Anifrolumab-fnia
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Anifrolumab-fnia is a type I interferon receptor antagonist that is FDA approved for the treatment of moderate-to severe systemic lupus erythematousus along with standard therapy. Common adverse reactions include bronchitis, nasopharyngitis, herpes zoster, upper respiratory tract infections, infusion related reactions, and cough.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- 300 mg is the recommended dosage of Anifrolumab-fnia.
- Recommended dosage should be given every 4 weeks as an intravenous infusion that takes 30 minutes.
- Administer recommended dosage as soon as possible if a dosage of Anifrolumab-fnia is missed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Anifrolumab-fnia in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anifrolumab-fnia in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Anifrolumab-fnia FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Anifrolumab-fnia in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anifrolumab-fnia in pediatric patients.
Contraindications
- Patients that have a history of anaphylaxis when taking Anifrolumab-fnia are contradicted.
Warnings
Serious Infections
- Some patients have reported serious and fatal infections when treated with Anifrolumab-fnia.
- Clinical studies show that patients treated with Anifrolumab-fnia were more likely to experience a fatal infection in comparison to patients treated with a placebo.
- Clinical studies show similar reports of experiencing serious infections when comparing the Anifrolumab-fnia to the placebo group.
- Clinical studies showed that risk of herpes zoster and respiratory infections are increased in patients taking Anifrolumab-fnia.
- Advise patients about the risks and benefits of Anifrolumab-fnia treatment.
- Anifrolumab-fnia treatment should be avoided in patients with any clinically significant active infection until infection is resolved.
- Anifrolumab-fnia treatment may be interrupted if a patients is experiencing is not responding to standard anti-infective therapy or develops an infection.
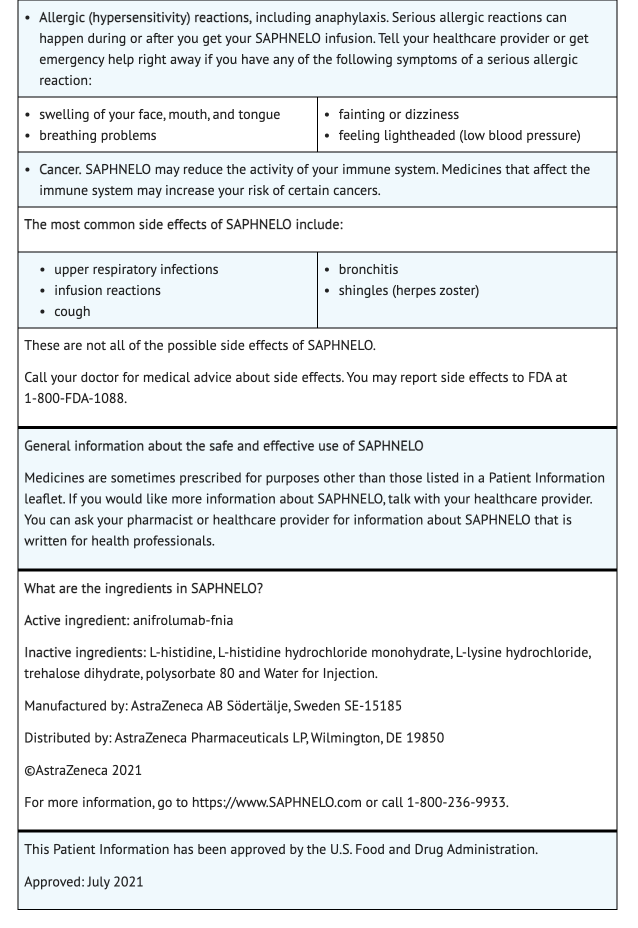
Hypersensitivity Reactions Including Anaphylaxis
- Patients have experienced serious hypersensitivity reactions, infusion-related reactions, and angioedema when treated Anifrolumab-fnia.
- Advise patients that their healthcare provider who can manage hypersensitivity reactions should administer Anifrolumab-fnia treatment.
- Advise patients to seek immediate therapy if they experience hypersensitivity reactions or serious infusion-related reactions when taking Anifrolumab-fnia.
Malignancy
- Immunosuppressants use can increase risk of malignancies.
- Development of potential malignancies during Anifrolumab-fnia treatment is still unknown.
- Advise patients about the risks and benefits of Anifrolumab-fnia treatment if malignancies develop.
Immunization
- Advise patients who have had a live or live-attenuated vaccines should avoid concurrent use with Anifrolumab-fnia.
- Advise patients prior to Anifrolumab-fnia treatment should update immunizations record.
Not Recommended for Concomitant Use with Other Biologic Therapies
- Advise patients to avoid a combination of biologic therapies and Anifrolumab-fnia treatment.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Patients that had moderate to severe SLE were used in a 52 week clinical study to test the safety of Anifrolumab-fnia. 466 patients were given a placebo while 459 patients were given 300 mg of Anifrolumab-fnia every 4 weeks through an intravenous infusion. The patient population was largely Caucasian (60%), included 93% of females, and had a mean age of 41 years of age.
- 79% of patients in the placebo group experienced signs of adverse reactions.
- 87% of patients who received Anifrolumab-fnia experienced signs of adverse reactions.
Table 1 summarizes the Adverse Reactions Experienced in the Clinical Studies.

Specific Adverse Reactions
Infections
- 69.7% of patients who received Anifrolumab-fnia reported signs of infections in the clinical studies.
- 55.4% of patients in the placebo group reported signs of infections in the clinical studies.
Serious Infections
- 4.8% of patients who received Anifrolumab-fnia reported signs of serious infections in the clinical studies.
- 5.6% of patients in the placebo group reported signs of serious infections in the clinical studies.
- Pneumonia was the most common serious infection reported in patients.
- 0.4% of patients who received Anifrolumab-fnia reported signs of fatal infections in the clinical studies.
- 0.2% of patients in the placebo group reported signs of fatal infections in the clinical studies.
Herpes Zoster
- 6.1% of patients who received Anifrolumab-fnia reported signs of herpes zoster in the clinical studies.
- 1.3% of patients in the placebo group reported signs of herpes zoster in the clinical studies.
- 2 patients in the clinical study treated with Anifrolumab-fnia needed hospitalization after experiencing disseminated disease.
Hypersensitivity Reactions Including Anaphylaxis
- 1 patient who received 150 mg of Anifrolumab-fnia reported an anaphylactic reaction.
- 2 patients who received 300 mg of Anifrolumab-fnia reported angioedema.
- 2.8% of patients who received Anifrolumab-fnia reported signs of hypersensitivity reactions in the clinical studies.
- 0.6% of patients in the placebo group reported signs of hypersensitivity reactions in the clinical studies.
- 0.6% of patients who received Anifrolumab-fnia reported signs of serious hypersensitivity reactions in the clinical studies.
Infusion-related Reactions
- Vomiting, dizziness, headache, fatigue, and nausea are the most common signs reported in patients who experienced infusion-related reactions.
- 9.4% of patients who received Anifrolumab-fnia reported signs of infusion-related reactions in the clinical studies.
- 7.1% of patients in the placebo group reported signs of infusion-related reactions in the clinical studies.
Malignancies
- 0.7% of patients who received Anifrolumab-fnia reported signs of malignancies in the clinical studies.
- 0.6% of patients in the placebo group reported signs of malignancies in the clinical studies.
- 1.3% of patients who received Anifrolumab-fnia reported signs of malignant neoplasm in the clinical studies.
- 0.6% of patients in the placebo group reported signs of malignant neoplasm in the clinical studies.
Immunogenicity
- 1.7% of patients receiving Anifrolumab-fnia showed signs of anti-anifrolumab-fnia antibodies in Trial 2 and 3 studies.
Postmarketing Experience
There is limited information regarding Anifrolumab-fnia Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Anifrolumab-fnia Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is not enough data done on pregnant women treated with Anifrolumab-fnia to determine the effects of Anifrolumab-fnia on miscarriage, fetal outcomes, major birth defects, and adverse maternal outcomes. The exposure of Anifrolumab-fnia to the fetus may be higher in the third trimester during pregnancy. No evidence of fetal malformations or embryotoxicity in pregnant cynomolgus monkeys that received intravenous administration of Anifrolumab-fnia. No evidence was found in pregnant cynomolgus monkeys of embryo-fetal toxicity, maternal toxicity, or post-natal developmental effects when receiving either 30 or 60 mg/kg once every 2 weeks of Anifrolumab-fnia from Gestation Day 20 to 1‑month post-partum. There are indications from animal studies that show Anifrolumab-fnia can be transferred to the fetus through the placenta.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Anifrolumab-fnia in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Anifrolumab-fnia during labor and delivery.
Nursing Mothers
No data is present on the effects done on the breastfed child and the effects on milk production when treated with Anifrolumab-fnia. Animal studies done on female cynomolgus monkeys show indications of Anifrolumab-fnia in milk. Advise nursing patients of the risks and benefits of taking Anifrolumab-fnia.
Pediatric Use
There is no FDA guidance on the use of Anifrolumab-fnia in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Anifrolumab-fnia in geriatric settings.
Gender
There is no FDA guidance on the use of Anifrolumab-fnia with respect to specific gender populations.
Race
There is no FDA guidance on the use of Anifrolumab-fnia with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Anifrolumab-fnia in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Anifrolumab-fnia in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Anifrolumab-fnia in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Anifrolumab-fnia in patients who are immunocompromised.
Administration and Monitoring
Administration
Instructions for Preparation and Administration
- Vial of Anifrolumab-fnia should be clear to opalescent, colorless to slightly yellow, solution.
- From the 100 mL 0.9% Sodium Chloride Injection, USP infusion bag, discard 2 mL of solution.
- Add 2 ml of solution from the vial to the infusion bag.
- Infusion solution should be administered immediately.
- Using an infusion line containing a sterile, low-protein binding 0.2 or 0.22 micron in-line filter, infuse the solution intravenously over a 30-minute time frame.
- Flush, using 25 mL of 0.9% Sodium Chloride Injection (USP), infusion set.
- Infusion solution, if not administered immediately, may be refrigerated (36°F to 46°F, 2°C to 8°C) for up to 24 hours or stored at room temperature (59°F to 77°F, 15°C to 25°C) for up to 4 hours.
Monitoring
There is limited information regarding Anifrolumab-fnia Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Anifrolumab-fnia and IV administrations.
Overdosage
There is limited information regarding Anifrolumab-fnia overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Anifrolumab-fnia?
| |
| Therapeutic monoclonal antibody | |
| Source | u |
| Target | Interferon α/β receptor |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | MEDI-546, anifrolumab-fnia |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(AU) |
| Legal status |
Prescription Only (S4)(AU) ?(CA) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous |
Mechanism of Action
- Anifrolumab-fnia is a human IgG1κ monoclonal antibody.
- I IFN signaling is inhibited by Anifrolumab-fnia which stops biologic activity of type I IFNs.
- IFN responsive gene expression is inhibited with Anifrolumab-fnia.
Structure
- Anifrolumab-fnia is a type I interferon (IFN) receptor antagonist.
- 148 kDa is the molecular weight of Anifrolumab-fnia.
Pharmacodynamics
- Type I IFN gene signature was observed from Week 4 to Week 52 in SLE patients who received 300 mg dose Anifrolumab-fnia.
- Reductions in anti-dsDNA antibodies were seen in SLE patients with positive anti-dsDNA antibodies who received 300 mg of Anifrolumab-fnia.
- Increases in complement levels were seen in patients with low complement levels that received Anifrolumab-fnia.
Pharmacokinetics
- There is a non-linear PK in the dose range of 100 mg to 1000 mg of Anifrolumab-fnia.
- Steady state of Anifrolumab-fnia was reached at day 85 after patients received 300 mg of Anifrolumab-fnia through intravenous administration every 4 weeks.
- 1.36 is the accumulation ratio of Cmax for Anifrolumab-fnia.
- 2.49 is the accumulation ratio of Ctrough for Anifrolumab-fnia.
Distribution
- In patients with SLE, 6.23 L is the the estimated volume of distribution at steady state of Anifrolumab-fnia.
Elimination
- Non-linear PK was seen in Anifrolumab-fnia due to clearance of IFNAR1-mediated drug.
- 0.193 L/day is the estimated systemic clearance in patients who received 300 mg of Anifrolumab-fnia every 4 weeks via intravenous infusion.
Specific Populations
- Race, IFN status or body weight, ethnicity, gender, age, or region did not create any clinically meaningful differences in systemic clearance.
Age:
- Clearance of Anifrolumab-fnia was not affected by patients in the age range of 18 to 69 years.
- Limited data is available on patients of the age of 65 years or older.
Renal Impairment:
- Studies on the effect of renal impairment on Anifrolumab-fnia has not been conducted.
- SLE patients with mild and moderate decrease in eGFR values had comparable clearance with patients who have normal renal function.
- The affect of Anifrolumab-fnia by increased urine protein/creatinine ratio was not significant.
Hepatic Impairment:
- Studies on the effect of hepatic impairment on Anifrolumab-fnia has not been conducted.
- Anifrolumab-fnia clearance is not expected to be influenced by hepatic function changes.
- Anifrolumab-fnia clearance had no clinically relevant changes by baseline hepatic function biomarkers.
Drug Interactions
- Drug-drug interaction studies have not been formally conducted with Anifrolumab-fnia.
- The PK of Anifrolumab-fnia was not significantly affected by concomitant use of NSAIDs, ACE inhibitors, anti-malarials, immunosuppressants, HMG-CoA reductase inhibitors, or oral corticosteroids.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Genotoxic potential or carcinogenic have not been evaluated of Anifrolumab-fnia.
- Increased carcinogenic potential was found in rodent models of IFNAR1 blockade.
- There have been no direct effects done on male and female fertility when taking Anifrolumab-fnia.
- Cynomolgus monkeys studies indicate indirectly no adverse effects of male or female fertility when treated with Anifrolumab-fnia.
Clinical Studies
Trials 1,2 and 3
- Three 52-week treatment period, multicenter, randomized, double-blind, placebo-controlled studies were conducted on SLE patients to test the safety and efficacy of Anifrolumab-fnia.
- British Isles Lupus Assessment Group based Composite Lupus Assessment and SLE Responder Index (SRI‑4) were used in the clinical trials to test the efficacy of Anifrolumab-fnia.
BICLA Response Requirements:
- Reduction of all baseline BILAG A to B/C/D and baseline BILAG B to C/D, and no BILAG worsening in other organ systems, as defined by ≥1 new BILAG A or ≥2 new BILAG B.
- No worsening from baseline in SLEDAI-2K, where worsening is defined as an increase from baseline of >0 points in SLEDAI-2K.
- No worsening from baseline in patients’ lupus disease activity, where worsening is defined by an increase ≥0.30 points on a 3-point PGA VAS.
- No discontinuation of treatment.
- No use of restricted medication beyond the protocol-allowed threshold.
SRI‑4 Response Requirements:
- Reduction from baseline of ≥4 points in the SLEDAI-2K.
- No new organ system affected as defined by 1 or more BILAG A or 2 or more BILAG B items compared to baseline.
- No worsening from baseline in the patients’ lupus disease activity defined by an increase ≥0.30 points on a 3‑point PGA visual analogue scale (VAS).
- No discontinuation of treatment.
- No use of restricted medication beyond the protocol-allowed threshold.
Trial 1:
- Trial 1 included 305 patients that received either 300 mg or 1000 mg of Anifrolumab-fnia or a placebo for 52 weeks.
- Sustained reduction in OCS and assessment of the SRI-4 was the primary endpoint of Trial 1 at Week 24.
Trial 2:
- Trial 2 included 457 patients that received either 150 mg or 300 mg of Anifrolumab-fnia or a placebo for 52 weeks.
- The primary endpoint uses SRI‑4 which was helpful to measure improvement in disease activity during the 52 week period.
- Flare rate, maintenance of OCS reduction, and improvement in cutaneous SLE activity were the secondary efficacy endpoints used.
Trial 3:
- Trial 3 included 362 patients that received either 300 mg of Anifrolumab-fnia or a placebo for 52 weeks.
- The primary endpoint uses BICLA which was helpful to measure improvement in disease activity during the 52 week period.
- Flare rate, maintenance of OCS reduction, and improvement in cutaneous SLE activity were the secondary efficacy endpoints used.
Table 2 shows the Baseline and Demographics of Trials 1,2, and 3.

Table 3 shows the BICLA results of the Trials.

Figure 1 shows the Proportion (%) of BICLA Responders in Trial 3.

Table 4 summarizes the SRI-4 Response Rate of the Trials in Week 52.

Effect on Concomitant Steroid Treatment
- Trial 3 data shows statistically significant differences in reduction of OCS use by at least 25% to ≤7.5 mg/day at Week 40 and maintain the reduction through Week 52 that was more common in patients part of the Anifrolumab-fnia than the placebo group.
- Effect of reduction of OCS use seen in Trial 1 and 2 were not statistically significant between the patients part of the Anifrolumab-fnia and the placebo group.
How Supplied
- Injection that contains sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution.
- Injection is part of a 2 mL clear glass vial containing 150 mg/mL of Anifrolumab-fnia.
- One single-dose vial of Anifrolumab-fnia are available.
Storage
- Store in a refrigerator at 36°F to 46°F (2°C to 8°C).
- Protect against light through use of original Anifrolumab-fnia carton.
- Do not freeze.
- Do not shake.
Images
Drug Images
{{#ask: Page Name::Anifrolumab-fnia |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Anifrolumab-fnia |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Serious Infections
- The ability to fight infections may be decreased when treated with Anifrolumab-fnia.
- Herpes zoster and respiratory infections risk increases when treated with Anifrolumab-fnia.
- Symptoms of infection includes muscle aches; cough; diarrhea or stomach pain; fever or flu-like symptoms; shingles; shortness of breath; burning when they urinate or urinating more often than usual which should be reported to healthcare provider if they appear.
Hypersensitivity Reactions/Anaphylaxis
- Advise patients that patients treated with Anifrolumab-fnia have experienced serious hypersensitivity reactions that includes anaphylaxis.
- Advise patients to seek medical attention if they experience any symptoms and signs of allergic reactions which also includes anaphylaxis.
- Feeling lightheaded, breathing difficulties, and/or fainting, swelling of the face, tongue, or mouth, and dizziness are symptoms associated with hypersensitivity reactions.
Immunizations
- Advise patients that during Anifrolumab-fnia treatment, patients should avoid live or live-attenuated vaccines.
- Advise patients to consult with their medical provider about receiving immunizations while Anifrolumab-fnia treatment.
Pregnancy
- Advise patients to inform their medical providers about any potential or ongoing pregnancies during Anifrolumab-fnia treatment.
Anifrolumab-fnia Package Insert:

Precautions with Alcohol
Alcohol-Anifrolumab-fnia interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- SAPHNELO
Look-Alike Drug Names
There is limited information regarding Anifrolumab-fnia Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.