Avalglucosidase alfa-ngpt
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, AND RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS
See full prescribing information for complete Boxed Warning.
Hypersensitivity Reactions Including Anaphylaxis
Infusion-Associated Reactions (IARs)
Risk of Acute Cardiorespiratory Failure in Susceptible Patients
|
Overview
Avalglucosidase alfa-ngpt is a hydrolytic lysosomal glycogen-specific enzyme that is FDA approved for the treatment of late-onset Pompe disease. There is a Black Box Warning for this drug as shown here. Common adverse reactions include erythema, pruritus, urticaria, nausea, headache, dizziness, paresthesia, myalgia, arthralgia, diarrhea, dyspnea, and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dosage
- Avalglucosidase alfa-ngpt dosage is given through an intravenous infusion and is based on a patients body weight.
- Avalglucosidase alfa-ngpt should be both diluted and reconstituted before administering dosage into patients.
- Use of antipyretics, corticosteroids, or antihistamines may be used and considered before the administration of Avalglucosidase alfa-ngpt dosage into patients.
- The recommended dosage of patients ≥30 kg is 20 mg/kg every 2 weeks.
- The recommended dosage of patients <30 kg is 40 mg/kg every 2 weeks.
- 1 mg/kg/hour is the initial infusion rate that should be gradually increased every 30 minutes if there are no IARs seen in patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Avalglucosidase alfa-ngpt in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Avalglucosidase alfa-ngpt in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Avalglucosidase alfa-ngpt FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Avalglucosidase alfa-ngpt in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Avalglucosidase alfa-ngpt in pediatric patients.
Contraindications
There are no contraindications associated with Avalglucosidase alfa-ngpt.
Warnings
|
SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, AND RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS
See full prescribing information for complete Boxed Warning.
Hypersensitivity Reactions Including Anaphylaxis
Infusion-Associated Reactions (IARs)
Risk of Acute Cardiorespiratory Failure in Susceptible Patients
|
Hypersensitivity Reactions Including Anaphylaxis
- Cardiopulmonary resuscitation equipment and other medical support should be on the ready during Avalglucosidase alfa-ngpt dosage administration.
- Stop Avalglucosidase alfa-ngpt treatment and seek proper medical attention if patient experiences a severe hypersensitivity reaction.
- Advise patients to consider both the risks and benefits prior to restarting Avalglucosidase alfa-ngpt treatment.
- A desensitization procedure during Avalglucosidase alfa-ngpt may be considered in patients who experienced a severe hypersensitivity reaction.
- Monitor patients during infusion if restarting Avalglucosidase alfa-ngpt treatment.
- Patients may be returned to recommended dosage of Avalglucosidase alfa-ngpt if they can tolerate the infusion.
- Advise patients to either slow or temporary stop infusion rate if patient experiences a mild or moderate hypersensitivity reaction.
- 48% of patients treated with Avalglucosidase alfa-ngpt reported experiencing hypersensitivity reactions during clinical studies.
- 4% of patients treated with Avalglucosidase alfa-ngpt reported experiencing severe hypersensitivity reactions during clinical studies.
- 2% of patients treated with Avalglucosidase alfa-ngpt reported experiencing anaphylaxis during clinical studies.
- 1% of patients treated with Avalglucosidase alfa-ngpt reported experiencing anaphylaxis that led to discontinuation from the clinical study.
- Chest discomfort, dysphagia, lip swelling, respiratory distress, rash, swollen tongue, flushing, erythema, pruritus, and cough are symptoms of anaphylaxis in patients.
- Tongue edema, erythema, rash, respiratory distress, and urticaria are symptoms of severe hypersensitivity reactions in patients.
- Patients with higher antidrug antibody titers are more likely to experience hypersensitivity reactions when treated with Avalglucosidase alfa-ngpt.
Infusion-Associated Reactions
- Patients receiving pretreatment such as antipyretics, antihistamines, or corticosteroids may still experience infusion-associated reactions.
- Stop Avalglucosidase alfa-ngpt treatment and seek proper medical attention if patient experiences an infusion-associated reaction.
- Advise patients to consider both the risks and benefits prior to restarting Avalglucosidase alfa-ngpt treatment.
- Decrease or stop the infusion rate if patient is experiencing a mild or moderate infusion-associated reaction.
- 34% of patients treated with Avalglucosidase alfa-ngpt reported experiencing infusion-associated reaction during clinical studies.
- 4% of patients treated with Avalglucosidase alfa-ngpt reported experiencing 10 severe infusion-associated reaction during clinical studies.
- Dysphagia, tongue edema, increased blood pressure, respiratory distress, nausea, urticaria, erythema, and chest discomfort are some of the symptoms reported by patients experiencing a severe infusion-associated reaction.
- Cough, ocular hyperemia, dizziness, chest discomfort, flushing, respiratory distress, nausea, and erythema are the symptoms reported by patients with infusion-associated reactions that led to discontinuation of Avalglucosidase alfa-ngpt treatment.
- Patients with higher ADA titers were more likely to experience an infusion-associated reaction during Avalglucosidase alfa-ngpt treatment.
- Acute underlying illness in patients could pose a greater risk to experiencing an infusion-associated reaction during Avalglucosidase alfa-ngpt treatment.
- Severe complications of infusion-associated reactions increase in patients with advanced Pompe disease.
Risk of Acute Cardiorespiratory Failure in Susceptible Patients
- The risk of serious exacerbation of cardiac or respiratory status in patients may increase in patients with acute underlying respiratory illness, susceptible to fluid volume overload, and compromised cardiac or respiratory function.
- Monitor patients for serious exacerbation of cardiac or respiratory status.
Adverse Reactions
Clinical Trials Experience
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials in the Pompe Disease Population
- 118 adult and 23 pediatric patients were treated with Avalglucosidase_alfa-ngpt in 4 clinical trials that tested the safety of NEXVIAZYME treatment.
- Chills, respiratory distress, and pyrexia were some of the adverse reactions reported in at least 2 patients treated with NEXVIAZYME.
- Discontinuation of NEXVIAZYME treatment due to adverse reactions occurred in 5 patients with 2 of them experiencing serious adverse reactions.
- Arthralgia, myalgia, dizziness, rash, cough, urticaria, dyspnea, hypertension, vomiting, pyrexia, abdominal pain, pruritus, erythema, abdominal pain upper, chills, headache, diarrhea, nausea, fatigue, and hypotension were the most common adverse reactions reported in the clinical trials.
- 34% of patients experienced infusion-associated reactions when treated with NEXVIAZYME in the clinical trials.
- Pruritus, rash, rash erythematous, tachycardia, urticaria, vomiting, chest discomfort, dizziness, hyperhidrosis, lip swelling, diarrhea, erythema, fatigue, headache, influenza-like illness, nausea, ocular hyperemia, pain in extremity, oxygen saturation decreased, pain, palmar erythema, swollen tongue, abdominal pain upper, burning sensation, eyelid edema, chills, cough, feeling cold, flushing, respiratory distress, throat irritation, and tremor are some of the infusion-associated reactions reported in more than 1 patient treated with NEXVIAZYME.
Adverse Reactions from Clinical Trials in Late-Onset Pompe Disease (LOPD)
- Study 1 looked into the effects of giving 20 mg/kg of NEXVIAZYME or 20 mg/kg of Alglucosidase alfa in 100 patients with LOPD.
- 49 patients were given 20 mg/kg of Alglucosidase alfa while 51 patients were given 20 mg/kg of NEXVIAZYME.
- Each dosage was given every other week for 49 weeks that would be followed by an open-label extension period.
- 6% of patients reported serious adverse reactions when treated with Alglucosidase alfa.
- 2% of patients reported serious adverse reactions when treated with NEXVIAZYME.
- Diarrhea, nausea, arthralgia, dizziness, myalgia, paresthesia, urticaria, headache, fatigue, pruritus, vomiting, dyspnea, and erythema were the most common adverse reactions reported in the study.
- 25% of patients reported mild or moderate infusion-associated reactions when treated with NEXVIAZYME.
- No patient treated with NEXVIAZYME reported severe infusion-associated reactions.
- Pruritus, rash, headache, urticaria, and diarrhea were the most common infusion-associated reactions in patients treated with NEXVIAZYME.
- 33% of patients reported infusion-associated reactions when treated with Alglucosidase alfa.
- Pruritis, rash, erythema, dizziness, flushing, dyspnea, chills, nausea, and feeling hot were the most common mild to severe infusion-associated reactions in patients treated with Alglucosidase alfa.
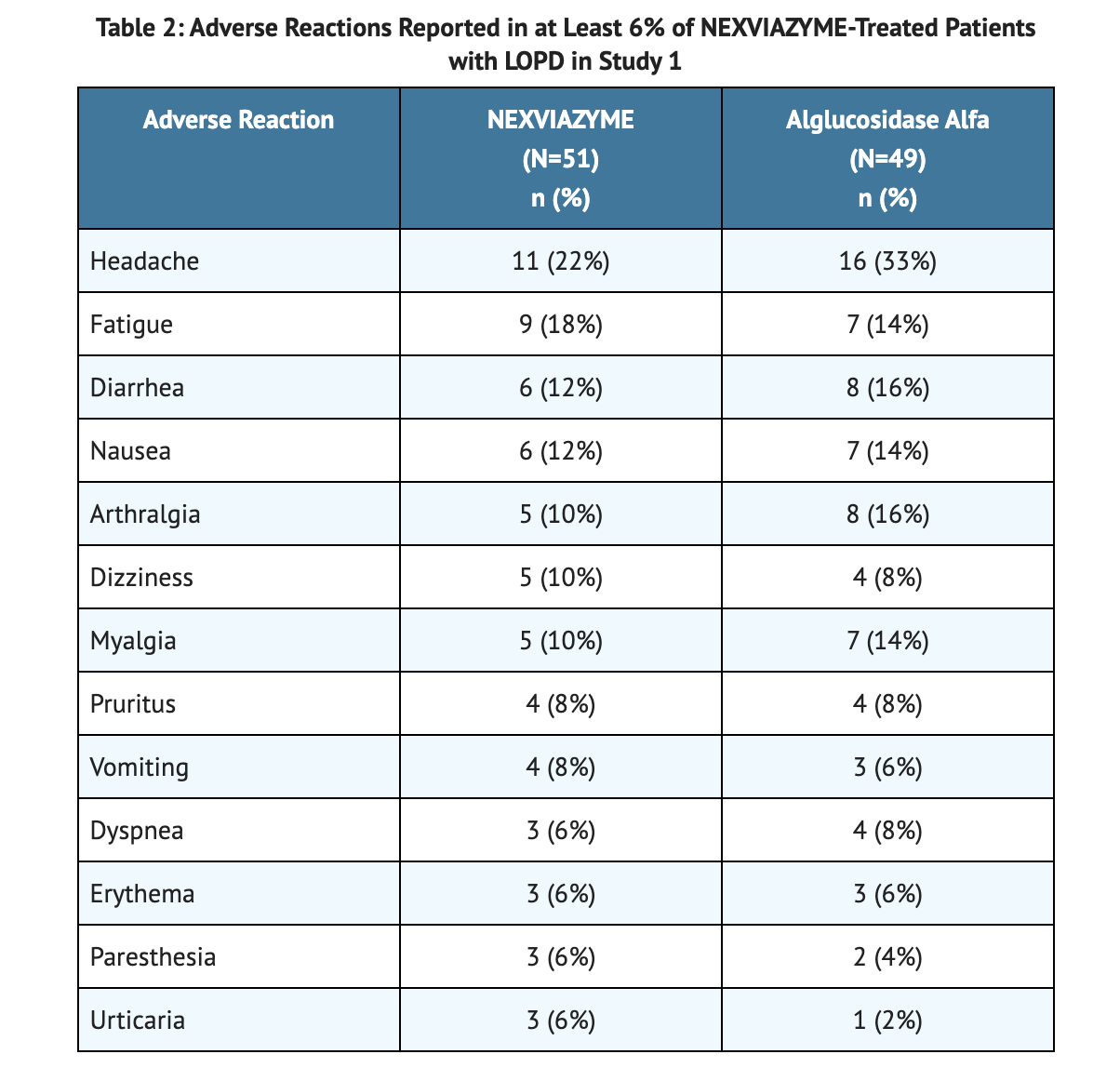
Table 2 summarizes the Adverse Reactions Reported in Study 1.
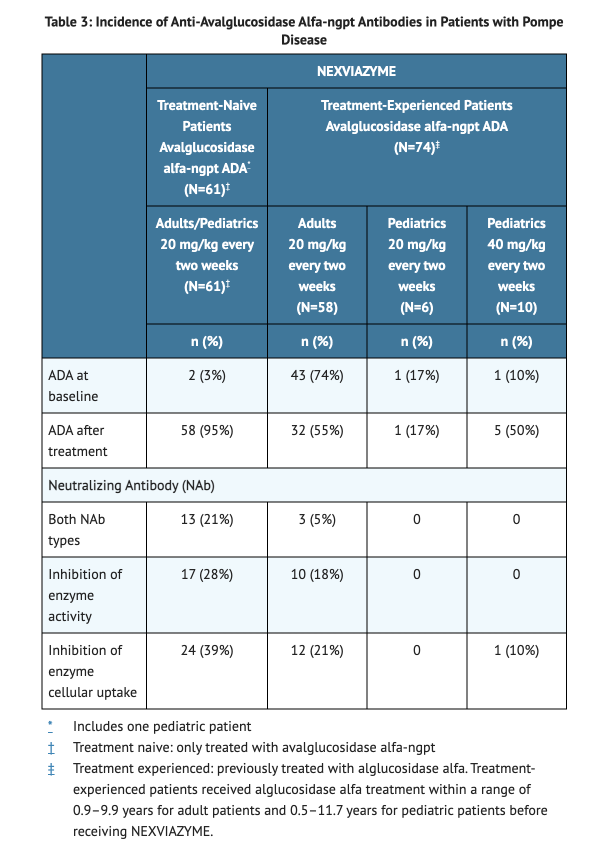
Immunogenicity
- 62% was the incidence of IAR in patients treated with NEXVIAZYME.
- Patients with higher ADA titer were more likely to experience hypersensitive reactions in comparison to patients with a low ADA titer.
- Patients who developed ADA were more likely to experience hypersensitive reactions and IAR compared to patients who are ADA negative.
- 8 weeks is the median time to seroconversion.
Table 3 Incidence of Anti-avalglucosidase alfa-ngpt Antibodies in Pompe Disease Patients.
Postmarketing Experience
There is limited information regarding Avalglucosidase alfa-ngpt Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Avalglucosidase alfa-ngpt Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is not enough data done on pregnant women treated with Avalglucosidase alfa-ngpt to determine the effects of Avalglucosidase alfa-ngpt on miscarriage, fetal outcomes, major birth defects, and adverse maternal outcomes. There have been no indications from postmarketing reports that Avalglucosidase alfa-ngpt causes risk of adverse pregnancy outcomes in pregnant women. Individualize Avalglucosidase alfa-ngpt treatment in pregnant women. Pregnant mice studies showed signs of maternal toxicity that was related to an immunologic response when treated with Avalglucosidase alfa-ngpt. The studies on pregnant mice also showed how Avalglucosidase alfa-ngpt treatment on mice did not result in the drug crossing over the placenta. Pregnant rabbit studies showed how Avalglucosidase alfa-ngpt treatment did not cause adverse effects on the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Avalglucosidase alfa-ngpt in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Avalglucosidase alfa-ngpt during labor and delivery.
Nursing Mothers
No data is present on the effects done on the breastfed child and the effects on milk production when treated with Avalglucosidase alfa-ngpt. There has been signs of Alglucosidase alfa found in human milk from other published literatures. Advise female patients who are nursing about the potential adverse reactions associated with Avalglucosidase alfa-ngpt.
Pediatric Use
Studies have been done on pediatric patients 1 year of age and older to test the safety and effectiveness of Avalglucosidase alfa-ngpt. The safety profiles of Avalglucosidase alfa-ngpt treatment on patients 1 to 12 years old with Pompe disease compared to older patients is similar. Studies have not been done on pediatric patients younger than 1 year of age to test the safety and effectiveness of Avalglucosidase alfa-ngpt in this age group.
Geriatic Use
The recommended dosage of Avalglucosidase alfa-ngpt is the same in for younger adult patients and geriatric patients as found in clinical studies.
Gender
There is no FDA guidance on the use of Avalglucosidase alfa-ngpt with respect to specific gender populations.
Race
There is no FDA guidance on the use of Avalglucosidase alfa-ngpt with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Avalglucosidase alfa-ngpt in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Avalglucosidase alfa-ngpt in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Avalglucosidase alfa-ngpt in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Avalglucosidase alfa-ngpt in patients who are immunocompromised.
Administration and Monitoring
Administration
Reconstitution and Dilution Instructions
Reconstitute the Lyophilized Powder:
- Use the recommended dosage and the patients weight to determine how many vials need to be reconstituted.
- Allow for 30 minutes after the vials are taken out of the fridge.
- Inject 10mL of sterile water for injection into each vial that needs to be reconstituted while making sure water does not come into contact with the lyophilized powder directly.
- Avoid foaming of lyophilized powder when tilting and rolling each vial.
- Advise patients to make sure each reconstituted vial is colorless to pale-yellow and clear.
Storage of the Reconstituted Solution:
- Without delay, dilute the reconstituted solution.
- Reconstituted solution can be stored in a refrigerator thats temperature ranges from 36°F to 46°F for up to 24 hours.
- Advise patients that reconstituted solution cannot be freezed.
Dilute the Reconstituted Solution:
- Add the the volume of reconstituted solution that is based on the patients weight into 5% Dextrose Injection slowly.
- Avoid air and foaming in the infusion bag.
- Add the infusion volume based on the patients weight and mix the bag with all its content through inversion of the bag.
- Advise patients to administer, without delays, the diluted solution.
Storage of the Diluted Solution:
- Refrigerate the diluted solution if not used immediately for up to 24 hours in 36°F to 46°F conditions.
- Within 9 hours of removal of the reconstituted solution from the fridge, completely infuse the solution.
- Diluted solution cannot be restored in the refrigerator after it has been taken out of the refrigerator previously.
- Diluted solution should be discarded from use if the solution has not been completely infused within 9 hours or has been refrigerated more than 24 hours.
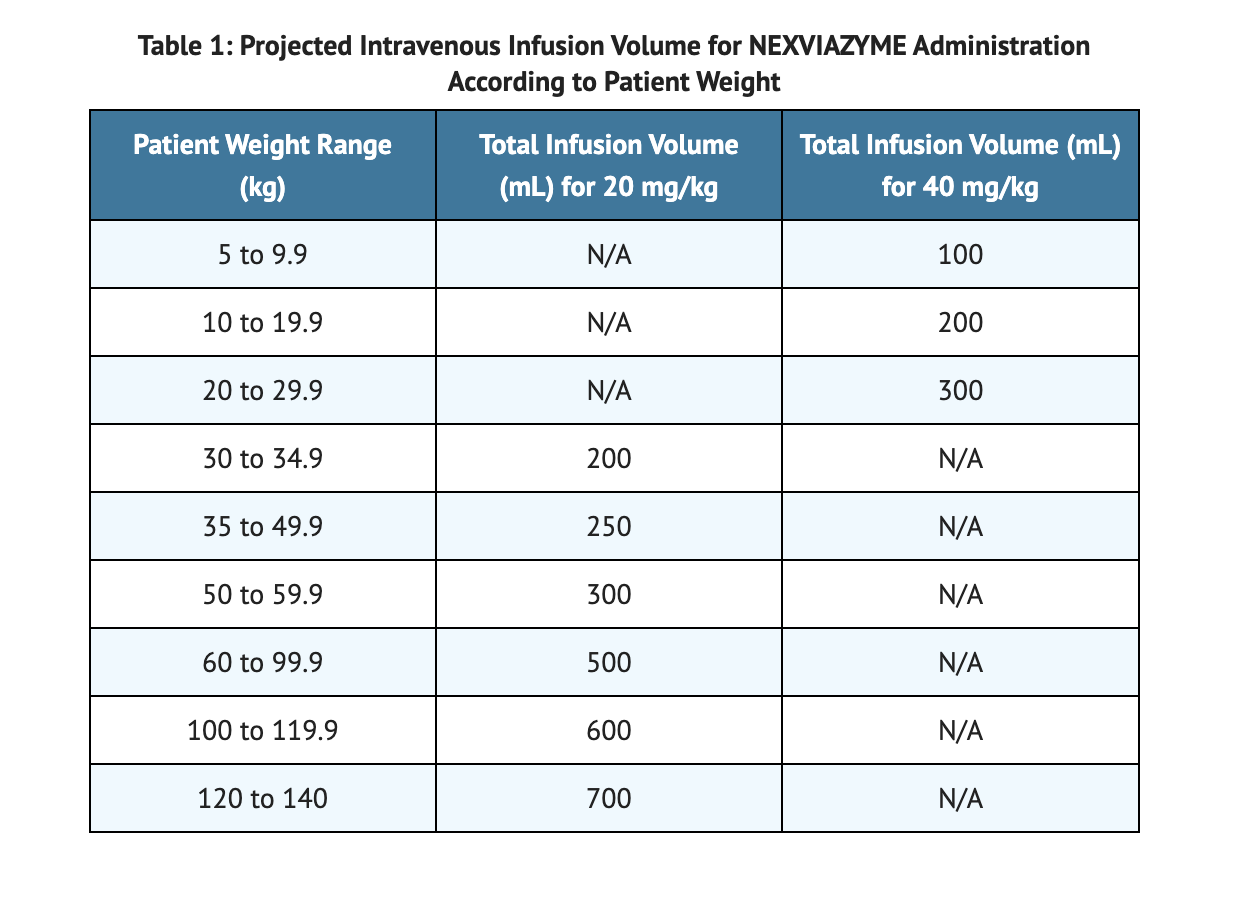
Table 1 shows the Infusion Volume given based on a Patients Weight.
Administration Instructions
When the recommended dose is 20 mg/kg:
Initial and Subsequent Infusions:
- Infusion rate should start off at 1 mg/kg/hour.
- Gradually increase the infusion rate every 30 minutes until it eventually becomes 7 mg/kg/hour.
- Advise patients to not increase infusion rate if they experience signs of Infusion-Associated Reactions.
- 4 to 5 hours is how long total infusion duration should last.
When the recommended dose is 40 mg/kg:
Initial Infusion:
- Infusion rate should start off at 1 mg/kg/hour.
- Gradually increase the infusion rate every 30 minutes until it eventually becomes 7 mg/kg/hour.
- Advise patients to not increase infusion rate if they experience signs of Infusion-Associated Reactions.
- 7 hours is how long total infusion duration should last.
Subsequent Infusions:
- Infusion rate should start off at 1 mg/kg/hour.
- Gradually increase the infusion rate every 30 minutes until it eventually becomes 10 mg/kg/hour.
- Advise patients to not increase infusion rate if they experience signs of Infusion-Associated Reactions.
- 5 hours is how long total infusion duration should last.
Monitoring
Dosage and Administration Modifications Due to Hypersensitivity Reactions and/or Infusion-Associated Reactions
- Advise patients to discontinue Avalglucosidase alfa-ngpt treatment and seek proper medical help if patients experiences either a severe infusion-associated reaction or a severe hypersensitivity reaction when treated with Avalglucosidase alfa-ngpt.
- Advise patients to temporarily slow or hold infusion rate and seek proper medical help if patients experiences either a mild to moderate infusion-associated reaction or a mild to moderate hypersensitivity reaction when treated with Avalglucosidase alfa-ngpt.
IV Compatibility
There is limited information regarding the compatibility of Avalglucosidase alfa-ngpt and IV administrations.
Overdosage
There is limited information regarding Avalglucosidase alfa-ngpt overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Avalglucosidase alfa-ngpt
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | GZ-402666, avalglucosidase alfa-ngpt |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B1(AU) |
| Legal status |
Prescription Only (S4)(AU) ?(CA) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous |
Mechanism of Action
- Avalglucosidase alfa-ngpt is a hydrolytic lysosomal glycogen-specific enzyme.
- An exogenous source of GAA is provided by Avalglucosidase alfa-ngpt.
Structure
- Avalglucosidase alfa-ngpt is a hydrolytic lysosomal glycogen-specific enzyme.
- 124 kDa is the molecular weight of Avalglucosidase alfa-ngpt.
Pharmacodynamics
- Clinical studies have shown that patients have seen a reduction of urinary Glc4 concentrations when treated with NEXVIAZYME.
- In NEXVIAZYME, 12.7 mmol/mol is the baseline mean urinary Glc4 concentration which over a 49 week period had a mean percentage change of -54%.
- In Alglucosidase alfa, 8.7 mmol/mol is the baseline mean urinary Glc4 concentration which over a 49 week period had a mean percentage change of -11%.
Pharmacokinetics
- When doses increase of Avalglucosidase alfa-ngpt from a a range from 5 to 20 mg/kg, the exposure of Avalglucosidase alfa-ngpt increases.
- 259 ± 72 µg/mL is the mean ± SD plasma Cmax of Avalglucosidase alfa-ngpt at Week 1 when patients, LOPD patients weighing ≥30 kg, were given intravenous infusion of 20 mg/kg of NEXVIAZYME.
- 242 ± 81 µg/m is the mean ± SD plasma Cmax of Avalglucosidase alfa-ngpt at Week 49 when patients, LOPD patients weighing ≥30 kg, were given intravenous infusion of 20 mg/kg of NEXVIAZYME.
- 1,290 ± 420 µg∙h/mL is the mean ± SD plasma AUC of Avalglucosidase alfa-ngpt at Week 1 when patients, LOPD patients weighing ≥30 kg, were given intravenous infusion of 20 mg/kg of NEXVIAZYME.
- 1,250 ± 433 µg∙h/mL is the mean ± SD plasma AUC of Avalglucosidase alfa-ngpt at Week 49 when patients, LOPD patients weighing ≥30 kg, were given intravenous infusion of 20 mg/kg of NEXVIAZYME.
Distribution
- 3.4 L is the volume of distribution in LOPD patients treated with Avalglucosidase alfa-ngpt.
Elimination
- 1.6 hours is the mean plasma elimination half-life in LOPD patients treated with Avalglucosidase alfa-ngpt.
- 0.9 L/hour is the mean clearance in LOPD patients treated with Avalglucosidase alfa-ngpt.
Metabolism
- The metabolic pathway is not characterized yet for Avalglucosidase alfa-ngpt.
- Via catabolic pathways, amino acids and small peptides are expected to be metabolized by the protein portion of Avalglucosidase alfa-ngpt.
Antidrug Antibody Effects on Pharmacokinetics
- Treatment emergent ADA was experienced by 96% of treatment-naive LOPD patients when given 20mg/kg every two weeks of NEXVIAZYME.
- Median AUC of patients experiencing ADA was similar between Week 1 and Week 49 treatment.
- Patients who have higher ADA peak titers are more likely to experience Infusion-Associated Reactions.
Specific Populations
- The pharmacokinetics in patients from the ages 1 to 78 years was not influenced by sex or age when treated with Avalglucosidase alfa-ngpt.
- 175 to 189 µg/mL is the Cmax in pediatric patients following a 4-hour intravenous infusion of NEXVIAZYME 20 mg/kg every two weeks.
- 250 to 403 µg/mLis the Cmax in pediatric patients following a 7-hour intravenous infusion of NEXVIAZYME 40 mg/kg every two weeks.
- 805 to 923 µg∙hr/mL is the mean AUClast in pediatric patients receiving 20mg/kg of NEXVIAZYME every 2 weeks.
- 1,720 to 2,630 µg∙hr/mL is the mean AUClast in pediatric patients receiving 40mg/kg of NEXVIAZYME every 2 weeks.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenic potential of Avalglucosidase alfa-ngpt studies on animals have not been conducted.
- Mutagenic potential of Avalglucosidase alfa-ngpt studies on animals have not been conducted.
- Mice studies show no signs of adverse effects on fertility in either female or male mices when given Avalglucosidase alfa-ngpt.
Clinical Studies
Clinical Trial in Patients with Late-Onset Pompe Disease
- Study 1 looked into the safety and efficacy of NEXVIAZYME to Alglucosidase alfa in a randomized, double-blinded, multinational, multicenter trial.
- The study consisted of 100 LOPD patients.
- Patients would be split up and receive either 20 mg/kg of NEXVIAZYME or 20 mg/kg Alglucosidase alfa every 2 weeks for 49 weeks.
- The patient population included 52 males, 76.4 kg as the mean baseline weight, and 49 years of age is the mean age.
- 62.1% is the mean FVC at baseline, and 388.9 meters is the mean 6MWT at baseline for the study.
Endpoints and Results from the 49-Week Active-Controlled Period in Study 1
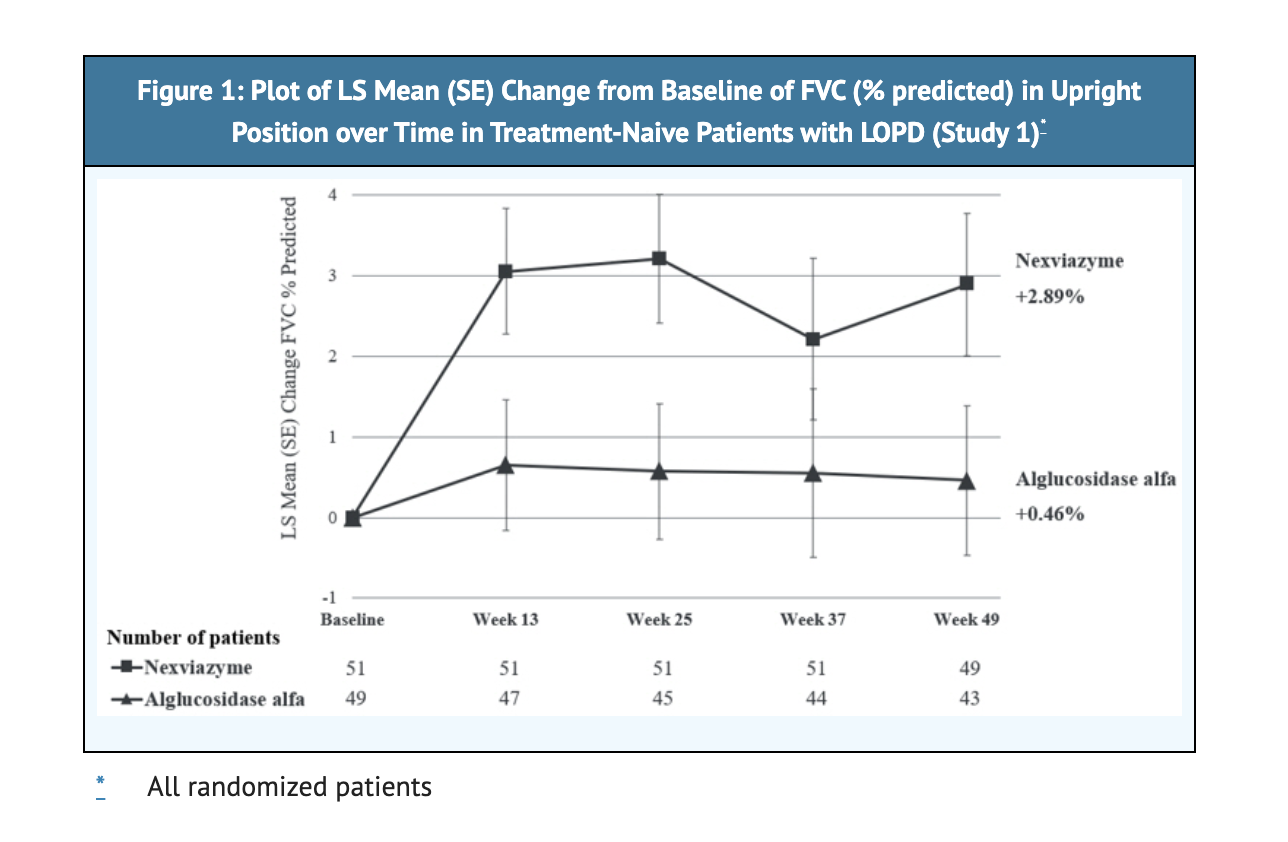
- 2.9% is the least squares mean change at Week 49 in patients who were treated with NEXVIAZYME.
- 0.5% is the least squares mean change at Week 49 in patients who were treated with Alglucosidase alfa.
Table 4 shows Results of FVC in Study 1.
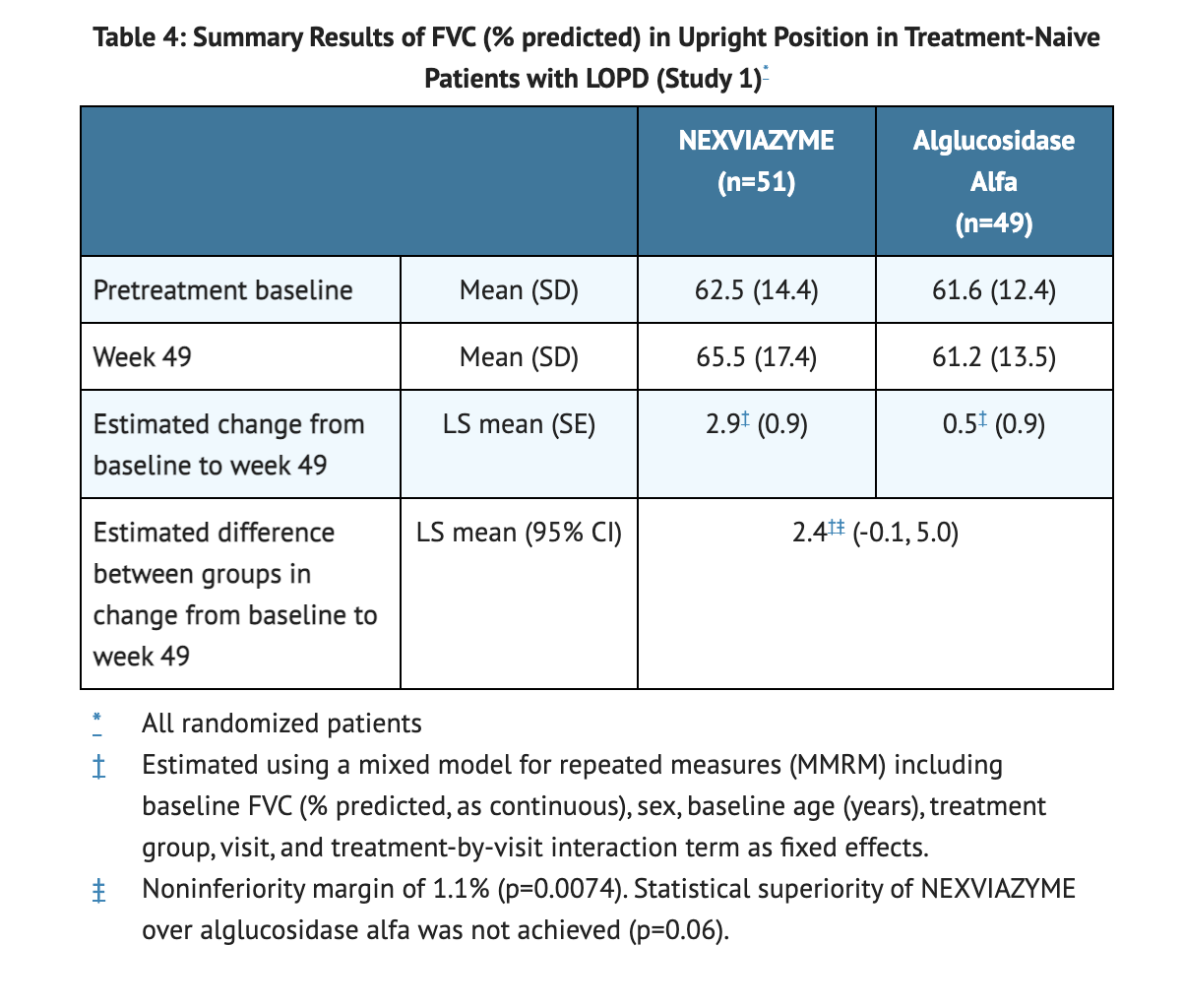
Figure 1 shows the LS Mean Change from Baseline in FVC in Study 1.
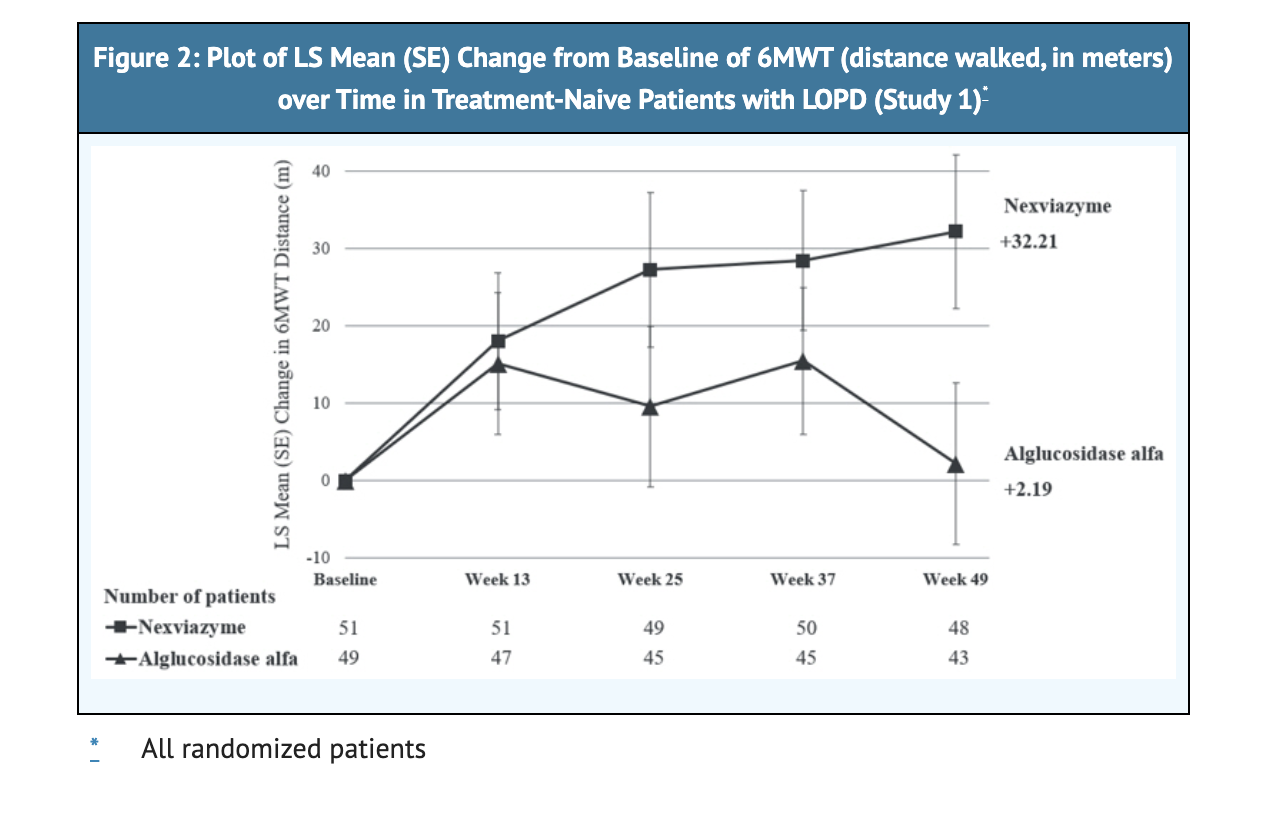
- For patients on NEXVIAZYME treatment, 32.2 meters is the LS mean change from baseline in 6MWT.
- For patients on Alglucosidase alfa, 2.2 meters is the LS mean change from baseline in 6MWT.
Table 5 shows the Summary of the 6-Minute Walk Test conducted in Study 1.
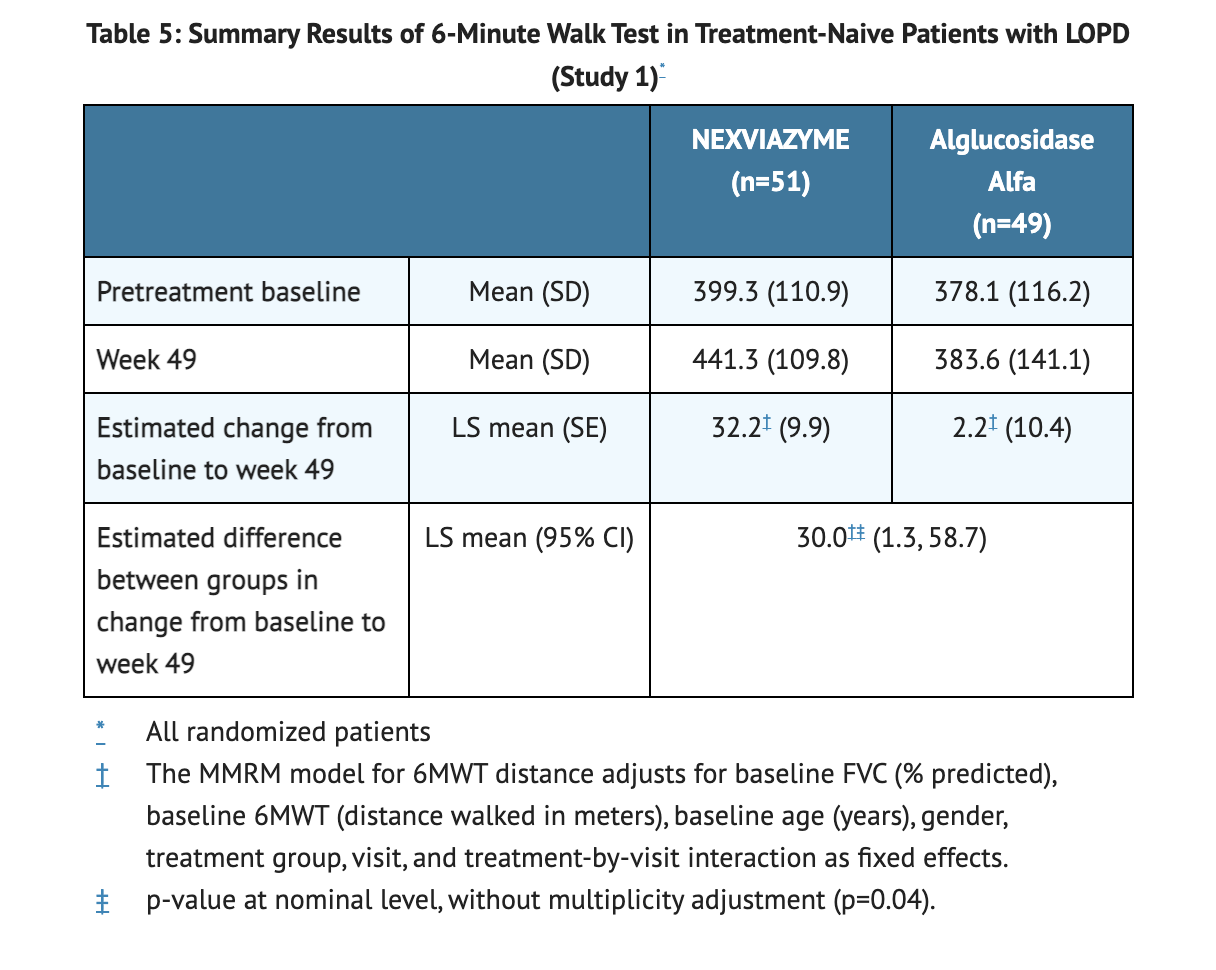
Figure 2 shows LS mean change from Baseline in 6MWT Distance over Time in Study 1.
How Supplied
- One 100 mg vial in a carton of Avalglucosidase alfa-ngpt.
- Injection is given as a single-dose vial that is white to pale-yellow lyophilized powder and sterile.
Storage
- Vials of Avalglucosidase alfa-ngpt should be refrigerated at 36°F to 46°F.
Images
Drug Images
{{#ask: Page Name::Avalglucosidase alfa-ngpt |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
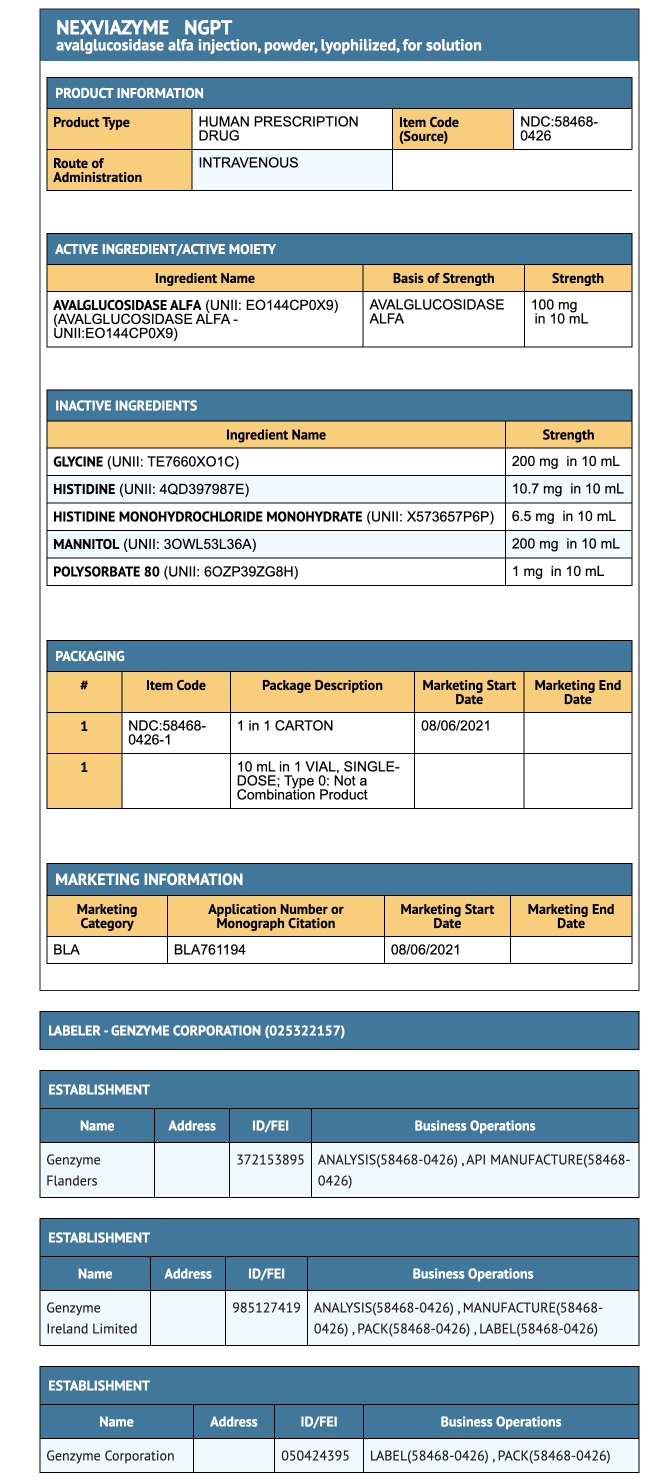
{{#ask: Label Page::Avalglucosidase alfa-ngpt |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hypersensitivity Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs)
- Hypersensitivity reactions, anaphylactic reactions, and infusion-associated reactions may occur in patients who are treated with Avalglucosidase alfa-ngpt.
- Advise patients about the symptoms associated with infusion-associated reactions and hypersensitivity reactions.
- Advise patients to seek medical attention from their doctor if they experience any signs or symptoms of infusion-associated reactions and hypersensitivity reactions.
Risk of Acute Cardiorespiratory Failure
- Acute cardiorespiratory failure may occur in patients that have compromised respiratory function, underlying respiratory illness, and compromised cardiac function when treated with Avalglucosidase alfa-ngpt.
NEXVIAZYME Exposure During Pregnancy or Lactation
- Advise female patients who are nursing or pregnant to immediately contact their doctor if exposed to NEXVIAZYME.
Pompe Registry
- Monitor patients for long-term effects of Avalglucosidase alfa-ngpt.
- Advise patients that Pompe Registry is used to understand progression and variability of Pompe disease.
- Participation in Pompe Registry is voluntary and may require additional long-term follow-up.
- Call 1-800-745-4447, extension 15500 for more information on Pompe Registry.
Precautions with Alcohol
Alcohol-Avalglucosidase alfa-ngpt interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NEXVIAZYME
Look-Alike Drug Names
There is limited information regarding Avalglucosidase alfa-ngpt Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.