Psoriasis pathophysiology: Difference between revisions
No edit summary |
|||
| (42 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Psoriasis}} | {{Psoriasis}} | ||
{{CMG}} | {{CMG}}; {{AE}} {{HK}} | ||
==Overview== | ==Overview== | ||
Psoriasis is an immune-mediated disease with genetic predisposition, | Psoriasis is an [[immune-mediated disease]] with a [[genetic predisposition]], though no specific [[Immunogenicity|immunogen]] has been implicated in the development of psoriasis. The [[pathophysiology]] of psoriasis consists of interactions between [[Cytokine|cytokines]], [[Dendritic cell|dendritic cells]], and [[T lymphocytes]] (particularly [[Th1]] and [[Th17]]).<ref name="pmid24655295">{{cite journal |vauthors=Lowes MA, Suárez-Fariñas M, Krueger JG |title=Immunology of psoriasis |journal=Annu. Rev. Immunol. |volume=32 |issue= |pages=227–55 |year=2014 |pmid=24655295 |pmc=4229247 |doi=10.1146/annurev-immunol-032713-120225 |url=}}</ref> Common triggers of psoriasis include injury to the [[skin]], [[Physical trauma|trauma]], [[infection]], and [[Medication|medications]]. [[T cell|T cells]] play a key role in the pathogenesis of psoriasis via the production of [[Proinflammatory|pro-inflammatory]] [[cytokines]]. Certain [[genes]] increase the likelihood of developing psoriasis; the first [[gene]] that was discovered to be linked to the development of psoriasis was [[Human leukocyte antigen|HLA-Cw6]], which is located at PSORS1 at [[chromosomal]] position 6p21.3. Microscopically, [[skin]] affected by psoriasis displays parakeratosis, acanthosis, [[hyperkeratosis]], Kogoj pustules, and Munro's [[Abscesses|microabscesses]]. The red appearance of psoriatic lesions is due to [[Vasodilation|dilated blood vessels]] in the [[skin]]. | ||
==Pathophysiology== | ==Pathophysiology== | ||
There are two main hypotheses about the development of psoriasis. The first hypothesis considers psoriasis as primarily a disorder of excessive growth and reproduction of [[skin]] [[cells]], in which psoriasis is a manifestation of a fault of the [[epidermis (skin)|epidermis]] and its [[keratinocytes]]. The second hypothesis views the [[disease]] as an [[immune-mediated disease|immune-mediated disorder]] in which the excessive reproduction of skin cells is secondary to factors produced by the [[immune system]]. [[T cell]]s (which normally help protect the body against [[infection]]) become active, migrate to the [[dermis]], and trigger the release of [[cytokines]] ([[tumor necrosis factor-alpha]] [TNFα] in particular), which cause [[inflammation]] and the rapid production of skin cells. It is not known what initiates the activation of the [[T cells]]. | |||
==Pathogenesis== | |||
=== Cutaneous psoriasis === | |||
=== | The [[Immune mediated inflammatory diseases|immune-mediated]] nature of psoriasis has been demonstrated by multiple studies in which various treatments that target and inhibit the proliferation and activation of [[T cell|T cells]] have been used successfully.<ref name="pmid10225967">{{cite journal |vauthors=Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S |title=CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris |journal=J. Clin. Invest. |volume=103 |issue=9 |pages=1243–52 |year=1999 |pmid=10225967 |pmc=408469 |doi=10.1172/JCI5857 |url=}}</ref><ref name="pmid15671179">{{cite journal |vauthors=Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Cardinale I, Khatcherian A, Novitskaya I, Wittkowski KM, Krueger JG |title=Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=102 |issue=6 |pages=2075–80 |year=2005 |pmid=15671179 |pmc=545584 |doi=10.1073/pnas.0409569102 |url=}}</ref><ref name="pmid17555598">{{cite journal |vauthors=Chamian F, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Cardinale I, Khatcherian A, Novitskaya I, Wittkowski KM, Krueger JG, Lowes MA |title=Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis |journal=J Transl Med |volume=5 |issue= |pages=27 |year=2007 |pmid=17555598 |pmc=1906741 |doi=10.1186/1479-5876-5-27 |url=}}</ref> | ||
==== Triggers ==== | |||
*Psoriasis can be triggered by many factors, including:<ref name="pmid24655295">{{cite journal |vauthors=Lowes MA, Suárez-Fariñas M, Krueger JG |title=Immunology of psoriasis |journal=Annu. Rev. Immunol. |volume=32 |issue= |pages=227–55 |year=2014 |pmid=24655295 |pmc=4229247 |doi=10.1146/annurev-immunol-032713-120225 |url=}}</ref> | *Psoriasis can be triggered by many factors, including:<ref name="pmid24655295">{{cite journal |vauthors=Lowes MA, Suárez-Fariñas M, Krueger JG |title=Immunology of psoriasis |journal=Annu. Rev. Immunol. |volume=32 |issue= |pages=227–55 |year=2014 |pmid=24655295 |pmc=4229247 |doi=10.1146/annurev-immunol-032713-120225 |url=}}</ref> | ||
**Injury | **[[Genetic]] susceptibility | ||
**Trauma (termed the Koebner effect) | **[[Injury]] | ||
**Infection | **[[Physical trauma|Trauma]] (termed the [[Koebner phenomenon|"Koebner effect]]") | ||
**[[Infection]] | |||
**Medications | **Medications | ||
**Topical biological response modifier imiquimod (a TLR7 agonist) | **Topical biological response modifier [[imiquimod]] (a [[TLR 7|TLR7]] agonist)<ref name="pmid19380832">{{cite journal |vauthors=van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E |title=Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis |journal=J. Immunol. |volume=182 |issue=9 |pages=5836–45 |year=2009 |pmid=19380832 |doi=10.4049/jimmunol.0802999 |url=}}</ref> | ||
==== Role of Dendritic Cells ==== | |||
*[[Tumor necrosis factor-alpha|TNFα]] and [[nitric oxide]] [[synthase]] [[isoform]] (iNOS)-producing [[inflammatory]] [[Dendritic cell|dendritic cells]] [[Infiltration (medical)|infiltrate]] [[psoriatic]] [[skin]]. These [[Dendritic cell|dendritic cells]] have the ability to activate [[T-cells]] to differentiate into [[Th1]] and [[Th17]] cell lines.<ref name="pmid8040262">{{cite journal |vauthors=Nestle FO, Turka LA, Nickoloff BJ |title=Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines |journal=J. Clin. Invest. |volume=94 |issue=1 |pages=202–9 |year=1994 |pmid=8040262 |pmc=296298 |doi=10.1172/JCI117308 |url=}}</ref><ref name="pmid26215033">{{cite journal |vauthors=Harden JL, Krueger JG, Bowcock AM |title=The immunogenetics of Psoriasis: A comprehensive review |journal=J. Autoimmun. |volume=64 |issue= |pages=66–73 |year=2015 |pmid=26215033 |pmc=4628849 |doi=10.1016/j.jaut.2015.07.008 |url=}}</ref><ref name="pmid19322214">{{cite journal |vauthors=Di Cesare A, Di Meglio P, Nestle FO |title=The IL-23/Th17 axis in the immunopathogenesis of psoriasis |journal=J. Invest. Dermatol. |volume=129 |issue=6 |pages=1339–50 |year=2009 |pmid=19322214 |doi=10.1038/jid.2009.59 |url=}}</ref><ref name="pmid16380428">{{cite journal |vauthors=Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Wittkowski KM, Papp K, Garovoy M, Dummer W, Steinman RM, Krueger JG |title=Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=102 |issue=52 |pages=19057–62 |year=2005 |pmid=16380428 |pmc=1323218 |doi=10.1073/pnas.0509736102 |url=}}</ref> | |||
*[[Macrophage|Macrophages]] and [[Immune cells|innate immune cells]], as well as an increased number of [[endothelial cells]] ([[angiogenesis]]), have also been implicated in the [[pathogenesis]] of psoriasis. | |||
*[[Inflammatory]] [[myeloid dendritic cells]] release IL-23 and [[Interleukin 12|IL-12]] to activate [[Interleukin 17|IL-17]]-producing [[T cells]], [[Th1 cell|Th1 cells]], and Th22 cells to produce numerous psoriatic [[cytokines]], which include [[Interleukin 17|IL-17]], [[Interferon-gamma|IFN-γ]], [[Tumor necrosis factor-alpha|TNF]], and IL-22. These [[Cytokine|cytokines]] mediate effects on [[Keratinocyte|keratinocytes]] to augment psoriatic [[inflammation]]. | |||
*Injury to the skin causes [[cell death]] and the production of the [[Cathelicidin]] LL-37 (anti-microbial protein LL37) by [[Keratinocyte|keratinocytes]]. DNA/LL37 complexes bind to intracellular [[Toll-like receptor|toll-like receptor 9]] ([[Toll-like receptor|TLR9]]) in [[Dendritic cell|dendritic cells]] ([[Dendritic cell|DCs]]), which causes activation and production of type I [[interferons]] [[IFN-α]] and -β. | |||
* Activation and differentiation of T cell subsets are maintained by IL-12 and IL-23, which appear to be produced mainly from myeloid DC subsets in the skin. Psoriasis lesions contain T cells that produce IFN-γ, IL-17, and IL-22, produced by Th1, Th17, and Th22, respectively. There are also CD8+ T cell populations that make the same types of cytokines. | *[[Myeloid dendritic cells|Myeloid DCs]] can be activated by the LL37/RNA complex, as well as by type 1 [[interferons]], leading to [[T cell]] proliferation, activation, and the production of [[Cytokine|cytokines]] found in psoriasis. | ||
*In response to these cytokines, keratinocytes in the skin upregulate the production of mRNAs, which lead to the formation of many pro-inflammatory products. | |||
*Chemokines produced by keratinocytes | ==== Role of T Cells ==== | ||
* | * Activation and [[differentiation]] of [[T cell]] subsets are maintained by [[Interleukin 12|IL-12]] and IL-23, which appear to be produced mainly from [[Myeloid dendritic cells|myeloid DC]] subsets in the [[skin]]. Psoriasis [[lesions]] contain [[T cell|T cells]] that produce [[Interferon-gamma|IFN-γ]], [[Interleukin 17|IL-17]], and IL-22, produced by [[Th1 cell|Th1]], [[T helper 17 cell|Th17]], and Th22, respectively. There are also [[CD8+ T cells|CD8+ T cell]] populations that make the same types of [[Cytokine|cytokines]]. | ||
*Genes in the NF-κB pathway | *In response to these [[Cytokine|cytokines]], [[Keratinocyte|keratinocytes]] in the skin upregulate the production of [[Messenger RNA|mRNAs]], which lead to the formation of many pro-inflammatory products. | ||
*[[Chemokine|Chemokines]] produced by [[Keratinocyte|keratinocytes]] cause the migration of many [[leukocyte]] subsets (e.g., [[Dendritic cell|dendritic cells]] ([[Dendritic cells|DCs]]) and [[neutrophils]]). | |||
*The [[innate immune system]] is also thought to play an important role in the development of psoriasis. | |||
==== NF-κB Pathway ==== | |||
*Genes in the [[NF-κB]] pathway are associated with psoriasis.<ref name="pmid23219896">{{cite journal |vauthors=Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF |title=NF-κB: an essential transcription factor in psoriasis |journal=J. Dermatol. Sci. |volume=69 |issue=2 |pages=89–94 |year=2013 |pmid=23219896 |doi=10.1016/j.jdermsci.2012.11.002 |url=}}</ref><ref name="pmid15955104">{{cite journal |vauthors=Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, Gottlieb AB |title=Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept |journal=J. Invest. Dermatol. |volume=124 |issue=6 |pages=1275–83 |year=2005 |pmid=15955104 |doi=10.1111/j.0022-202X.2005.23735.x |url=}}</ref> | |||
*[[IκBα|IκB]] is an inhibitor of the [[NF-κB]] pathway. After the initiation of [[NF-κB]] signaling by [[cytokines]] such as [[Tumor necrosis factor-alpha|TNF-alpha]], [[IκBα|IκB]] is [[Phosphorylation|phosphorylated]] by [[IκB kinase]] (IKK) and subsequently targeted for proteosomal degradation. The degradation of [[IκBα|IκB]] releases [[NF-κB]] for translocation to the [[Cell nucleus|nucleus]], consequently leading to [[gene expression]] for pro-inflammatory products.<ref name="pmid17183360">{{cite journal |vauthors=Perkins ND |title=Integrating cell-signalling pathways with NF-kappaB and IKK function |journal=Nat. Rev. Mol. Cell Biol. |volume=8 |issue=1 |pages=49–62 |year=2007 |pmid=17183360 |doi=10.1038/nrm2083 |url=}}</ref> | |||
=== Psoriatic arthritis === | |||
The pathogenesis of psoriatic arthritis (PsA) involves the following events:<ref name="pmid12639988">{{cite journal |vauthors=Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM |title=Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis |journal=J. Clin. Invest. |volume=111 |issue=6 |pages=821–31 |year=2003 |pmid=12639988 |pmc=153764 |doi=10.1172/JCI16069 |url=}}</ref> | |||
* In [[joints]] there is a prominent [[lymphocytic]] infiltrate, limited to the [[dermal papillae]] in [[skin]] and to the underlying [[stroma]]. | |||
* [[T lymphocytes]], particularly [[CD4+ cell|CD4 cells]], are the most common [[inflammatory]] [[Cell (biology)|cells]] in the [[skin]] and [[joints]], with a [[CD4]]/[[CD8]] ratio of 2:1. | |||
* High levels of [[Tumor necrosis factor-alpha|tumor necrosis factor alpha]] ([[TNF]]), [[Interleukin 8|IL-8]], [[Interleukin 6|IL-6]], [[IL-1]], [[Interleukin 10|IL-10]], and [[Matrix metalloproteinase|matrix metalloproteinases]] are present in the joint fluid of patients with early PsA. | |||
* [[Collagenase]] mediated degradation of [[cartilage]] [[collagen]] begins in early phases of the disease and may be the result of the [[proteases]] produced as a result of above mentioned [[cytokines]]. | |||
==== Osteoclast mediated joint destruction ==== | |||
* The elevated levels of [[Tumor necrosis factors|TNF]] leads to a high number of [[Osteoclast|osteoclast precursor cells]] circulating in the [[blood]]. | |||
* Osteoclast precursors migrate to the [[joint]] where they encounter increased expression of receptor activator of nuclear factor kappa B ligand ( [[NF-κB]]), which favors the [[differentiation]] and activation of [[Osteoclast|osteoclasts]]. | |||
* [[Osteoclast|Osteoclasts]] eventually lead to the [[joint]] destruction seen in psoriatic arthritis. | |||
==Genetics== | |||
*The first [[gene]] that was discovered to be linked to the development of psoriasis was [[Human leukocyte antigen|HLA-Cw6]], which is located at PSORS1 at [[chromosomal]] position 6p21.3.<ref name="pmid16124855">{{cite journal |vauthors=Bowcock AM |title=The genetics of psoriasis and autoimmunity |journal=Annu Rev Genomics Hum Genet |volume=6 |issue= |pages=93–122 |year=2005 |pmid=16124855 |doi=10.1146/annurev.genom.6.080604.162324 |url=}}</ref> | |||
*HLA-Cw6 codes for a [[Major histocompatibility complex|major histocompatibility complex I]] ([[Major histocompatibility complex|MHCI]]) [[allele]]. | |||
*Presentation of intracellular [[proteins]] by [[Major histocompatibility complex|MHCI]] leads to the activation of [[Cytotoxic T cell|cytotoxic T cells]] ([[CD8+ T cells]]). This [[T-cell]] priming plays a key role in the pathogenesis of psoriasis. | |||
*The ERAP1 [[Locus (genetics)|loci]] has also been shown to be linked to the development of psoriasis and is found in individuals carrying the HLA-Cw6 [[mutation]].<ref name="pmid20953190">{{cite journal |vauthors=Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, Barton A, Band G, Bellenguez C, Bergboer JG, Blackwell JM, Bramon E, Bumpstead SJ, Casas JP, Cork MJ, Corvin A, Deloukas P, Dilthey A, Duncanson A, Edkins S, Estivill X, Fitzgerald O, Freeman C, Giardina E, Gray E, Hofer A, Hüffmeier U, Hunt SE, Irvine AD, Jankowski J, Kirby B, Langford C, Lascorz J, Leman J, Leslie S, Mallbris L, Markus HS, Mathew CG, McLean WH, McManus R, Mössner R, Moutsianas L, Naluai AT, Nestle FO, Novelli G, Onoufriadis A, Palmer CN, Perricone C, Pirinen M, Plomin R, Potter SC, Pujol RM, Rautanen A, Riveira-Munoz E, Ryan AW, Salmhofer W, Samuelsson L, Sawcer SJ, Schalkwijk J, Smith CH, Ståhle M, Su Z, Tazi-Ahnini R, Traupe H, Viswanathan AC, Warren RB, Weger W, Wolk K, Wood N, Worthington J, Young HS, Zeeuwen PL, Hayday A, Burden AD, Griffiths CE, Kere J, Reis A, McVean G, Evans DM, Brown MA, Barker JN, Peltonen L, Donnelly P, Trembath RC |title=A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1 |journal=Nat. Genet. |volume=42 |issue=11 |pages=985–90 |year=2010 |pmid=20953190 |pmc=3749730 |doi=10.1038/ng.694 |url=}}</ref> | |||
*MICA ([[MHC class I]] [[polypeptide]]-related sequence A) is also associated with psoriasis.<ref name="pmid25087609">{{cite journal |vauthors=Okada Y, Han B, Tsoi LC, Stuart PE, Ellinghaus E, Tejasvi T, Chandran V, Pellett F, Pollock R, Bowcock AM, Krueger GG, Weichenthal M, Voorhees JJ, Rahman P, Gregersen PK, Franke A, Nair RP, Abecasis GR, Gladman DD, Elder JT, de Bakker PI, Raychaudhuri S |title=Fine mapping major histocompatibility complex associations in psoriasis and its clinical subtypes |journal=Am. J. Hum. Genet. |volume=95 |issue=2 |pages=162–72 |year=2014 |pmid=25087609 |pmc=4129407 |doi=10.1016/j.ajhg.2014.07.002 |url=}}</ref> | |||
*The [[gene]] DDX58 (DEAD (Asp-Glu-Ala-Asp) box polypeptide 58), which encodes the [[protein]] RIG-I, and [[IFIH1]], which encodes the protein MDA5, have also been implicated in the [[pathogenesis]] of psoriasis.<ref name="pmid23143594">{{cite journal |vauthors=Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, Kang HM, Allen MH, McManus R, Novelli G, Samuelsson L, Schalkwijk J, Ståhle M, Burden AD, Smith CH, Cork MJ, Estivill X, Bowcock AM, Krueger GG, Weger W, Worthington J, Tazi-Ahnini R, Nestle FO, Hayday A, Hoffmann P, Winkelmann J, Wijmenga C, Langford C, Edkins S, Andrews R, Blackburn H, Strange A, Band G, Pearson RD, Vukcevic D, Spencer CC, Deloukas P, Mrowietz U, Schreiber S, Weidinger S, Koks S, Kingo K, Esko T, Metspalu A, Lim HW, Voorhees JJ, Weichenthal M, Wichmann HE, Chandran V, Rosen CF, Rahman P, Gladman DD, Griffiths CE, Reis A, Kere J, Nair RP, Franke A, Barker JN, Abecasis GR, Elder JT, Trembath RC |title=Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity |journal=Nat. Genet. |volume=44 |issue=12 |pages=1341–8 |year=2012 |pmid=23143594 |pmc=3510312 |doi=10.1038/ng.2467 |url=}}</ref> | |||
*Activation of RIG-I or MDA5 results in [[gene expression]] changes mainly mediated by [[NF-κB|NF-κB pathway]].<ref name="pmid25101084">{{cite journal |vauthors=Reikine S, Nguyen JB, Modis Y |title=Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5 |journal=Front Immunol |volume=5 |issue= |pages=342 |year=2014 |pmid=25101084 |pmc=4107945 |doi=10.3389/fimmu.2014.00342 |url=}}</ref> | |||
*Two [[Cytokine|cytokines]] known to be significant mediators of psoriasis, [[Tumor necrosis factor-alpha|TNFα]] and/or [[Interferon gamma|IFNγ]], can increase expression of RIG-I and MDA5 expression in [[keratinocytes]].<ref name="pmid17182220">{{cite journal |vauthors=Kitamura H, Matsuzaki Y, Kimura K, Nakano H, Imaizumi T, Satoh K, Hanada K |title=Cytokine modulation of retinoic acid-inducible gene-I (RIG-I) expression in human epidermal keratinocytes |journal=J. Dermatol. Sci. |volume=45 |issue=2 |pages=127–34 |year=2007 |pmid=17182220 |doi=10.1016/j.jdermsci.2006.11.003 |url=}}</ref> | |||
*[[Gene|Genes]] such as CARD14 and ZC3H12C are found to not only potentially alter [[immune cell]] or [[keratinocyte]] behavior, but also the biology of the [[vasculature]]. These [[Mutation|mutations]] might, therefore, play a part in the [[cardiovascular]] comorbidities linked to psoriasis.<ref name="pmid22521418">{{cite journal |vauthors=Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, Ryan C, Duan S, Helms CA, Liu Y, Chen Y, McBride AA, Hwu WL, Wu JY, Chen YT, Menter A, Goldbach-Mansky R, Lowes MA, Bowcock AM |title=PSORS2 is due to mutations in CARD14 |journal=Am. J. Hum. Genet. |volume=90 |issue=5 |pages=784–95 |year=2012 |pmid=22521418 |pmc=3376640 |doi=10.1016/j.ajhg.2012.03.012 |url=}}</ref><ref name="pmid22521419">{{cite journal |vauthors=Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, Duffin KC, Stuart PE, Goldgar D, Hayashi G, Olfson EH, Feng BJ, Pullinger CR, Kane JP, Wise CA, Goldbach-Mansky R, Lowes MA, Peddle L, Chandran V, Liao W, Rahman P, Krueger GG, Gladman D, Elder JT, Menter A, Bowcock AM |title=Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis |journal=Am. J. Hum. Genet. |volume=90 |issue=5 |pages=796–808 |year=2012 |pmid=22521419 |pmc=3376540 |doi=10.1016/j.ajhg.2012.03.013 |url=}}</ref> | |||
*Approximately one-third of people with psoriasis report a [[family history (medicine)|family history]] of the disease. Studies of [[twin|monozygotic twins]] suggest a 70% chance of a twin developing psoriasis if the other twin has psoriasis. The [[concordance (genetics)|concordance]] is 20% for [[twin|dizygotic twins]]. These findings suggest both a [[genetic predisposition]] and an environmental component in the development of psoriasis.<ref name="Krueger">{{cite journal |author=Krueger G, Ellis CN |title=Psoriasis--recent advances in understanding its pathogenesis and treatment |journal=J. Am. Acad. Dermatol. |volume=53 |issue=1 Suppl 1 |pages=S94-100 |year=2005 |pmid=15968269 |doi=10.1016/j.jaad.2005.04.035}}</ref> | |||
[[Image:Psorpatho.gif|500px|center|frame|'''Pathogenesis of psoriasis''']] | |||
== Associated conditions == | |||
Psoriasis is associated with the following conditions:<ref name="pmid20415823">{{cite journal |vauthors=Gisondi P, Del Giglio M, Cozzi A, Girolomoni G |title=Psoriasis, the liver, and the gastrointestinal tract |journal=Dermatol Ther |volume=23 |issue=2 |pages=155–9 |year=2010 |pmid=20415823 |doi=10.1111/j.1529-8019.2010.01310.x |url=}}</ref><ref name="pmid19380659">{{cite journal |vauthors=Qureshi AA, Choi HK, Setty AR, Curhan GC |title=Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses |journal=Arch Dermatol |volume=145 |issue=4 |pages=379–82 |year=2009 |pmid=19380659 |pmc=2849106 |doi=10.1001/archdermatol.2009.48 |url=}}</ref><ref name="pmid23197207">{{cite journal |vauthors=Fraga NA, Oliveira Mde F, Follador I, Rocha Bde O, Rêgo VR |title=Psoriasis and uveitis: a literature review |journal=An Bras Dermatol |volume=87 |issue=6 |pages=877–83 |year=2012 |pmid=23197207 |pmc=3699904 |doi= |url=}}</ref><ref name="pmid17465464">{{cite journal |vauthors=Abenavoli L, Leggio L, Gasbarrini G, Addolorato G |title=Celiac disease and skin: psoriasis association |journal=World J. Gastroenterol. |volume=13 |issue=14 |pages=2138–9 |year=2007 |pmid=17465464 |pmc=4319141 |doi= |url=}}</ref><ref name="pmid26982772">{{cite journal |vauthors=Machado-Pinto J, Diniz Mdos S, Bavoso NC |title=Psoriasis: new comorbidities |journal=An Bras Dermatol |volume=91 |issue=1 |pages=8–14 |year=2016 |pmid=26982772 |pmc=4782640 |doi=10.1590/abd1806-4841.20164169 |url=}}</ref><ref name="pmid25848461">{{cite journal |vauthors=Ganzetti G, Campanati A, Offidani A |title=Non-alcoholic fatty liver disease and psoriasis: So far, so near |journal=World J Hepatol |volume=7 |issue=3 |pages=315–26 |year=2015 |pmid=25848461 |pmc=4381160 |doi=10.4254/wjh.v7.i3.315 |url=}}</ref><ref name="pmid26959083">{{cite journal |vauthors=Egeberg A, Mallbris L, Warren RB, Bachelez H, Gislason GH, Hansen PR, Skov L |title=Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study |journal=Br. J. Dermatol. |volume=175 |issue=3 |pages=487–92 |year=2016 |pmid=26959083 |doi=10.1111/bjd.14528 |url=}}</ref><ref name="pmid17911986">{{cite journal |vauthors=Passarini B, Infusino SD, Barbieri E, Varotti E, Gionchetti P, Rizzello F, Morselli C, Tambasco R, Campieri M |title=Cutaneous manifestations in inflammatory bowel diseases: eight cases of psoriasis induced by anti-tumor-necrosis-factor antibody therapy |journal=Dermatology (Basel) |volume=215 |issue=4 |pages=295–300 |year=2007 |pmid=17911986 |doi=10.1159/000107622 |url=}}</ref><ref name="pmid8076391">{{cite journal |vauthors=Kahn MF, Khan MA |title=The SAPHO syndrome |journal=Baillieres Clin Rheumatol |volume=8 |issue=2 |pages=333–62 |year=1994 |pmid=8076391 |doi= |url=}}</ref><ref name="pmid18637897">{{cite journal |vauthors=Dreiher J, Weitzman D, Shapiro J, Davidovici B, Cohen AD |title=Psoriasis and chronic obstructive pulmonary disease: a case-control study |journal=Br. J. Dermatol. |volume=159 |issue=4 |pages=956–60 |year=2008 |pmid=18637897 |doi=10.1111/j.1365-2133.2008.08749.x |url=}}</ref><ref name="pmid15891254">{{cite journal |vauthors=Behnam SM, Behnam SE, Koo JY |title=Smoking and psoriasis |journal=Skinmed |volume=4 |issue=3 |pages=174–6 |year=2005 |pmid=15891254 |doi= |url=}}</ref><ref name="pmid26386630">{{cite journal |vauthors=Egeberg A, Mallbris L, Hilmar Gislason G, Skov L, Riis Hansen P |title=Increased risk of migraine in patients with psoriasis: A Danish nationwide cohort study |journal=J. Am. Acad. Dermatol. |volume=73 |issue=5 |pages=829–35 |year=2015 |pmid=26386630 |doi=10.1016/j.jaad.2015.08.039 |url=}}</ref> | |||
* [[Depression]] | |||
* [[Psoriatic arthritis]] | |||
* [[Inflammatory bowel disease|Chronic inflammatory bowel disease]] | |||
* [[Non-alcoholic fatty liver disease]] | |||
* [[Celiac disease]] | |||
* [[Sensorineural hearing loss]] | |||
* [[Osteopenia]] and [[osteoarthritis]] | |||
* [[Diabetes mellitus|Diabetes]] | |||
* [[Hypertension]] | |||
* [[Conjunctivitis]] | |||
* [[Uveitis]] | |||
* [[Metabolic syndrome]] | |||
* [[SAPHO syndrome]] ([[synovitis]], [[acne]], [[pustulosis]], [[hyperostosis]], and [[osteitis]]) | |||
* [[Alcohol abuse]] | |||
* [[Smoking]] | |||
* [[Migraine]] | |||
== Gross pathology== | |||
*On gross inspection, psoriatic [[lesions]] have the following characteristics: | |||
**Red or salmon-colored [[Plaque|plaques]] | |||
**Well-defined borders | |||
**Silvery-white dry scale | |||
**Usually located on the [[Dorsal|extensor]] surfaces like [[elbows]], [[knees]], and [[scalp]], and in the [[Lumbosacral trunk|lumbosacral area]]<ref name="pmid24655295">{{cite journal |vauthors=Lowes MA, Suárez-Fariñas M, Krueger JG |title=Immunology of psoriasis |journal=Annu. Rev. Immunol. |volume=32 |issue= |pages=227–55 |year=2014 |pmid=24655295 |pmc=4229247 |doi=10.1146/annurev-immunol-032713-120225 |url=}}</ref> | |||
*The surface area of the body affected by psoriasis can be measured roughly as a percentage of body area using the palm to represent 1% of the body. One-third of patients present with at least 10 percent body involvement, which is referred to as moderate-to-severe psoriasis. | |||
[[Image:Trunkpsor.jpg|100px|left|frame|'''Psoriasis gross examination''', courtesy of regionalderm.com]] | |||
<br style="clear:left"> | |||
== Microscopic pathology== | |||
=== Cutaneous psoriasis === | |||
*The [[Epidermis (skin)|epidermis]] is greatly thickened ([[Acanthosis nigricans|acanthosis]]) as the [[Keratinocyte|keratinocytes]] migrate through the [[Epidermis (skin)|epidermis]] over 4–5 days.<ref name="pmid24655295">{{cite journal |vauthors=Lowes MA, Suárez-Fariñas M, Krueger JG |title=Immunology of psoriasis |journal=Annu. Rev. Immunol. |volume=32 |issue= |pages=227–55 |year=2014 |pmid=24655295 |pmc=4229247 |doi=10.1146/annurev-immunol-032713-120225 |url=}}</ref> | |||
*There is a loss of the normal [[granular layer]] of the [[skin]] and a thickening of the [[stratum corneum]] ([[hyperkeratosis]]). | |||
*There is retention of [[Cell nucleus|nuclei]] in the upper layers and [[stratum corneum]] (parakeratosis).<ref name="pmid4323599">{{cite journal |vauthors=Zhdanov VM, Ershov FI, Bukrinskaia AG, Uryvaev LV |title=[Characteristics of viral RNA isolated from the polyribosomes of infected cells] |language=Russian |journal=Vopr. Virusol. |volume=15 |issue=4 |pages=467–73 |year=1970 |pmid=4323599 |doi= |url=}}</ref> | |||
*There is [[Neutrophil|neutrophilic]] [[Infiltration (medical)|infiltration]] in the [[Epidermis (skin)|epidermis]] and [[stratum corneum]] (Kogoj pustules and Munro's [[Abscesses|microabscesses]]).<ref name="pmid17828343">{{cite journal |vauthors=De Rosa G, Mignogna C |title=The histopathology of psoriasis |journal=Reumatismo |volume=59 Suppl 1 |issue= |pages=46–8 |year=2007 |pmid=17828343 |doi= |url=}}</ref> | |||
* | *In the [[dermis]], there are abundant [[mononuclear cells]] (mainly [[myeloid cells]] and [[T cell|T cells]]). | ||
*The red-colored appearance of psoriatic [[lesions]] is due to dilated [[Blood vessel|blood vessels]]. | |||
* | [[Image:Psoriasis_mid_power.jpg|400px|left|thumb|'''Psoriasis microscopic pathology''', courtesy ucsf.edu]] | ||
* | <br style="clear:left"> | ||
=== Psoriatic arthritis === | |||
<ref name= | * Psoriatic arthritis (PsA) affected [[synovial]] [[Tissue (biology)|tissue]] is characterized by a [[T cell|T-cell]] infiltrate with an increase in [[vascularity]].<ref name="urlPsoriasis clinical guideline | American Academy of Dermatology">{{cite web |url=https://www.aad.org/practicecenter/quality/clinical-guidelines/psoriasis |title=Psoriasis clinical guideline | American Academy of Dermatology |format= |work= |accessdate=}}</ref> | ||
* There is a reduction in [[macrophages]] compared with the [[synovial]] [[Tissue (biology)|tissue]] found in [[rheumatoid arthritis]]. | |||
==References== | ==References== | ||
Latest revision as of 07:03, 19 May 2020
|
Psoriasis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Psoriasis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Psoriasis pathophysiology |
|
Risk calculators and risk factors for Psoriasis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]
Overview
Psoriasis is an immune-mediated disease with a genetic predisposition, though no specific immunogen has been implicated in the development of psoriasis. The pathophysiology of psoriasis consists of interactions between cytokines, dendritic cells, and T lymphocytes (particularly Th1 and Th17).[1] Common triggers of psoriasis include injury to the skin, trauma, infection, and medications. T cells play a key role in the pathogenesis of psoriasis via the production of pro-inflammatory cytokines. Certain genes increase the likelihood of developing psoriasis; the first gene that was discovered to be linked to the development of psoriasis was HLA-Cw6, which is located at PSORS1 at chromosomal position 6p21.3. Microscopically, skin affected by psoriasis displays parakeratosis, acanthosis, hyperkeratosis, Kogoj pustules, and Munro's microabscesses. The red appearance of psoriatic lesions is due to dilated blood vessels in the skin.
Pathophysiology
There are two main hypotheses about the development of psoriasis. The first hypothesis considers psoriasis as primarily a disorder of excessive growth and reproduction of skin cells, in which psoriasis is a manifestation of a fault of the epidermis and its keratinocytes. The second hypothesis views the disease as an immune-mediated disorder in which the excessive reproduction of skin cells is secondary to factors produced by the immune system. T cells (which normally help protect the body against infection) become active, migrate to the dermis, and trigger the release of cytokines (tumor necrosis factor-alpha [TNFα] in particular), which cause inflammation and the rapid production of skin cells. It is not known what initiates the activation of the T cells.
Pathogenesis
Cutaneous psoriasis
The immune-mediated nature of psoriasis has been demonstrated by multiple studies in which various treatments that target and inhibit the proliferation and activation of T cells have been used successfully.[2][3][4]
Triggers
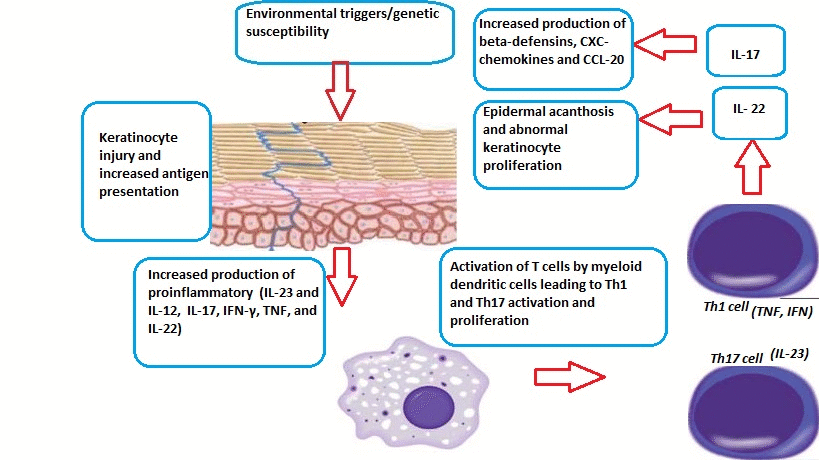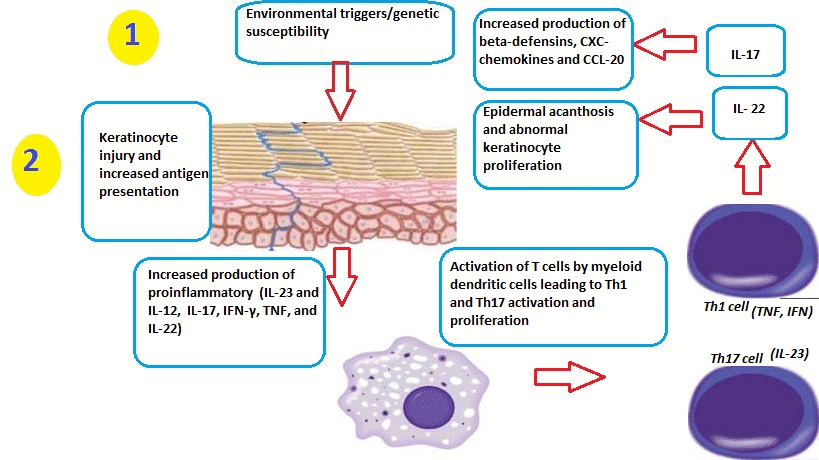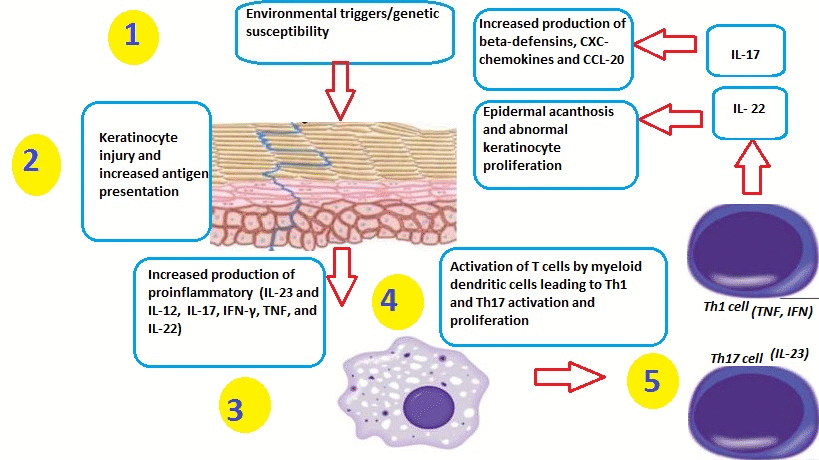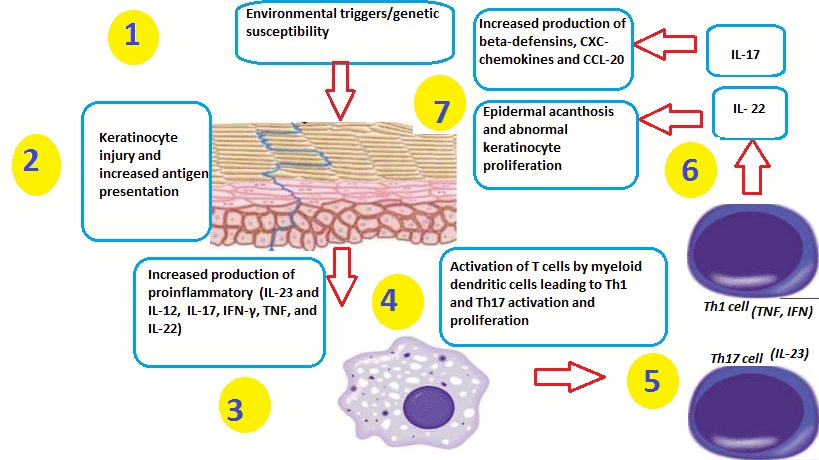
- Psoriasis can be triggered by many factors, including:[1]
Role of Dendritic Cells
- TNFα and nitric oxide synthase isoform (iNOS)-producing inflammatory dendritic cells infiltrate psoriatic skin. These dendritic cells have the ability to activate T-cells to differentiate into Th1 and Th17 cell lines.[6][7][8][9]
- Macrophages and innate immune cells, as well as an increased number of endothelial cells (angiogenesis), have also been implicated in the pathogenesis of psoriasis.
- Inflammatory myeloid dendritic cells release IL-23 and IL-12 to activate IL-17-producing T cells, Th1 cells, and Th22 cells to produce numerous psoriatic cytokines, which include IL-17, IFN-γ, TNF, and IL-22. These cytokines mediate effects on keratinocytes to augment psoriatic inflammation.
- Injury to the skin causes cell death and the production of the Cathelicidin LL-37 (anti-microbial protein LL37) by keratinocytes. DNA/LL37 complexes bind to intracellular toll-like receptor 9 (TLR9) in dendritic cells (DCs), which causes activation and production of type I interferons IFN-α and -β.
- Myeloid DCs can be activated by the LL37/RNA complex, as well as by type 1 interferons, leading to T cell proliferation, activation, and the production of cytokines found in psoriasis.
Role of T Cells
- Activation and differentiation of T cell subsets are maintained by IL-12 and IL-23, which appear to be produced mainly from myeloid DC subsets in the skin. Psoriasis lesions contain T cells that produce IFN-γ, IL-17, and IL-22, produced by Th1, Th17, and Th22, respectively. There are also CD8+ T cell populations that make the same types of cytokines.
- In response to these cytokines, keratinocytes in the skin upregulate the production of mRNAs, which lead to the formation of many pro-inflammatory products.
- Chemokines produced by keratinocytes cause the migration of many leukocyte subsets (e.g., dendritic cells (DCs) and neutrophils).
- The innate immune system is also thought to play an important role in the development of psoriasis.
NF-κB Pathway
- Genes in the NF-κB pathway are associated with psoriasis.[10][11]
- IκB is an inhibitor of the NF-κB pathway. After the initiation of NF-κB signaling by cytokines such as TNF-alpha, IκB is phosphorylated by IκB kinase (IKK) and subsequently targeted for proteosomal degradation. The degradation of IκB releases NF-κB for translocation to the nucleus, consequently leading to gene expression for pro-inflammatory products.[12]
Psoriatic arthritis
The pathogenesis of psoriatic arthritis (PsA) involves the following events:[13]
- In joints there is a prominent lymphocytic infiltrate, limited to the dermal papillae in skin and to the underlying stroma.
- T lymphocytes, particularly CD4 cells, are the most common inflammatory cells in the skin and joints, with a CD4/CD8 ratio of 2:1.
- High levels of tumor necrosis factor alpha (TNF), IL-8, IL-6, IL-1, IL-10, and matrix metalloproteinases are present in the joint fluid of patients with early PsA.
- Collagenase mediated degradation of cartilage collagen begins in early phases of the disease and may be the result of the proteases produced as a result of above mentioned cytokines.
Osteoclast mediated joint destruction
- The elevated levels of TNF leads to a high number of osteoclast precursor cells circulating in the blood.
- Osteoclast precursors migrate to the joint where they encounter increased expression of receptor activator of nuclear factor kappa B ligand ( NF-κB), which favors the differentiation and activation of osteoclasts.
- Osteoclasts eventually lead to the joint destruction seen in psoriatic arthritis.
Genetics
- The first gene that was discovered to be linked to the development of psoriasis was HLA-Cw6, which is located at PSORS1 at chromosomal position 6p21.3.[14]
- HLA-Cw6 codes for a major histocompatibility complex I (MHCI) allele.
- Presentation of intracellular proteins by MHCI leads to the activation of cytotoxic T cells (CD8+ T cells). This T-cell priming plays a key role in the pathogenesis of psoriasis.
- The ERAP1 loci has also been shown to be linked to the development of psoriasis and is found in individuals carrying the HLA-Cw6 mutation.[15]
- MICA (MHC class I polypeptide-related sequence A) is also associated with psoriasis.[16]
- The gene DDX58 (DEAD (Asp-Glu-Ala-Asp) box polypeptide 58), which encodes the protein RIG-I, and IFIH1, which encodes the protein MDA5, have also been implicated in the pathogenesis of psoriasis.[17]
- Activation of RIG-I or MDA5 results in gene expression changes mainly mediated by NF-κB pathway.[18]
- Two cytokines known to be significant mediators of psoriasis, TNFα and/or IFNγ, can increase expression of RIG-I and MDA5 expression in keratinocytes.[19]
- Genes such as CARD14 and ZC3H12C are found to not only potentially alter immune cell or keratinocyte behavior, but also the biology of the vasculature. These mutations might, therefore, play a part in the cardiovascular comorbidities linked to psoriasis.[20][21]
- Approximately one-third of people with psoriasis report a family history of the disease. Studies of monozygotic twins suggest a 70% chance of a twin developing psoriasis if the other twin has psoriasis. The concordance is 20% for dizygotic twins. These findings suggest both a genetic predisposition and an environmental component in the development of psoriasis.[22]
Associated conditions
Psoriasis is associated with the following conditions:[23][24][25][26][27][28][29][30][31][32][33][34]
- Depression
- Psoriatic arthritis
- Chronic inflammatory bowel disease
- Non-alcoholic fatty liver disease
- Celiac disease
- Sensorineural hearing loss
- Osteopenia and osteoarthritis
- Diabetes
- Hypertension
- Conjunctivitis
- Uveitis
- Metabolic syndrome
- SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis)
- Alcohol abuse
- Smoking
- Migraine
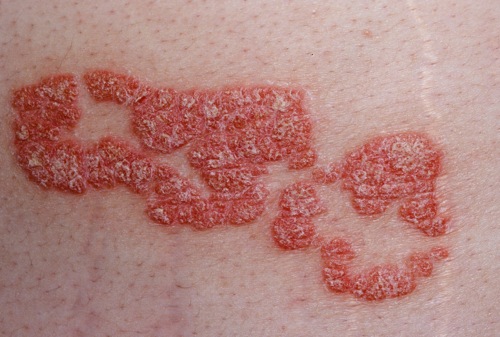
Gross pathology
- On gross inspection, psoriatic lesions have the following characteristics:
- The surface area of the body affected by psoriasis can be measured roughly as a percentage of body area using the palm to represent 1% of the body. One-third of patients present with at least 10 percent body involvement, which is referred to as moderate-to-severe psoriasis.
Microscopic pathology
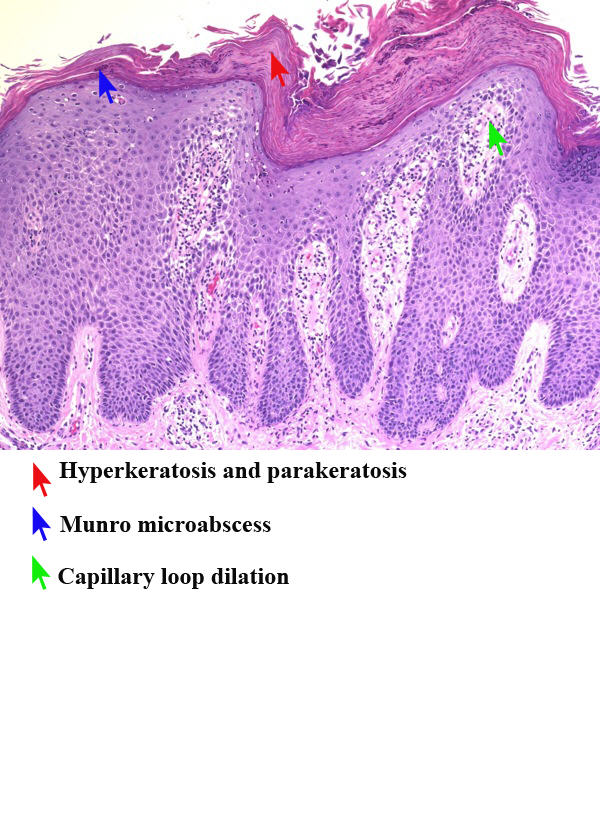
Cutaneous psoriasis
- The epidermis is greatly thickened (acanthosis) as the keratinocytes migrate through the epidermis over 4–5 days.[1]
- There is a loss of the normal granular layer of the skin and a thickening of the stratum corneum (hyperkeratosis).
- There is retention of nuclei in the upper layers and stratum corneum (parakeratosis).[35]
- There is neutrophilic infiltration in the epidermis and stratum corneum (Kogoj pustules and Munro's microabscesses).[36]
- In the dermis, there are abundant mononuclear cells (mainly myeloid cells and T cells).
- The red-colored appearance of psoriatic lesions is due to dilated blood vessels.
Psoriatic arthritis
- Psoriatic arthritis (PsA) affected synovial tissue is characterized by a T-cell infiltrate with an increase in vascularity.[37]
- There is a reduction in macrophages compared with the synovial tissue found in rheumatoid arthritis.
References
- ↑ 1.0 1.1 1.2 1.3 Lowes MA, Suárez-Fariñas M, Krueger JG (2014). "Immunology of psoriasis". Annu. Rev. Immunol. 32: 227–55. doi:10.1146/annurev-immunol-032713-120225. PMC 4229247. PMID 24655295.
- ↑ Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S (1999). "CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris". J. Clin. Invest. 103 (9): 1243–52. doi:10.1172/JCI5857. PMC 408469. PMID 10225967.
- ↑ Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Cardinale I, Khatcherian A, Novitskaya I, Wittkowski KM, Krueger JG (2005). "Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris". Proc. Natl. Acad. Sci. U.S.A. 102 (6): 2075–80. doi:10.1073/pnas.0409569102. PMC 545584. PMID 15671179.
- ↑ Chamian F, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Cardinale I, Khatcherian A, Novitskaya I, Wittkowski KM, Krueger JG, Lowes MA (2007). "Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis". J Transl Med. 5: 27. doi:10.1186/1479-5876-5-27. PMC 1906741. PMID 17555598.
- ↑ van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E (2009). "Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis". J. Immunol. 182 (9): 5836–45. doi:10.4049/jimmunol.0802999. PMID 19380832.
- ↑ Nestle FO, Turka LA, Nickoloff BJ (1994). "Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines". J. Clin. Invest. 94 (1): 202–9. doi:10.1172/JCI117308. PMC 296298. PMID 8040262.
- ↑ Harden JL, Krueger JG, Bowcock AM (2015). "The immunogenetics of Psoriasis: A comprehensive review". J. Autoimmun. 64: 66–73. doi:10.1016/j.jaut.2015.07.008. PMC 4628849. PMID 26215033.
- ↑ Di Cesare A, Di Meglio P, Nestle FO (2009). "The IL-23/Th17 axis in the immunopathogenesis of psoriasis". J. Invest. Dermatol. 129 (6): 1339–50. doi:10.1038/jid.2009.59. PMID 19322214.
- ↑ Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Wittkowski KM, Papp K, Garovoy M, Dummer W, Steinman RM, Krueger JG (2005). "Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a)". Proc. Natl. Acad. Sci. U.S.A. 102 (52): 19057–62. doi:10.1073/pnas.0509736102. PMC 1323218. PMID 16380428.
- ↑ Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF (2013). "NF-κB: an essential transcription factor in psoriasis". J. Dermatol. Sci. 69 (2): 89–94. doi:10.1016/j.jdermsci.2012.11.002. PMID 23219896.
- ↑ Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, Gottlieb AB (2005). "Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept". J. Invest. Dermatol. 124 (6): 1275–83. doi:10.1111/j.0022-202X.2005.23735.x. PMID 15955104.
- ↑ Perkins ND (2007). "Integrating cell-signalling pathways with NF-kappaB and IKK function". Nat. Rev. Mol. Cell Biol. 8 (1): 49–62. doi:10.1038/nrm2083. PMID 17183360.
- ↑ Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM (2003). "Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis". J. Clin. Invest. 111 (6): 821–31. doi:10.1172/JCI16069. PMC 153764. PMID 12639988.
- ↑ Bowcock AM (2005). "The genetics of psoriasis and autoimmunity". Annu Rev Genomics Hum Genet. 6: 93–122. doi:10.1146/annurev.genom.6.080604.162324. PMID 16124855.
- ↑ Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, Barton A, Band G, Bellenguez C, Bergboer JG, Blackwell JM, Bramon E, Bumpstead SJ, Casas JP, Cork MJ, Corvin A, Deloukas P, Dilthey A, Duncanson A, Edkins S, Estivill X, Fitzgerald O, Freeman C, Giardina E, Gray E, Hofer A, Hüffmeier U, Hunt SE, Irvine AD, Jankowski J, Kirby B, Langford C, Lascorz J, Leman J, Leslie S, Mallbris L, Markus HS, Mathew CG, McLean WH, McManus R, Mössner R, Moutsianas L, Naluai AT, Nestle FO, Novelli G, Onoufriadis A, Palmer CN, Perricone C, Pirinen M, Plomin R, Potter SC, Pujol RM, Rautanen A, Riveira-Munoz E, Ryan AW, Salmhofer W, Samuelsson L, Sawcer SJ, Schalkwijk J, Smith CH, Ståhle M, Su Z, Tazi-Ahnini R, Traupe H, Viswanathan AC, Warren RB, Weger W, Wolk K, Wood N, Worthington J, Young HS, Zeeuwen PL, Hayday A, Burden AD, Griffiths CE, Kere J, Reis A, McVean G, Evans DM, Brown MA, Barker JN, Peltonen L, Donnelly P, Trembath RC (2010). "A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1". Nat. Genet. 42 (11): 985–90. doi:10.1038/ng.694. PMC 3749730. PMID 20953190.
- ↑ Okada Y, Han B, Tsoi LC, Stuart PE, Ellinghaus E, Tejasvi T, Chandran V, Pellett F, Pollock R, Bowcock AM, Krueger GG, Weichenthal M, Voorhees JJ, Rahman P, Gregersen PK, Franke A, Nair RP, Abecasis GR, Gladman DD, Elder JT, de Bakker PI, Raychaudhuri S (2014). "Fine mapping major histocompatibility complex associations in psoriasis and its clinical subtypes". Am. J. Hum. Genet. 95 (2): 162–72. doi:10.1016/j.ajhg.2014.07.002. PMC 4129407. PMID 25087609.
- ↑ Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, Kang HM, Allen MH, McManus R, Novelli G, Samuelsson L, Schalkwijk J, Ståhle M, Burden AD, Smith CH, Cork MJ, Estivill X, Bowcock AM, Krueger GG, Weger W, Worthington J, Tazi-Ahnini R, Nestle FO, Hayday A, Hoffmann P, Winkelmann J, Wijmenga C, Langford C, Edkins S, Andrews R, Blackburn H, Strange A, Band G, Pearson RD, Vukcevic D, Spencer CC, Deloukas P, Mrowietz U, Schreiber S, Weidinger S, Koks S, Kingo K, Esko T, Metspalu A, Lim HW, Voorhees JJ, Weichenthal M, Wichmann HE, Chandran V, Rosen CF, Rahman P, Gladman DD, Griffiths CE, Reis A, Kere J, Nair RP, Franke A, Barker JN, Abecasis GR, Elder JT, Trembath RC (2012). "Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity". Nat. Genet. 44 (12): 1341–8. doi:10.1038/ng.2467. PMC 3510312. PMID 23143594.
- ↑ Reikine S, Nguyen JB, Modis Y (2014). "Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5". Front Immunol. 5: 342. doi:10.3389/fimmu.2014.00342. PMC 4107945. PMID 25101084.
- ↑ Kitamura H, Matsuzaki Y, Kimura K, Nakano H, Imaizumi T, Satoh K, Hanada K (2007). "Cytokine modulation of retinoic acid-inducible gene-I (RIG-I) expression in human epidermal keratinocytes". J. Dermatol. Sci. 45 (2): 127–34. doi:10.1016/j.jdermsci.2006.11.003. PMID 17182220.
- ↑ Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, Ryan C, Duan S, Helms CA, Liu Y, Chen Y, McBride AA, Hwu WL, Wu JY, Chen YT, Menter A, Goldbach-Mansky R, Lowes MA, Bowcock AM (2012). "PSORS2 is due to mutations in CARD14". Am. J. Hum. Genet. 90 (5): 784–95. doi:10.1016/j.ajhg.2012.03.012. PMC 3376640. PMID 22521418.
- ↑ Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, Duffin KC, Stuart PE, Goldgar D, Hayashi G, Olfson EH, Feng BJ, Pullinger CR, Kane JP, Wise CA, Goldbach-Mansky R, Lowes MA, Peddle L, Chandran V, Liao W, Rahman P, Krueger GG, Gladman D, Elder JT, Menter A, Bowcock AM (2012). "Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis". Am. J. Hum. Genet. 90 (5): 796–808. doi:10.1016/j.ajhg.2012.03.013. PMC 3376540. PMID 22521419.
- ↑ Krueger G, Ellis CN (2005). "Psoriasis--recent advances in understanding its pathogenesis and treatment". J. Am. Acad. Dermatol. 53 (1 Suppl 1): S94–100. doi:10.1016/j.jaad.2005.04.035. PMID 15968269.
- ↑ Gisondi P, Del Giglio M, Cozzi A, Girolomoni G (2010). "Psoriasis, the liver, and the gastrointestinal tract". Dermatol Ther. 23 (2): 155–9. doi:10.1111/j.1529-8019.2010.01310.x. PMID 20415823.
- ↑ Qureshi AA, Choi HK, Setty AR, Curhan GC (2009). "Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses". Arch Dermatol. 145 (4): 379–82. doi:10.1001/archdermatol.2009.48. PMC 2849106. PMID 19380659.
- ↑ Fraga NA, Oliveira Mde F, Follador I, Rocha Bde O, Rêgo VR (2012). "Psoriasis and uveitis: a literature review". An Bras Dermatol. 87 (6): 877–83. PMC 3699904. PMID 23197207.
- ↑ Abenavoli L, Leggio L, Gasbarrini G, Addolorato G (2007). "Celiac disease and skin: psoriasis association". World J. Gastroenterol. 13 (14): 2138–9. PMC 4319141. PMID 17465464.
- ↑ Machado-Pinto J, Diniz Mdos S, Bavoso NC (2016). "Psoriasis: new comorbidities". An Bras Dermatol. 91 (1): 8–14. doi:10.1590/abd1806-4841.20164169. PMC 4782640. PMID 26982772.
- ↑ Ganzetti G, Campanati A, Offidani A (2015). "Non-alcoholic fatty liver disease and psoriasis: So far, so near". World J Hepatol. 7 (3): 315–26. doi:10.4254/wjh.v7.i3.315. PMC 4381160. PMID 25848461.
- ↑ Egeberg A, Mallbris L, Warren RB, Bachelez H, Gislason GH, Hansen PR, Skov L (2016). "Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study". Br. J. Dermatol. 175 (3): 487–92. doi:10.1111/bjd.14528. PMID 26959083.
- ↑ Passarini B, Infusino SD, Barbieri E, Varotti E, Gionchetti P, Rizzello F, Morselli C, Tambasco R, Campieri M (2007). "Cutaneous manifestations in inflammatory bowel diseases: eight cases of psoriasis induced by anti-tumor-necrosis-factor antibody therapy". Dermatology (Basel). 215 (4): 295–300. doi:10.1159/000107622. PMID 17911986.
- ↑ Kahn MF, Khan MA (1994). "The SAPHO syndrome". Baillieres Clin Rheumatol. 8 (2): 333–62. PMID 8076391.
- ↑ Dreiher J, Weitzman D, Shapiro J, Davidovici B, Cohen AD (2008). "Psoriasis and chronic obstructive pulmonary disease: a case-control study". Br. J. Dermatol. 159 (4): 956–60. doi:10.1111/j.1365-2133.2008.08749.x. PMID 18637897.
- ↑ Behnam SM, Behnam SE, Koo JY (2005). "Smoking and psoriasis". Skinmed. 4 (3): 174–6. PMID 15891254.
- ↑ Egeberg A, Mallbris L, Hilmar Gislason G, Skov L, Riis Hansen P (2015). "Increased risk of migraine in patients with psoriasis: A Danish nationwide cohort study". J. Am. Acad. Dermatol. 73 (5): 829–35. doi:10.1016/j.jaad.2015.08.039. PMID 26386630.
- ↑ Zhdanov VM, Ershov FI, Bukrinskaia AG, Uryvaev LV (1970). "[Characteristics of viral RNA isolated from the polyribosomes of infected cells]". Vopr. Virusol. (in Russian). 15 (4): 467–73. PMID 4323599.
- ↑ De Rosa G, Mignogna C (2007). "The histopathology of psoriasis". Reumatismo. 59 Suppl 1: 46–8. PMID 17828343.
- ↑ "Psoriasis clinical guideline | American Academy of Dermatology".