Topiramate drug interactions: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
==Drug Interactions== | ==Drug Interactions== | ||
In vitro studies indicate that topiramate does not inhibit enzyme activity for CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4/5 isozymes. In vitro studies indicate that topiramate is a mild inhibitor of CYP2C19 and a mild inducer of CYP3A4. Drug interactions with some antiepileptic drugs, CNS depressants and oral contraceptives are described here. For other drug interactions, please refer to Clinical Pharmacology (12.3). | In vitro studies indicate that topiramate does not inhibit enzyme activity for [[CYP1A2]], [[CYP2A6]], [[CYP2B6]], [[CYP2C9]], [[CYP2D6]], [[CYP2E1]], and [[CYP3A4]]/5 isozymes. In vitro studies indicate that topiramate is a mild inhibitor of [[CYP2C19]] and a mild inducer of CYP3A4. Drug interactions with some antiepileptic drugs, CNS depressants and oral contraceptives are described here. For other drug interactions, please refer to Clinical Pharmacology (12.3). | ||
===Antiepileptic Drugs=== | ===Antiepileptic Drugs=== | ||
| Line 15: | Line 15: | ||
Concomitant administration of valproic acid and topiramate | Concomitant administration of valproic acid and topiramate | ||
has been associated with hyperammonemia with and without encephalopathy. Concomitant administration of topiramate with valproic acid has also been associated with hypothermia (with and without hyperammonemia) in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see Warnings and Precautions (5.9), (5.11) or Clinical Pharmacology (12.3)]. | has been associated with [[hyperammonemia]] with and without encephalopathy. Concomitant administration of topiramate with [[valproic acid]] has also been associated with hypothermia (with and without [[hyperammonemia]]) in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see Warnings and Precautions (5.9), (5.11) or Clinical Pharmacology (12.3)]. | ||
====Concomitant administration of valproic acid and topiramate==== | ====Concomitant administration of valproic acid and topiramate==== | ||
| Line 25: | Line 25: | ||
===Oral Contraceptives=== | ===Oral Contraceptives=== | ||
Exposure to ethinyl estradiol was statistically significantly decreased at doses of 200, 400, and 800 mg/day (18%, 21%, and 30%, respectively) when topiramate was given as adjunctive therapy in patients taking [[valproic acid]]. However, norethindrone exposure was not significantly affected. In another pharmacokinetic interaction study in healthy volunteers with a concomitantly administered combination oral contraceptive product containing 1 mg [[norethindrone]] (NET) plus 35 mcg [[ethinyl estradiol]] (EE), topiramate, given in the absence of other medications at doses of 50 to 200 mg/day, was not associated with statistically significant changes in mean exposure (AUC) to either component of the [[oral contraceptive]]. The possibility of decreased contraceptive efficacy and increased breakthrough bleeding should be considered in patients taking combination [[oral contraceptive]] products with topiramate. Patients taking estrogen-containing contraceptives should be asked to report any change in their bleeding patterns. Contraceptive efficacy can be decreased even in the absence of breakthrough bleeding [see Clinical Pharmacology (12.3)]. | Exposure to [[ethinyl estradiol]] was statistically significantly decreased at doses of 200, 400, and 800 mg/day (18%, 21%, and 30%, respectively) when topiramate was given as adjunctive therapy in patients taking [[valproic acid]]. However, [[norethindrone]] exposure was not significantly affected. In another pharmacokinetic interaction study in healthy volunteers with a concomitantly administered combination oral contraceptive product containing 1 mg [[norethindrone]] (NET) plus 35 mcg [[ethinyl estradiol]] (EE), topiramate, given in the absence of other medications at doses of 50 to 200 mg/day, was not associated with statistically significant changes in mean exposure (AUC) to either component of the [[oral contraceptive]]. The possibility of decreased contraceptive efficacy and increased breakthrough bleeding should be considered in patients taking combination [[oral contraceptive]] products with topiramate. Patients taking estrogen-containing contraceptives should be asked to report any change in their bleeding patterns. Contraceptive efficacy can be decreased even in the absence of breakthrough bleeding [see Clinical Pharmacology (12.3)]. | ||
===Metformin=== | ===Metformin=== | ||
Topiramate treatment can frequently cause metabolic acidosis, a condition for which the use of metformin is contraindicated [see Clinical Pharmacology. | Topiramate treatment can frequently cause [[metabolic acidosis]], a condition for which the use of [[metformin]] is contraindicated [see Clinical Pharmacology. | ||
===Lithium=== | ===Lithium=== | ||
In patients, lithium levels were unaffected during treatment with topiramate at doses of 200 mg/day; however, there was an observed increase in systemic exposure of lithium (27% for Cmax and 26% for AUC) following topiramate doses of up to 600 mg/day. Lithium levels should be monitored when coadministered with high-dose topiramate [see Clinical Pharmacology. | In patients, [[lithium]] levels were unaffected during treatment with topiramate at doses of 200 mg/day; however, there was an observed increase in systemic exposure of [[lithium]] (27% for Cmax and 26% for AUC) following topiramate doses of up to 600 mg/day. [[Lithium]] levels should be monitored when coadministered with high-dose topiramate [see Clinical Pharmacology. | ||
===Other Carbonic Anhydrase Inhibitors=== | ===Other Carbonic Anhydrase Inhibitors=== | ||
| Line 37: | Line 37: | ||
* As topiramate inhibits [[carbonic anhydrase]], the concomitant use of other inhibitors of carbonic anhydrase (e.g. [[acetazolamide]]) may lead to an increased risk of renal | * As topiramate inhibits [[carbonic anhydrase]], the concomitant use of other inhibitors of [[carbonic anhydrase]] (e.g. [[acetazolamide]]) may lead to an increased risk of [[renal stone]]s. | ||
* Enzyme inductors (e.g. carbamazepine) : The elimination of topiramate may be increased, possibly requiring dose escalations of topiramate. | * Enzyme inductors (e.g. [[carbamazepine]]) : The elimination of topiramate may be increased, possibly requiring dose escalations of topiramate. | ||
* [[Phenytoin]] : Topiramate may increase the plasma-levels of phenytoin. | * [[Phenytoin]] : Topiramate may increase the plasma-levels of [[phenytoin]]. | ||
* Topiramate itself is a weak inhibitor of | * Topiramate itself is a weak inhibitor of [[CYP2C19]] and induces CYP 3A4. Under topiramate a decrease of plasma-levels of [[estrogens]] (e.g. 'the pill') and [[digoxin]] have been noted. | ||
* Alcohol may cause increased sedation or drowsiness, and increase the risk of having a seizure. | * Alcohol may cause increased sedation or [[drowsiness]], and increase the risk of having a [[seizure]]. | ||
* As listed in the 06/29/2005 label posted at the Drugs@FDA website page 14,'conditions or therapies that predispose to acidosis may be additive to the bicarbonate lowering effects of Topiramate'. Absent from this label is any direct discussion of narcotic (drugs known to promote respiratory acidosis) interactions. This discussion on page 14 is under the topic of Metabolic Acidosis, and is not repeated under the topic of interactions.<ref>[Shttp://www.fda.gov/cder/foi/label/2005/020505s018lbl.pdf FDA.gov]</ref> | * As listed in the 06/29/2005 label posted at the Drugs@FDA website page 14,'conditions or therapies that predispose to acidosis may be additive to the bicarbonate lowering effects of Topiramate'. Absent from this label is any direct discussion of narcotic (drugs known to promote respiratory acidosis) interactions. This discussion on page 14 is under the topic of Metabolic Acidosis, and is not repeated under the topic of interactions.<ref>[Shttp://www.fda.gov/cder/foi/label/2005/020505s018lbl.pdf FDA.gov]</ref> | ||
| Line 51: | Line 51: | ||
* Oral contraceptives: Decreased contraceptive efficacy and increased breakthrough bleeding should be considered, especially at doses greater than 200 mg/day . | * Oral contraceptives: Decreased contraceptive efficacy and increased breakthrough bleeding should be considered, especially at doses greater than 200 mg/day . | ||
* Metformin is contraindicated with metabolic acidosis, an effect of topiramate. | * [[Metformin]] is contraindicated with [[metabolic acidosis]], an effect of topiramate. | ||
* Lithium levels should be monitored when coadministered with high-dose topiramate. | * [[Lithium]] levels should be monitored when coadministered with high-dose topiramate. | ||
* Other carbonic anhydrase inhibitors: Monitor the patient for the appearance or worsening of metabolic acidosis | * Other carbonic anhydrase inhibitors: Monitor the patient for the appearance or worsening of [[metabolic acidosis]].<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TOPIRAMATE (TOPIRAMATE ) TABLET, FILM COATED [AUROBINDO PHARMA LIMITED] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=32b48ea0-a215-43b8-83b4-a5435a686d68 | publisher = | date = | accessdate = 6 February 2014 }}</ref> | ||
==References== | ==References== | ||
Revision as of 03:12, 7 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
For patient information, click here.
Drug Interactions
In vitro studies indicate that topiramate does not inhibit enzyme activity for CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4/5 isozymes. In vitro studies indicate that topiramate is a mild inhibitor of CYP2C19 and a mild inducer of CYP3A4. Drug interactions with some antiepileptic drugs, CNS depressants and oral contraceptives are described here. For other drug interactions, please refer to Clinical Pharmacology (12.3).
Antiepileptic Drugs
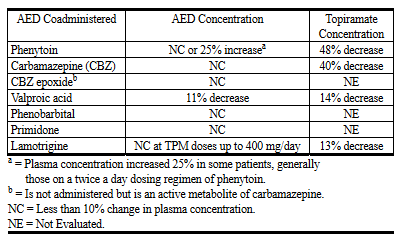
Potential interactions between topiramate and standard AEDs were assessed in controlled clinical pharmacokinetic studies in patients with epilepsy. Concomitant administration of phenytoin or carbamazepine with topiramate decreased plasma concentrations of topiramate by 48% and 40%, respectively when compared to topiramate given alone [see Clinical Pharmacology (12.3)].
Concomitant administration of valproic acid and topiramate
has been associated with hyperammonemia with and without encephalopathy. Concomitant administration of topiramate with valproic acid has also been associated with hypothermia (with and without hyperammonemia) in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see Warnings and Precautions (5.9), (5.11) or Clinical Pharmacology (12.3)].
Concomitant administration of valproic acid and topiramate
Has been associated with hyperammonemia with and without encephalopathy. Concomitant administration of topiramate with valproic acid has also been associated with hypothermia (with and without hyperammonemia) in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see Warnings and Precautions (5.9), (5.11) or Clinical Pharmacology (12.3)].
CNS Depressants
Concomitant administration of topiramate and alcohol or other CNS depressant drugs has not been evaluated in clinical studies. Because of the potential of topiramate to cause CNS depression, as well as other cognitive and/or neuropsychiatric adverse reactions, topiramate should be used with extreme caution if used in combination with alcohol and other CNS depressants.
Oral Contraceptives
Exposure to ethinyl estradiol was statistically significantly decreased at doses of 200, 400, and 800 mg/day (18%, 21%, and 30%, respectively) when topiramate was given as adjunctive therapy in patients taking valproic acid. However, norethindrone exposure was not significantly affected. In another pharmacokinetic interaction study in healthy volunteers with a concomitantly administered combination oral contraceptive product containing 1 mg norethindrone (NET) plus 35 mcg ethinyl estradiol (EE), topiramate, given in the absence of other medications at doses of 50 to 200 mg/day, was not associated with statistically significant changes in mean exposure (AUC) to either component of the oral contraceptive. The possibility of decreased contraceptive efficacy and increased breakthrough bleeding should be considered in patients taking combination oral contraceptive products with topiramate. Patients taking estrogen-containing contraceptives should be asked to report any change in their bleeding patterns. Contraceptive efficacy can be decreased even in the absence of breakthrough bleeding [see Clinical Pharmacology (12.3)].
Metformin
Topiramate treatment can frequently cause metabolic acidosis, a condition for which the use of metformin is contraindicated [see Clinical Pharmacology.
Lithium
In patients, lithium levels were unaffected during treatment with topiramate at doses of 200 mg/day; however, there was an observed increase in systemic exposure of lithium (27% for Cmax and 26% for AUC) following topiramate doses of up to 600 mg/day. Lithium levels should be monitored when coadministered with high-dose topiramate [see Clinical Pharmacology.
Other Carbonic Anhydrase Inhibitors
Concomitant use of topiramate, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor (e.g., zonisamide, acetazolamide, or dichlorphenamide) may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation. Therefore, if topiramate is given concomitantly with another carbonic anhydrase inhibitor, the patient should be monitored for the appearance or worsening of metabolic acidosis.
- As topiramate inhibits carbonic anhydrase, the concomitant use of other inhibitors of carbonic anhydrase (e.g. acetazolamide) may lead to an increased risk of renal stones.
- Enzyme inductors (e.g. carbamazepine) : The elimination of topiramate may be increased, possibly requiring dose escalations of topiramate.
- Phenytoin : Topiramate may increase the plasma-levels of phenytoin.
- Topiramate itself is a weak inhibitor of CYP2C19 and induces CYP 3A4. Under topiramate a decrease of plasma-levels of estrogens (e.g. 'the pill') and digoxin have been noted.
- Alcohol may cause increased sedation or drowsiness, and increase the risk of having a seizure.
- As listed in the 06/29/2005 label posted at the Drugs@FDA website page 14,'conditions or therapies that predispose to acidosis may be additive to the bicarbonate lowering effects of Topiramate'. Absent from this label is any direct discussion of narcotic (drugs known to promote respiratory acidosis) interactions. This discussion on page 14 is under the topic of Metabolic Acidosis, and is not repeated under the topic of interactions.[1]
 |
- Oral contraceptives: Decreased contraceptive efficacy and increased breakthrough bleeding should be considered, especially at doses greater than 200 mg/day .
- Metformin is contraindicated with metabolic acidosis, an effect of topiramate.
- Lithium levels should be monitored when coadministered with high-dose topiramate.
- Other carbonic anhydrase inhibitors: Monitor the patient for the appearance or worsening of metabolic acidosis.[2]
References
- ↑ [Shttp://www.fda.gov/cder/foi/label/2005/020505s018lbl.pdf FDA.gov]
- ↑ "TOPIRAMATE (TOPIRAMATE ) TABLET, FILM COATED [AUROBINDO PHARMA LIMITED]". Retrieved 6 February 2014.