Intravascular device related infections
| Intravascular device related infections |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor in Chief: Cafer Zorkun, M.D., Ph.D. [2]
Intravascular catheters are indispensable in modern-day medical practice, particularly in intensive care units (ICUs). Although such catheters provide necessary vascular access, their use puts patients at risk for local and systemic infectious complications, including local site infection, catheter-related bloodstream infections (CRBSI), septic thrombophlebitis, endocarditis, and other metastatic infections (e.g., lung abscess, brain abscess, osteomyelitis, and endophthalmitis). [1]
Health-care institutions purchase millions of intravascular catheters each year. The incidence of CRBSI varies considerably by type of catheter, frequency of catheter manipulation, and patient-related factors (e.g., underlying disease and acuity of illness). Peripheral venous catheters are the devices most frequently used for vascular access. Although the incidence of local or bloodstream infections (BSIs) associated with peripheral venous catheters is usually low, serious infectious complications produce considerable annual morbidity because of the frequency with which such catheters are used. However, the majority of serious catheter-related infections are associated with central venous catheters (CVCs), especially those that are placed in patients in ICUs.
In the ICU setting, the incidence of infection is often higher than in the less acute in-patient or ambulatory setting. In the ICU, central venous access might be needed for extended periods of time; patients can be colonized with hospital-acquired organisms; and the catheter can be manipulated multiple times per day for the administration of fluids, drugs, and blood products. Moreover, some catheters can be inserted in urgent situations, during which optimal attention to aseptic technique might not be feasible. Certain catheters (e.g., pulmonary artery catheters and peripheral arterial catheters) can be accessed multiple times per day for hemodynamic measurements or to obtain samples for laboratory analysis, augmenting the potential for contamination and subsequent clinical infection.
The magnitude of the potential for CVCs to cause morbidity and mortality resulting from infectious complications has been estimated in several studies [2]. In the United States, 15 million CVC days (i.e., the total number of days of exposure to CVCs by all patients in the selected population during the selected time period) occur in ICUs each year [3]. If the average rate of CVC-associated BSIs is 5.3 per 1,000 catheter days in the ICU [4], approximately 80,000 CVC-associated BSIs occur in ICUs each year in the United States. The attributable mortality for these BSIs has ranged from no increase in mortality in studies that controlled for severity of illness [5] [6] [7], to 35% increase in mortality in prospective studies that did not use this control [8] [9] Thus, the attributable mortality remains unclear. The attributable cost per infection is an estimated $34,508–$56,000 [10] [11] and the annual cost of caring for patients with CVC-associated BSIs ranges from $296 million to $2.3 billion [12]
A total of 250,000 cases of CVC-associated BSIs have been estimated to occur annually if entire hospitals are assessed rather than ICUs exclusively [13]. In this case, attributable mortality is an estimated 12%–25% for each infection, and the marginal cost to the health-care system is $25,000 per episode [14]. Therefore, by several analyses, the cost of CVC-associated BSI is substantial, both in terms of morbidity and in terms of financial resources expended. To improve patient outcome and reduce health-care costs, strategies should be implemented to reduce the incidence of these infections. This effort should be multidisciplinary, involving health-care professionals who insert and maintain intravascular catheters, health-care managers who allocate resources, and patients who are capable of assisting in the care of their catheters. Although several individual strategies have been studied and shown to be effective in reducing CRBSI, studies using multiple strategies have not been conducted. Thus, it is not known whether implementing multiple strategies will have an additive effect in reducing CRBSI, but it is logical to use multiple strategies concomitantly.
Terminology and Estimates of Risk
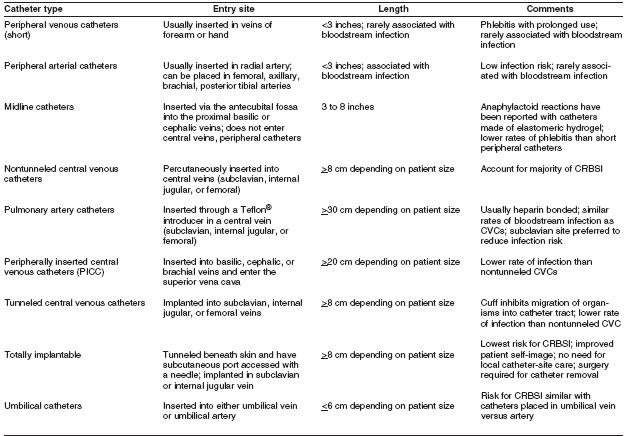
The terminology used to identify different types of catheters is confusing, because many clinicians and researchers use different aspects of the catheter for informal reference. A catheter can be designated by the type of vessel it occupies (e.g., peripheral venous, central venous, or arterial); its intended life span (e.g., temporary or short-term versus permanent or long-term); its site of insertion (e.g., subclavian, femoral, internal jugular, peripheral, and peripherally inserted central catheter [PICC]); its pathway from skin to vessel (e.g., tunneled versus nontunneled); its physical length (e.g., long versus short); or some special characteristic of the catheter (e.g., presence or absence of a cuff, impregnation with heparin, antibiotics or antiseptics, and the number of lumens). To accurately define a specific type of catheter, all of these aspects should be described.
The rate of all catheter-related infections (including local infections and systemic infections) is difficult to determine. Although CRBSI is an ideal parameter because it represents the most serious form of catheter-related infection, the rate of such infection depends on how CRBSI is defined.
Health-care professionals should recognize the difference between surveillance definitions and clinical definitions. The surveillance definitions for catheter-associated BSI includes all BSIs that occur in patients with CVCs, when other sites of infection have been excluded (Appendix A). That is, the surveillance definition overestimates the true incidence of CRBSI because not all BSIs originate from a catheter. Some bacteremias are secondary BSIs from undocumented sources (e.g., postoperative surgical sites, intra-abdominal infections, and hospital-associated pneumonia or urinary tract infections).
Thus, surveillance definitions are really definitions for catheter-associated BSIs. A more rigorous definition might include only those BSIs for which other sources were excluded by careful examination of the patient record, and where a culture of the catheter tip demonstrated substantial colonies of an organism identical to those found in the bloodstream. Such a clinical definition would focus on catheter-related BSIs. Therefore, to accurately compare a health-care facility’s infection rate to published data, comparable definitions also should be used.
CDC and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommend that the rate of catheter-associated BSIs be expressed as the number of catheter associated BSIs per 1,000 CVC days [15] [16] This parameter is more useful than the rate expressed as the number of catheter-associated infections per 100 catheters (or percentage of catheters studied), because it accounts for BSIs over time and therefore adjusts risk for the number of days the catheter is in use.
Epidemiology and Microbiology
Since 1970, CDC’s National Nosocomial Infection Surveillance System (NNIS) has been collecting data on the incidence and etiologies of hospital-acquired infections, including CVC-associated BSIs in a group of nearly 300 U.S. hospitals. The majority of hospital-acquired BSIs are associated with the use of a CVC, with BSI rates being substantially higher among patients with CVCs than among those without CVCs. Rates of CVC-associated BSI vary considerably by hospital size, hospital service/unit, and type of CVC.
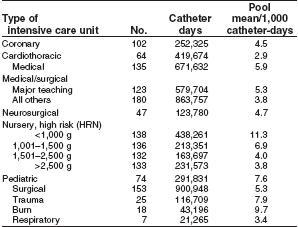
During 1992–2001, NNIS hospitals reported ICU rates of CVC-associated BSI ranging from 2.9 (in a cardiothoracic ICU) to 11.3 (in a neonatal nursery for infants weighing <1,000 g) BSIs per 1,000 CVC days [17]
The relative risk of catheter-associated BSI also has been assessed in a meta-analysis of 223 prospective studies of adult patients. [18] Relative risk of infection was best determined by analyzing rates of infection both by BSIs per 100 catheters and BSIs per 1,000 catheter days. These rates, and the NNIS derived data, can be used as benchmarks by individual hospitals to estimate how their rates compare with other institutions.
Rates are influenced by patient-related parameters, such as severity of illness and type of illness (e.g., third-degree burns versus postcardiac surgery), and by catheter-related parameters, such as the condition under which the catheter was placed (e.g., elective versus urgent) and catheter type (e.g., tunneled versus nontunneled or subclavian versus jugular).
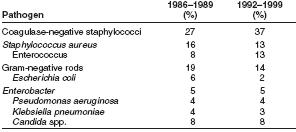
Types of organisms that most commonly cause hospital acquired BSIs change over time. During 1986–1989, coagulase negative staphylococci, followed by Staphylococcus aureus, were the most frequently reported causes of BSIs, accounting for 27% and 16% of BSIs, respectively [19]
Pooled data from 1992 through 1999 indicate that coagulase negative staphylococci, followed by enterococci, are now the most frequently isolated causes of hospital-acquired BSIs. [20]
Coagulase-negative staphylococci account for 37% [21] and S. aureus account for 12.6% of reported hospital-acquired BSIs [22] Also notable was the susceptibility pattern of S. aureus isolates. In 1999, for the first time since NNIS has been reporting susceptibilities, >50% of all S. aureus isolates from ICUs were resistant to oxacillin. [23]
In 1999, enterococci accounted for 13.5% of BSIs, an increase from 8% reported to NNIS during 1986–1989. The percentage of enterococcal ICU isolates resistant to vancomycin also is increasing, escalating from 0.5% in 1989 to 25.9% in 1999 [24]
Candida spp. caused 8% of hospital-acquired BSIs reported to NNIS during 1986–1989 [25] [26] and during 1992–1999. [27] [28] [29] Resistance of Candida spp. to commonly used antifungal agents is increasing. Although NNIS has not reported the percentage of BSIs caused by nonalbicans species or fluconazole susceptibility data, other epidemiologic and clinical data document that fluconazole resistance is an increasingly relevant consideration when designing empiric therapeutic regimens for CRBSIs caused by yeast. Data from the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) Program documented that 10% of C. albicans bloodstream isolates from hospitalized patients were resistant to fluconazole [30] Additionally, 48% of Candida BSIs were caused by nonalbicans species, including C. glabrata and C. krusei, which are more likely than C. albicans to demonstrate resistance to fluconazole and itraconazole [31] [32]
Gram-negative bacilli accounted for 19% of catheter associated BSIs during 1986–1989 [33] compared with 14% of catheter-associated BSIs during 1992–1999 [34] An increasing percentage of ICU-related isolates are caused by Enterobacteriaceae that produce extended-spectrum ß-lactamases (ESBLs), particularly Klebsiella pneumoniae [35] Such organisms not only are resistant to extended-spectrum cephalosporins, but also to frequently used, broad spectrum antimicrobial agents.
Pathogenesis
Migration of skin organisms at the insertion site into the cutaneous catheter tract with colonization of the catheter tip is the most common route of infection for peripherally inserted, short-term catheters [36] [37] Contamination of the catheter hub contributes substantially to intraluminal colonization of long-term catheters [38] [39] [40] Occasionally, catheters might become hematogenously seeded from another focus of infection. Rarely, infusate contamination leads to CRBSI.[41]
Important pathogenic determinants of catheter-related infection are;
- the material of which the device is made and
- the intrinsic virulence factors of the infecting organism.
In vitro studies demonstrate that catheters made of polyvinyl chloride or polyethylene are likely less resistant to the adherence of microorganisms than are catheters made of Teflon®, silicone elastomer, or polyurethane [42] [43] Therefore, the majority of catheters sold in the United States are no longer made of polyvinyl chloride or polyethylene. Some catheter materials also have surface irregularities that enhance the microbial adherence of certain species (e.g., coagulase-negative staphylococci, Acinetobacter calcoaceticus, and Pseudomonas aeruginosa) [44] [45] [46]; catheters made of these materials are especially vulnerable to microbial colonization and subsequent infection. Additionally, certain catheter materials are more thrombogenic than others, a characteristic that also might predispose to catheter colonization and catheter-related infection [47] [48] This association has led to emphasis on preventing catheter-related thrombus as an additional mechanism for reducing CRBSI.
The adherence properties of a given microorganism also are important in the pathogenesis of catheter-related infection. For example, S. aureus can adhere to host proteins (e.g., fibronectin) commonly present on catheters [49] [50] Also, coagulase-negative staphylococci adhere to polymer surfaces more readily than do other pathogens (e.g., Escherichia coli or S. aureus). Additionally, certain strains of coagulase-negative staphylococci produce an extracellular polysaccharide often referred to as “slime” [51] [52] In the presence of catheters, this slime potentiates the pathogenicity of coagulase-negative staphylococci by allowing them to withstand host defense mechanisms (e.g., acting as a barrier to engulfment and killing by polymorphonuclear leukocytes) or by making them less susceptible to antimicrobial agents (e.g., forming a matrix that binds antimicrobials before their contact with the organism cell wall) [53] Certain Candida spp., in the presence of glucose-containing fluids, might produce slime similar to that of their bacterial counterparts, potentially explaining the increased proportion of BSIs caused by fungal pathogens among patients receiving parenteral nutrition fluids [54]
Strategies for Prevention of Catheter Related Infections in Adult and Pediatric Patients
Quality Assurance and Continuing Education
Measures to minimize the risk for infection associated with intravascular therapy should strike a balance between patient safety and cost effectiveness. As knowledge, technology, and health-care settings change, infection control and prevention measures also should change. Well-organized programs that enable health-care providers to provide, monitor, and evaluate care and to become educated are critical to the success of this effort. Reports spanning the past two decades have consistently demonstrated that risk for infection declines following standardization of aseptic care (39–43), and that insertion and maintenance of intravascular catheters by inexperienced staff might increase the risk for catheter colonization and CRBSI (43, 44).
Specialized “IV teams” have shown unequivocal effectiveness in reducing the incidence of catheter-related infections and associated complications and costs (45–47). Additionally, infection risk increases with nursing staff reductions below a critical level (48).
Site of Catheter Insertion
The site at which a catheter is placed influences the subsequent risk for catheter-related infection and phlebitis. The influence of site on the risk for catheter infections is related in part to the risk for thrombophlebitis and density of local skin flora. Phlebitis has long been recognized as a risk for infection. For adults, lower extremity insertion sites are associated with a higher risk for infection than are upper extremity sites (49–51). In addition, hand veins have a lower risk for phlebitis than do veins on the wrist or upper arm (52).
The density of skin flora at the catheter insertion site is a major risk factor for CRBSI. Authorities recommend that CVCs be placed in a subclavian site instead of a jugular or femoral site to reduce the risk for infection. No randomized trial satisfactorily has compared infection rates for catheters placed in jugular, subclavian, and femoral sites. Catheters inserted into an internal jugular vein have been associated with higher risk for infection than those inserted into a subclavian or femoral vein (22,53,54).
Femoral catheters have been demonstrated to have relatively high colonization rates when used in adults (55). Femoral catheters should be avoided, when possible, because they are associated with a higher risk for deep venous thrombosis than are internal jugular or subclavian catheters (56–60) and because of a presumption that such catheters are more likely to become infected. However, studies in pediatric patients have demonstrated that femoral catheters have a low incidence of mechanical complications and might have an equivalent infection rate to that of nonfemoral catheters (61–63). Thus, in adult patients, a subclavian site is preferred for infection control purposes, although other factors (e.g., the potential for mechanical complications, risk for subclavian vein stenosis, and catheter-operator skill) should be considered when deciding where to place the catheter. In a meta-analysis of eight studies, the use of bedside ultrasound for the placement of CVCs substantially reduced mechanical complications compared with the standard landmark placement technique (relative risk [RR] = 0.22; 95% confidence interval [CI] = 0.10–0.45) (64). Consideration of comfort, security, and maintenance of asepsis as well as patient-specific factors (e.g., preexisting catheters, anatomic deformity, and bleeding diathesis), relative risk of mechanical complications (e.g., bleeding and pneumothorax), the availability of bedside ultrasound, and the risk for infection should guide site selection.
Type of Catheter Material Teflon® or polyurethane catheters have been associated with fewer infectious complications than catheters made of polyvinyl chloride or polyethylene (27, 65, 66). Steel needles used as an alternative to catheters for peripheral venous access have the same rate of infectious complications as do Teflon® catheters (67, 68). However, the use of steel needles frequently is complicated by infiltration of intravenous (IV) fluids into the subcutaneous tissues, a potentially serious complication if the infused fluid is a vesicant (68). Hand Hygiene and Aseptic Technique For short peripheral catheters, good hand hygiene before catheter insertion or maintenance, combined with proper aseptic technique during catheter manipulation, provides protection against infection. Good hand hygiene can be achieved through the use of either a waterless, alcohol-based product (69) or an antibacterial soap and water with adequate rinsing (70). Appropriate aseptic technique does not necessarily require sterile gloves; a new pair of disposable non sterile gloves can be used in conjunction with a “no-touch” technique for the insertion of peripheral venous catheters. However, gloves are required by the Occupational Safety and Health Administration as standard precautions for the prevention of blood borne pathogen exposure.
Compared with peripheral venous catheters, CVCs carry a substantially greater risk for infection; therefore, the level of barrier precautions needed to prevent infection during insertion of CVCs should be more stringent.
A maximal sterile barrier precaution (e.g., cap, mask, sterile gown, sterile gloves, and large sterile drape) during the insertion of CVCs substantially reduces the incidence of CRBSI compared with standard precautions (e.g., sterile gloves and small drapes) (22, 71).
Although the efficacy of such precautions for insertion of PICCs and midline catheters has not been studied, the use of maximal barrier precautions also is probably applicable to PICCs.
Skin Antisepsis
In the United States, povidone iodine has been the most widely used antiseptic for cleansing arterial catheter and CVC insertion sites (72). However, in one study, preparation of central venous and arterial sites with a 2% aqueous chlorhexidine gluconate lowered BSI rates compared with site preparation with 10% povidone-iodine or 70% alcohol (73). Commercially available products containing chlorhexidine have not been available until recently; in July 2000, the U.S. Food and Drug Administration (FDA) approved a 2% tincture of chlorhexidine preparation for skin antisepsis. Other preparations of chlorhexidine might not be as effective. Tincture of chlorhexidine gluconate 0.5% is no more effective in preventing CRBSI or CVC colonization than 10% povidone iodine, as demonstrated by a prospective, randomized study of adults (74).
However, in a study involving neonates, 0.5% chlorhexidine reduced peripheral IV colonization compared with povidone iodine (20/418 versus 38/408 catheters; p = 0.01) (75). This study, which did not include CVCs, had an insufficient number of participants to assess differences in BSI rates. A 1% tincture of chlorhexidine preparation is available in Canada and Australia, but not yet in the United States.
No published trials have compared a 1% chlorhexidine preparation to povidone-iodine.
Catheter Site Dressing Regimens
Transparent, semi permeable polyurethane dressings have become a popular means of dressing catheter insertion sites. Transparent dressings reliably secure the device, permit continuous visual inspection of the catheter site, permit patients to bathe and shower without saturating the dressing, and require less frequent changes than do standard gauze and tape dressings; the use of these dressings saves personnel time.
In the largest controlled trial of dressing regimens on peripheral catheters, the infectious morbidity associated with the use of transparent dressings on approximately 2,000 peripheral catheters was examined (65). Data from this study suggest that the rate of colonization among catheters dressed with transparent dressings (5.7%) is comparable to that of those dressed with gauze (4.6%) and that no clinically substantial differences exist in either the incidences of catheter site colonization or phlebitis. Furthermore, these data suggest that transparent dressings can be safely left on peripheral venous catheters for the duration of catheter insertion without increasing the risk for thrombophlebitis (65).
A meta-analysis has assessed studies that compared the risk for catheter-related BSIs for groups using transparent dressings versus groups using gauze dressing (76). The risk for CRBSIs did not differ between the groups. The choice of dressing can be a matter of preference. If blood is oozing from the catheter insertion site, gauze dressing might be preferred. In a multi-center study, a chlorhexidine-impregnated sponge (Biopatch™) placed over the site of short-term arterial and CVCs reduced the risk for catheter colonization and CRBSI (77). No adverse systemic effects resulted from use of this device.
Catheter Securement Devices
Sutureless securement devices can be advantageous over suture in preventing catheter-related BSIs. One study, which involved only a limited number of patients and was underpowered, compared a sutureless device with suture for the securement of PICCS; in this study, CRBSI was reduced in the group of patients that received the sutureless device (78).
In-Line Filters
In-line filters reduce the incidence of infusion-related phlebitis (79,80). No data support their efficacy in preventing infections associated with intravascular catheters and infusion systems. Proponents of filters cite several potential benefits to using these filters, including
- reducing the risk for infection from contaminated infusate or proximal contamination (i.e., introduced proximal to the filter);
- reducing the risk for phlebitis in patients who require high doses of medication or in those in whom infusion-related phlebitis already has occurred;
- removing particulate matter that might contaminate IV fluids (81); and
- filtering endotoxin produced by gram-negative organisms in contaminated infusate (82).
These theoretical advantages should be tempered by the knowledge that infusate-related BSI is rare and that filtration of medications or infusates in the pharmacy is a more practical and less costly way to remove the majority of particulates. Furthermore, in-line filters might become blocked, especially with certain solutions (e.g., dextran, lipids, and mannitol), thereby increasing the number of line manipulations and decreasing the availability of administered drugs (83). Thus, for reducing the risk for CRBSI, no strong recommendation can be made in favor of using in-line filters.
Antimicrobial / Antiseptic Impregnated Catheters and Cuffs
Certain catheters and cuffs that are coated or impregnated with antimicrobial or antiseptic agents can decrease the risk for CRBSI and potentially decrease hospital costs associated with treating CRBSIs, despite the additional acquisition cost of an antimicrobial/antiseptic impregnated catheter (84). All of the studies involving antimicrobial/antiseptic impregnated catheters have been conducted using triple-lumen, non-cuffed catheters in adult patients whose catheters remained in place <30 days. Although all of the studies have been conducted in adults, these catheters have been approved by FDA for use in patients weighing >3 kg.
No antiseptic or antimicrobial impregnated catheters currently are available for use in weighing <3 kg.
Chlorhexidine / Silver sulfadiazine
Catheters coated with chlorhexidine/silver sulfadiazine only on the external luminal surface have been studied as a means to reduce CRBSI. Two meta-analyses (2, 85) demonstrated that such catheters reduced the risk for CRBSI compared with standard non-coated catheters. The mean duration of catheter placement in one meta-analysis ranged from 5.1 to 11.2 days (86). The half-life of antimicrobial activity against S. epidermidis is 3 days in vitro for catheters coated with chlorhexidine/silver sulfadiazine; this antimicrobial activity decreases over time (87). The benefit for the patients who receive these catheters will be realized within the first 14 days (86). A second-generation catheter is now available with chlorhexidine coating both the internal and external luminal surfaces. The external surface has three times the amount of chlorhexidine and extended release of the surface bound antiseptics than that in the first generation catheters. The external surface coating of chlorhexidine is combined with silver-sulfadiazine, and the internal surface is coated with chlorhexidine alone. Preliminary studies indicate that prolonged anti-infective activity provides improved efficacy in preventing infections (88). Although rare, anaphylaxis has been reported with the use of these chlorhexidine/silver sulfadiazine catheters in Japan (89). Whether patients will become colonized or infected with organisms resistant to chlorhexidine / silver sulfadiazine has not been determined (86).
Chlorhexidine / silver sulfadiazine catheters are more expensive than standard catheters. However, one analysis has suggested that the use of chlorhexidine / silver sulfadiazine catheters should lead to a cost savings of $68 to $391 per catheter (90) in settings in which the risk for CRBSI is high despite adherence to other preventive strategies (e.g., maximal barrier precautions and aseptic techniques). Use of these catheters might be cost effective in ICU patients, burn patients, neutropenic patients, and other patient populations in which the rate of infection exceeds 3.3 per 1,000 catheter days (86).
Minocycline/Rifampin
In a multicenter randomized trial, CVCs impregnated on both the external and internal surfaces with minocycline/rifampin were associated with lower rates of CRBSI when compared with the first-generation chlorhexidine-silver sulfadiazine impregnated catheters (91).
The beneficial effect began after day 6 of catheterization. None of the catheters were evaluated beyond 30 days. No minocycline/rifampin-resistant organisms were reported. However, in vitro data indicate that these impregnated catheters could increase the incidence of minocycline and rifampin resistance among pathogens, especially staphylococci. The half life of antimicrobial activity against S. epidermidis is 25 days with catheters coated with minocycline / rifampin, compared with 3 days for the first-generation catheters coated with chlorhexidine / silver sulfadiazine in vitro (87). In vivo, the duration of antimicrobial activity of the minocycline / rifampin catheter is longer than that of the first-generation chlorhexidine / silver sulfadiazine catheter (91).
No comparative studies have been published using the second-generation chlorhexidine / silver sulfadiazine catheter. Studies are needed to evaluate whether the improved performance of the minocyline / rifampin catheters results from the antimicrobial agents used or from the coating of both the internal and external surfaces. As with chlorhexidine / silver sulfadiazine catheters, some clinicians have recommended that the minocycline / rifampin catheters be considered in patient populations when the rate of CRBSI exceeds 3.3 per 1,000 catheter days (86). Others suggest that reducing all rates of CRBSI should be the goal (92).
The decision to use chlorhexidine / silver sulfadiazine or minocycline / rifampin impregnated catheters should be based on the need to enhance prevention of CRBSI after standard procedures have been implemented (e.g., educating personnel, using maximal sterile barrier precautions, and using 2% chlorhexidine skin antisepsis) and then balanced against the concern for emergence of resistant pathogens and the cost of implementing this strategy.
Platinum/Silver
Ionic metals have broad antimicrobial activity and are being used in catheters and cuffs to prevent CRBSI. A combination platinum/silver impregnated catheter is available in Europe and has recently been approved by FDA for use in the United States. Although these catheters are being marketed for their antimicrobial properties, no published studies have been presented to support an antimicrobial effect.
Silver cuffs
Ionic silver has been used in subcutaneous collagen cuffs attached to CVCs (93). The ionic silver provides antimicrobial activity and the cuff provides a mechanical barrier to the migration of microorganisms along the external surface of the catheter. In studies of catheters left in place >20 days, the cuff failed to reduce the incidence of CRBSI (94, 95).
Two other studies of short-term catheters could not demonstrate efficacy because of the minimal number of CRBSIs observed (93, 96).
Systemic Antibiotic Prophylaxis
No studies have demonstrated that oral or parenteral antibacterial or antifungal drugs might reduce the incidence of CRBSI among adults (97–99). However, among low birth weight infants, two studies have assessed vancomycin prophylaxis; both demonstrated a reduction in CRBSI but no reduction in mortality (100,101). Because the prophylactic use of vancomycin is an independent risk factor for the acquisition of vancomycin-resistant enterococcus (VRE) (102), the risk for acquiring VRE likely outweighs the benefit of using prophylactic vancomycin.
Antibiotic / Antiseptic Ointments
Povidone-iodine ointment applied at the insertion site of hemodialysis catheters has been studied as a prophylactic intervention to reduce the incidence of catheter-related infections. One randomized study of 129 hemodialysis catheters demonstrated a reduction in the incidence of exit-site infections, catheter-tip colonization, and BSIs with the routine use of povidone-iodine ointment at the catheter insertion site compared with no ointment at the insertion site (103).
Several studies have evaluated the effectiveness of mupirocin ointment applied at the insertion sites of CVCs as a means to prevent CRBSI (104–106). Although mupirocin reduced the risk for CRBSI (106), mupirocin ointment also has been associated with mupirocin resistance (107,108), and might adversely affect the integrity of polyurethane catheters (109,110).
Nasal carriers of S. aureus have a higher risk for acquiring CRBSI than do noncarriers (103,111). Mupirocin ointment has been used intranasally to decrease nasal carriage of S. aureus and lessen the risk for CRBSI. However, resistance to mupirocin develops in both S. aureus and coagulase-negative staphylococci soon after routine use of mupirocin is instituted (107,108).
Other antibiotic ointments applied to the catheter insertion site also have been studied and have yielded conflicting results (112–114). In addition, rates of catheter colonization with Candida spp. might be increased with the use of antibiotic ointments that have no fungicidal activity (112,114). To avoid compromising the integrity of the catheter, any ointment that is applied to the catheter insertion site should be checked against the catheter and ointment manufacturers’ recommendations regarding compatibility.
Anticoagulants
Anticoagulant flush solutions are used widely to prevent catheter thrombosis. Because thrombi and fibrin deposits on catheters might serve as a nidus for microbial colonization of intravascular catheters (120,121), the use of anticoagulants might have a role in the prevention of CRBSI.
In a meta-analysis evaluating the benefit of heparin prophylaxis (3 U/ml in TPN, 5,000 U every 6 or 12 hours flush, or 2,500 U low molecular weight heparin subcutaneously) in patients with short-term CVCs, the risk for catheter-related central venous thrombosis was reduced with the use of prophylactic heparin (122). However, no substantial difference in the rate for CRBSI was observed. Because the majority of heparin solutions contain preservatives with antimicrobial activity, whether any decrease in the rate of RBSI is a result of the reduced thrombus formation, the preservative, or both is unclear.
The majority of pulmonary artery, umbilical, and central venous catheters are available with a heparin- bonded coating.
The majority are heparin-bonded with benzalkonium chloride, which provides the catheters with antimicrobial activity (123) and provides an anti-thrombotic effect (124).
Warfarin also has been evaluated as a means for reducing CRBSI by reducing thrombus formation on catheters (125,126). In patients with long-term CVCs, low-dose warfarin (i.e., 1 mg/day) reduced the incidence of catheter thrombus. No data demonstrate that warfarin reduces the incidence of CRBSI.
Antibiotic Lock Prophylaxis
To prevent CRBSI, antibiotic lock prophylaxis has been attempted by flushing and filling the lumen of the catheter with an antibiotic solution and leaving the solution to dwell in the lumen of the catheter. Three studies have demonstrated the usefulness of such prophylaxis in neutropenic patients with long-term catheters (115–117). In two of the studies, patients received heparin alone (10 U/ml) or heparin plus 25 micrograms/ml of vancomycin. The third study compared vancomycin/ciprofloxacin/heparin (VCH) to vancomycin / heparin (VH) and then to heparin alone. The rate of CRBSI with vancomycin-susceptible organisms was significantly lower (VCH p = 0.022; VH p = 0.028) and the time to the first episode of bacteremia with vancomycin-susceptible organisms was substantially longer (VCH p = 0.036; VH p = 0.011) in patients receiving either vancomycin/ciprofloxacin/heparin or vancomycin/heparin compared with heparin alone (115–117).
One study involving a limited number of children revealed no difference in rates of CRBSI between children receiving a heparin flush compared with those receiving heparin and vancomycin (118). However, because the use of vancomycin is an independent risk factor for the acquisition of VRE (102), this practice is not recommended routinely.
An anticoagulant/antimicrobial combination comprising minocycline and ethylenediaminetetraraacetic acid (EDTA) has been proposed as a lock solution because it has antibiofilm and antimicrobial activity against gram-positive, gram negative, and Candida organisms (119), as well as anticoagulant properties. However, no controlled or randomized trials have demonstrated its efficacy.
Replacement of Catheters
Peripheral Venous Catheters
Scheduled replacement of intravascular catheters has been proposed as a method to prevent phlebitis and catheter related infections. Studies of short peripheral venous catheters indicate that the incidence of thrombophlebitis and bacterial colonization of catheters increases when catheters are left in place >72 hours (66, 67, 127). However, rates of phlebitis are not substantially different in peripheral catheters left in place 72 hours compared with 96 hours (128). Because phlebitis and catheter colonization have been associated with an increased risk for catheter-related infection, short peripheral catheter sites commonly are rotated at 72–96-hour intervals to reduce both the risk for infection and patient discomfort associated with phlebitis.
Midline Catheters
Midline catheters have been associated with lower rates of phlebitis than short peripheral catheters and with lower rates of infection than CVCs (129–131). In one prospective study of 140 midline catheters, their use was associated with a BSI rate of 0.8 per 1,000 catheter-days (131). No specific risk factors, including duration of catheterization, were associated with infection. Midline catheters were in place a median of 7 days, but for as long as 49 days. Although the findings of this study suggested that midline catheters can be changed only when there is a specific indication, no prospective, randomized studies have assessed the benefit of routine replacement as a strategy to prevent CRBSI associated with midline catheters.
CVCs, Including PICCs and Hemodialysis Catheters
Catheter replacement at scheduled time intervals as a method to reduce CRBSI has not lowered rates. Two trials have assessed a strategy of changing the catheter every 7 days compared with a strategy of changing catheters as needed (132,133).
One of these studies involved 112 surgical ICU patients needing CVCs, pulmonary artery catheters, or peripheral arterial catheters (132), whereas the other study involved only subclavian hemodialysis catheters (133). In both studies, no difference in CRBSI was observed in patients undergoing scheduled catheter replacement every 7 days compared with patients whose catheters were replaced as needed.
A scheduled guidewire exchange of CVCs is another proposed strategy for preventing CRBSI. The results of a meta-analysis of 12 randomized controlled trials assessing CVC management failed to prove any reduction of CRBSI rates through routine replacement of CVCs by guidewire exchange compared with catheter replacement on an as-needed basis (134). Thus, routine replacement of CVCs is not necessary for catheters that are functioning and have no evidence of causing local or systemic complications.
Catheter replacement over a guidewire has become an accepted technique for replacing a malfunctioning catheter or exchanging a pulmonary artery catheter for a CVC when invasive monitoring no longer is needed. Catheter insertion over a guidewire is associated with less discomfort and a significantly lower rate of mechanical complications than are those percutaneously inserted at a new site (135); in addition, this technique provides a means of preserving limited venous access in some patients. Replacement of temporary catheters over a guidewire in the presence of bacteremia is not an acceptable replacement strategy, because the source of infection is usually colonization of the skin tract from the insertion site to the vein (22,135). However, in selected patients with tunneled hemodialysis catheters and bacteriemia, catheter exchange over a guidewire, in combination with antibiotic therapy, might be an alternative as a salvage strategy in patients with limited venous access (136–139).
Hemodialysis Catheters
The use of catheters for hemodialysis is the most common factor contributing to bacteremia in dialysis patients (140,141). The relative risk for bacteremia in patients with dialysis catheters is sevenfold the risk for patients with primary arteriovenous fistulas (142). Despite the National Kidney Foundation’s effort to reduce the number of hemodialysis patients maintained with catheter access, catheter use increased from 12.7% in 1995 to 22.2% in 1999 (143). Rates for bacteremia per 100 patient months were 0.2 for arteriovenous fistulas, 0.5 for grafts, 5.0 for cuffed catheters, and 8.5 for non cuffed catheters
To reduce the rate of infection, hemodialysis catheters should be avoided in favor of arteriovenous fistulas and grafts. If temporary access is needed for dialysis, a cuffed catheter is preferable to a noncuffed catheter, even in the ICU setting, if the catheter is expected to stay in place for >3 weeks (11,144).
Pulmonary Artery Catheters
Pulmonary artery catheters are inserted through a Teflon® introducer and typically remain in place an average of 3 days. The majority of pulmonary artery catheters are heparin bonded, which reduces not only catheter thrombosis but also microbial adherence to the catheter (145). Meta-analysis indicates that standard nonheparin-bonded pulmonary artery catheter rates of CRBSI are 5.5 per 1,000 catheter days; for heparin bonded pulmonary artery catheters, this rate is 2.6 per 1,000 catheter days (11). Because the majority of pulmonary artery catheters are heparin-bonded, the relative risk of infection with these catheters is similar to that of CVC (2.6 versus 2.3 per 1,000 catheter days) (11).
A prospective study of 442 pulmonary artery catheters demonstrated an increased risk for CRBSI after 5 days (0/442 CRBSI before 5 days versus 5/442 CSBSI after 5 days; p < 0.001) (146). A prospective observational study of 71 pulmonary artery catheters demonstrated higher infection rates in catheters left in place longer than 7 days (2% before 7 days versus 16% after 7 days; p = 0.056) (147). However, no studies indicate that catheter replacement at scheduled time intervals is an effective method to reduce CRBSI (132,135). In patients who continue to require hemodynamic monitoring, pulmonary artery catheters do not need to be changed more frequently than every 7 days. No specific recommendation can be made regarding routine replacement of catheters that need to be in place for >7 days. Pulmonary artery catheters are usually packaged with a thin plastic sleeve that prevents touch contamination when placed over the catheter. In a study of 166 catheters, patients who were randomly assigned to have their catheters self-contained within this sleeve had a reduced risk for CRBSI compared with those who had a pulmonary artery catheter placed without the sleeve (p = 0.002) (148).
Peripheral Arterial Catheters
Peripheral arterial catheters are usually inserted into the radial or femoral artery and permit continuous blood pressure monitoring and blood gas measurements. The rate of CRBSI is comparable to that of temporary CVCs (2.9 versus 2.3 per 1,000 catheter days) (11). One study of peripheral arterial catheters demonstrated no difference in infection rates between changing catheters at scheduled times and changing arterial catheters on an as-needed basis (132). One observational study of 71 arterial catheters revealed that 10 local infections and four CRBSIs occurred in patients who had peripheral arterial catheters in place for >4 days compared with one local infection and no CRBSIs in patients whose catheters were in place <4 days (p < 0.05) (147). Because the risk for CRBSI is likely similar to that of short-term CVCs, arterial catheters can be approached in a similar way. No specific recommendation can be made regarding replacement of catheters that need to be in place for >5 days.
Replacement of Administration Sets
The optimal interval for routine replacement of IV administration sets has been examined in three well-controlled studies. Data from each of these studies reveal that replacing administration sets no more frequently than 72 hours after initiation of use is safe and cost-effective (149–151). Data from a more recent study demonstrated that rates of phlebitis were not substantially different if administration sets were left in place 96 hours compared with 72 hours (128). When a fluid that enhances microbial growth is infused (e.g., lipid emulsions and blood products), more frequent changes of administration sets are indicated, because these products have been identified as independent risk factors for CRBSI (152–158).
Stopcocks (used for injection of medications, administration of IV infusions, and collection of blood samples) represent a potential portal of entry for microorganisms into vascular access catheters and IV fluids. Stopcock contamination is common, occurring in 45% and 50% in the majority of series. Whether such contamination is a substantial entry point of CRBSI has been difficult to prove.
“Piggyback” systems are used as an alternative to stopcocks. However, they also pose a risk for contamination of the intravascular fluid if the device entering the rubber membrane of an injection port is exposed to air or comes into direct contact with non-sterile tape used to fix the needle to the port. Modified piggyback systems have the potential to prevent contamination at these sites (159).
Needleless Intravascular Catheter Systems
Attempts to reduce the incidence of sharp injuries and the resultant risk for transmission of blood borne infections to health-care workers have led to the design and introduction of needleless infusion systems. When the devices are used according to manufacturers’ recommendations, they do not substantially affect the incidence of CRBSI (160–167).
Multidose Parenteral Medication Vials
Parenteral medications commonly are dispensed in multidose, parenteral medication vials that might be used for prolonged periods for one or more patients. Although the overall risk for extrinsic contamination of multidose vials is likely minimal (168), the consequences of contamination might result in life-threatening infection (169,170). Single-use vials are frequently preservative-free and might pose a risk for contamination if they are punctured several times.
References
- ↑ Pearson ML. Guideline for prevention of intravascular device-related infections. Part I. Intravascular device-related infections: an overview. The Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1996; 24:262–77.
- ↑ Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med 2000; 132:391–402.
- ↑ Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med 2000; 132:391–402.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from October 1986–April 1998, issued June 1998. Am J Infect Control 1998;26:522–33.
- ↑ Digiovine B, Chenoweth C, Watts C, Higgins M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med 1999;160:976–81.
- ↑ Rello J, Ochagavia A, Sabanes E, et al. Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am J Respir Crit Care Med 2000; 162:1027–30.
- ↑ Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol 1999; 20:396–401.
- ↑ Collignon PJ. Intravascular catheter associated sepsis: a common problem. The Australian Study on Intravascular Catheter Associated Sepsis. Med J Aust 1994; 161:374–8.
- ↑ Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1994; 271:1598–601.
- ↑ Rello J, Ochagavia A, Sabanes E, et al. Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am J Respir Crit Care Med 2000; 162:1027–30.
- ↑ Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg 2001; 136:229–34.
- ↑ Mermel LA. Correction: catheter related bloodstream-infections. Ann Intern Med 2000; 133:395.
- ↑ Kluger DM, Maki DG. The relative risk of intravascular device related bloodstream infections in adults [Abstract]. In: Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: American Society for Microbiology, 1999:514.
- ↑ Kluger DM, Maki DG. The relative risk of intravascular device related bloodstream infections in adults [Abstract]. In: Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: American Society for Microbiology, 1999:514.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ Joint Commission on the Accreditation of Healthcare Organizations. Accreditation manual for hospitals. In: Joint Commission on the Accreditation of Healthcare Organizations, ed. Chicago, IL: Joint Commission on the Accreditation of Healthcare Organizations, 1994:121–40.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992–June 2001, issued August 2001. Am J Infect Control 2001; 6: 404–21.
- ↑ Kluger DM, Maki DG. The relative risk of intravascular device related bloodstream infections in adults [Abstract]. In: Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: American Society for Microbiology, 1999:514.
- ↑ Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med 1991;91 (suppl):S72–S75.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med 1991;91 (suppl):S72–S75.
- ↑ Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med 1991; 91 (suppl):S86–S89.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 1998; 31:327–32.
- ↑ Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 1998; 30:121–9.
- ↑ Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 1998; 31:327–32.
- ↑ Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 1998; 30:121–9.
- ↑ Nguyen MH, Peacock JE Jr., Morris AJ, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med 1996;100:617–23.
- ↑ Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med 1991;91 (suppl):S72–S75.
- ↑ CDC. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27: 520–32.
- ↑ Fridkin SK, Gaynes RP. Antimicrobial resistance in intensive care units. Clin Chest Med 1999; 20:303–16.
- ↑ Maki DG, Weise CE, Sarafin HW. A semi quantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med 1977; 296:1305–9.
- ↑ Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med 1991;91(suppl): S197–S205.
- ↑ Sitges-Serra A, Linares J, Perez JL, Jaurrieta E, Lorente L. A randomized trial on the effect of tubing changes on hub contamination and catheter sepsis during parenteral nutrition. Parenter Enteral Nutr 1985; 9: 322–5.
- ↑ Linares J, Sitges-Serra A, Garau J, Perez JL, Martin R. Pathogenesis of catheter sepsis: a prospective study with quantitative and semi quantitative cultures of catheter hub and segments. J Clin Microbiol 1985; 21:357–60.
- ↑ Raad II, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis 1993; 168: 400–7.
- ↑ Maki DG. Infections associated with intravascular lines. In: Remington JS, ed. Current Clinical Topics in Infectious Diseases. New York: McGraw-Hill, 1982:309–63.
- ↑ Sheth NK, Franson TR, Rose HD, Buckmire FL, Cooper JA, Sohnle PG. Colonization of bacteria on polyvinyl chloride and Teflon intravascular catheters in hospitalized patients. J Clin Microbiol 1983; 18: 1061–3.
- ↑ Ashkenazi S, Weiss E, Drucker MM, Bodey GP. Bacterial adherence to intravenous catheters and needles and its influence by cannula type and bacterial surface hydrophobicity. J Lab Clin Med 1986; 107:136–40.
- ↑ Locci R, Peters G, Pulverer G. Microbial colonization of prosthetic devices. IV. Scanning electron microscopy of intravenous catheters invaded by yeasts. Zentralbl Bakteriol Mikrobiol Hyg [B] 1981;173:419–24.
- ↑ Locci R, Peters G, Pulverer G. Microbial colonization of prosthetic devices. I. Microtopographical characteristics of intravenous catheters as detected by scanning electron microscopy. Zentralbl Bakteriol Mikrobiol Hyg [B] 1981;173:285–92.
- ↑ Nachnani GH, Lessin LS, Motomiya T, Jensen WN, Bodey GP. Scanning electron microscopy of thrombogenesis on vascular catheter surfaces. N Engl J Med 1972;286:139–40.
- ↑ Nachnani GH, Lessin LS, Motomiya T, Jensen WN, Bodey GP. Scanning electron microscopy of thrombogenesis on vascular catheter surfaces. N Engl J Med 1972; 286:139–40.
- ↑ Stillman RM, Soliman F, Garcia L, Sawyer PN. Etiology of catheter associated sepsis. Correlation with thrombogenicity. Arch Surg 1977; 112: 1497–9.
- ↑ Herrmann M, Lai QJ, Albrecht RM, Mosher DF, Proctor RA. Adhesion of Staphylococcus aureus to surface-bound platelets: role of fibrinogen/fibrin and platelet integrins. J Infect Dis 1993; 167:312–22.
- ↑ Herrmann M, Suchard SJ, Boxer LA, Waldvogel FA, Lew PD. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun 1991;59:279–88.
- ↑ Ludwicka A, Uhlenbruck G, Peters G, et al. Investigation on extracellular slime substance produced by Staphylococcus epidermidis. Zentralbl Bakteriol Mikrobiol Hyg 1984; 258:256–67.
- ↑ Gray ED, Peters G, Verstegen M, Regelmann WE. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet 1984; 1:365–7.
- ↑ Farber BF, Kaplan MH, Clogston AG. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis 1990;161:37–40.
- ↑ Branchini ML, Pfaller MA, Rhine-Chalberg J, Frempong T, Isenberg HD. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J Clin Microbiol 1994; 32: 452–6.