs used to treat MDR-TB (with patients who discontinued or died considered as failures).
- Study 2 was a smaller placebo controlled study designed similarly to Study 1 except that bedaquiline fumarate or placebo was given for only 8 weeks instead of 24 weeks. Patients were randomized to either bedaquiline fumarate and other drugs used to treat MDR-TB (bedaquiline fumarate treatment group) (n=23) or placebo and other drugs used to treat MDR-TB (placebo treatment group) (n=24). Twenty-one patients randomized to the bedaquiline fumarate treatment group and 23 patients randomized to the placebo treatment group had confirmed MDR-TB based on subjects' baseline M. tuberculosis isolate obtained prior to randomization. The SIRTURO treatment group had a decreased time to culture conversion and improved culture conversion rates compared to the placebo treatment group at Week 8. At Weeks 8 and 24, the differences in culture conversion proportions were 38.9% (95% CI: [12.3%, 63.1%] and p-value: 0.004), 15.7% (95% CI: [-11.9%, 41.9%] and p-value: 0.32), respectively.
How Supplied
- Bedaquiline fumarate is supplied as uncoated white to almost white round biconvex 100 mg tablets with debossing of "T" over "207" on one side and "100" on the other side. The tablets are packaged in white high density polyethylene (HDPE) bottles with child-resistant polypropylene (PP) closure with induction seal liner. Each bottle contains 188 tablets.
NDC 59676-701-01
Storage
- Keep out of reach of children.
- Dispense in original container. Tablets dispensed outside the original container should be stored in a tight light-resistant container with an expiration date not to exceed 3 months.
- Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F). [See USP Controlled Room Temperature]
Images
Drug Images
{{#ask: Page Name::Bedaquiline fumarate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Bedaquiline fumarate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Tuberculosis is a serious disease that can be fatal; some patients with resistant TB may have limited treatment options. Patients should be advised that the following serious side effects can occur with bedaquiline fumarate: death, heart rhythm abnormalities, and/or hepatitis. In addition, patients should also be advised about other potential side effects: nausea, joint pain, headache, increased blood amylase, hemoptysis, chest pain, anorexia, and/or rash. Additional testing may be needed to monitor or reduce the likelihood of adverse effects.
- Take bedaquiline fumarate in combination with other antimycobacterial drugs as prescribed. Compliance with the full course of therapy must be emphasized. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the treatment and (2) increase the likelihood that their mycobacterium may develop resistance and the disease will not be treatable by bedaquiline fumarate or other antibacterial drugs in the future.
- If a dose is missed during the first 2 weeks of treatment, patients should not make up the missed dose but should continue the usual dosing schedule. From Week 3 onwards, if a 200 mg dose is missed, patients should take the missed dose as soon as possible, and then resume the 3 times a week regimen.
- Patients should be informed to take bedaquiline fumarate with food.
- Patients should be advised to abstain from alcohol, hepatotoxic medications or herbal products.
- Patients should be advised to discuss with their physician the other medications they are taking and other medical conditions before starting treatment with bedaquiline fumarate.
- Patients should be advised to consult with their doctor if they have a personal or family history of congenital QT prolongation or heart failure.
Medication Guide

Precautions with Alcohol
- Alcohol-Bedaquiline fumarate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- SIRTURO
Look-Alike Drug Names
- A® — B®[4]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Diacon AH, Pym A, Grobusch M; et al. (2009). "The diarylquinoline TMC207 for multidrug-resistant tuberculosis". N Engl J Med. 360 (23): 2397&ndash, 405. doi:10.1056/NEJMoa0808427. PMID 19494215.
- ↑ "Sirturo: Clinical Pharmacology". Retrieved 28 April 2014.
- ↑ 3.0 3.1 3.2 "Bedaquiline". Retrieved 28 April 2014.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Bedaquiline fumarate |Label Name=Sirturo image001.jpg
}}
{{#subobject:
|Label Page=Bedaquiline fumarate |Label Name=Sirturo ingredients and appearance.png
}}