Ophthalmia neonatorum: Difference between revisions
| Line 107: | Line 107: | ||
*Poor hygienic delivery conditions | *Poor hygienic delivery conditions | ||
==Screening== | ==Screening== | ||
[[Screening]] for ophthalmia neonatorum is not recommended. However, antibiotic ointment or eye drops is given to all babies immediately after birth to prevent conjunctivitis and other medical conditions in [[newborns]] | [[Screening]] for ophthalmia neonatorum is not recommended. However, antibiotic ointment or eye drops is given to all babies immediately after birth to prevent conjunctivitis and other medical conditions in [[newborns]]. Occasionally, this treatment causes a mild chemical conjunctivitis, which usually clears up on its own. Screening has been recommended by CDC in pregnant women for sexually transmitted diseases (STDs) to prevent spreading the infection to the baby.<ref name= Conjunctivitis-screening > Centers for Disease Control and Prevention (2015) http://www.cdc.gov/std/tg2015/screening-recommendations.htm Accessed on June 29, 2016</ref><ref name=Screening-Chlamydia-Neisseria > Centers for Disease Control and Prevention (2002) http://www.cdc.gov/mmwr/PDF/rr/rr5115.pdf Accessed on June 29, 2016</ref> | ||
{| style="border: 0px; font-size: 90%; margin: 3px;" align=center | {| style="border: 0px; font-size: 90%; margin: 3px;" align=center | ||
|+ | |+ | ||
Revision as of 19:59, 14 September 2016
Template:Ophthalmia neonatorum
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Sara Mehrsefat, M.D. [5]
Synonyms and keywords: Neonatal Conjunctivitis
Overview
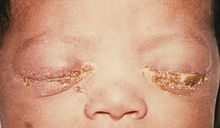
Ophthalmia neonatorum, or neonatal conjunctivitis, is a form of conjunctivitis that can be contracted by newborns during delivery. The baby can contract the disease if his/her eyes are contaminated during passage through the birth canal from a mother infected with either Neisseria gonorrhea, Chlamydia trachomatis, or Herpes simplex virus. It can also be caused by bacteria, viruses, and chemical irritants, such as silver nitrate. (It is for this reason that silver nitrate is no longer used frequently).[2] Ophthalmia neonatorum due to gonococci (Neisseria gonorrhoeae) typically manifests in the first 5 days of life and is associated with marked bilateral purulent discharge. In contrast, conjunctivitis secondary to infection with chlamydia (Chlamydia trachomatis) produces conjunctivitis after day 3 of life, but may occur up to 2 weeks after delivery. Babies infected with chlamydia may develop pneumonitis at a later stage (between 2 weeks and 19 weeks after delivery). Herpetic keratoconjunctivitis is a rare cause of neonatal conjunctivitis. It usually occurs within the first 2 weeks after birth. If left untreated, HSV conjunctivitis can cause corneal scarring and ulceration. Additionally, disseminated HSV infection can result in encephalitis. [3][4]
Prompt diagnosis is necessary in order to establish proper treatment and minimizing potential serious complications. Common laboratory studies for neonatal conjunctivitis may include Gram stain, cultures, Giemsa stain, and polymerase chain reaction (PCR) of conjunctival scrapings.[2][5] Effective measures for the prevention of ophthalmia neonatorum include ocular prophylaxis with 0.5% Erythromycin ointment or 1% Tetracycline hydrochloride, and educateing pregnant women on the importance of regular examinations to detect and treat sexually transmitted infections (STD). Empric antibiotic treatment for neonatal conjunctivitis includes Erythromycin ophthalmic ointment and Ceftriaxone IV or IM. Additionally, systemic treatment is indicated in cases of chlamydial and Herpes simplex conjunctivitis to avoid systemic complications.[6]
Historical Perspective
- In 1750, neonatal conjunctivitis (ophthalmia neonatorum) was first described by S.T. Quellmaz.[2]
- In 1881, Crédé introduced 2% silver nitrate for the first time as a prophylaxis treatment method for conjunctivitis in the newborns in Leipzig.[7]
Classification
According to the etiology ophthalmia neonatorum may be classified into:[2]
Pathophysiology
Pathogenesis
Neonatal conjunctivitis is occurring in a newborn during the first month of life, and often known as ophthalmia neonatorum. Neonatal conjunctivitis is mainly caused by sexually transmitted diseases agents such as Chlamydia trachomatis, Neisseria gonorrhoeae, and herpes simplex virus (HSV). Chlamydia trachomatis is the most common cause of ophthalmia neonatorum in the developed countries because of higher prevalence of chlamydia as a sexually transmitted disease.
The recognized routes of transmission of the organisms to the newborns include:[8]
- Infected birth canal during vaginal birth
- Transmembrane transmission of the infection
- Transplacental transmission of the infection
Additionally, silver nitrate drops (ocular prophylaxis) can cause ocular irritation and result in chemical conjunctivitis in newborn.[2]
Gross Pathology
On gross pathology, the following are characteristic findings of conjunctivitis:[9]
- Conjunctival injection
- Mucopurulent discharge or non-purulent discharge
- Pseudomembrane formation
- Chemosis
- Eyelid edema
Microscopic histopathological analysis
On microscopic histopathological analysis, the following are characteristic findings of infective conjunctivitis in neonate:[10]
- Mild spongiotic reaction pattern (low power view of the histology)
- Stromal infiltration by polymorphonuclear, leukocytes, plasma cells, mastocytes, and lymphocyte
- Numerous neutrophils (bacterial conjunctivitis)

Causes
Common causes of include:[2][12]
Differentiating ophthalmia neonatorum from Other Diseases
Neonatal conjunctivitis must be differentiated from:[2][12]
- Dacrocystitis
- Congenital glaucoma
- Nasolacrimal duct obstruction
- Preseptal/Orbital cellulitis
- Keratitis
Epidemiology and Demographics
Prevalence and Incidence
- Worldwide, neonatal conjunctivitis or ophthalmia neonatorum still blinds approximately 10,000 babies annually.[13]
Gender
- The incidence of neonatal conjunctivitis does not vary by gender.[14]
Developed Countries
The prevalence of neonatal conjunctivitis has decreased significantly in developed countries since the abandonment of silver nitrate as topical prophylaxis. Current prevalence of neonatal conjunctivitis in developed countries are 5 per 1000 live births.[15]
- In Belgium and Netherlands, the prevalence of neonatal conjunctivitis due to gonococcal infection was estimated 0.04 per 1000 live births.[2]
- In the United States, the prevalence of neonatal conjunctivitis due to gonococcal infection was estiamted 0.3 per 1000 live births.
- In the United States, the prevalence of neonatal conjunctivitis caused by chlamydial infection was estimated 5 to 60 cases per 1000 live births.
- In the United Kingdom, the prevalence of neonatal conjunctivitis caused by chlamydial infection was estimated 4 cases per 1000 live births.[16]
Developing Countries
- In African countries, the incidence of neonatal conjunctivitis is still high.[16]
Risk Factors
Common risk factors in the development of neonatal conjunctivitis include:[17][18][19]
- Maternal infections harbored in the mother's birth canal
- HIV infected mothers
- Exposure of the infant to infectious organisms
- Premature rupture of membranes (PROM)
- Inadequacy of ocular prophylaxis after birth
- Silver nitrate exposure
- Ocular trauma during delivery
- Mechanical ventilation
- Prematurity
- Poor prenatal care
- Poor hygienic delivery conditions
Screening
Screening for ophthalmia neonatorum is not recommended. However, antibiotic ointment or eye drops is given to all babies immediately after birth to prevent conjunctivitis and other medical conditions in newborns. Occasionally, this treatment causes a mild chemical conjunctivitis, which usually clears up on its own. Screening has been recommended by CDC in pregnant women for sexually transmitted diseases (STDs) to prevent spreading the infection to the baby.[20][21]
| Organism | Screening Recommendations in Pregnant Women |
|---|---|
| Chlamydia |
|
| Gonorrhea |
|
| Herpes simplex virus |
|
Natural History, Complications and Prognosis
Natural History
Neonatal conjunctivitis is one of the most common infections occurring in the first month of life. Chemical conjunctivitis secondary to silver nitrate solution application usually occurs in the first day of life, and disappears spontaneously within 2- 4 days. In the absence of adequate prophylaxis, 30% to 42% of infants born by vaginal delivery to infected mothers will develop gonococcal conjunctivitis. Gonococcal conjunctivitis tends to occur 2-7 days after birth, and tends to be more severe than other causes of ophthalmia neonatorum. It presents with severe bilateral purulent conjunctivitis, tearing, and eyelids swelling. If left untreated, it may cause corneal involvement such as corneal ulceration, diffuse opacification, and corneal perforation . This may lead to blindness, ultimately sepsis, or death. The onset of chlamydial conjunctivitis is usually later than gonococcal conjunctivitis. In the absence of adequate prophylaxis, 30% to 50% of infants born by vaginal delivery to infected mothers will develop chlamydial conjunctivitis. The incubation period is 5-14 days. Chlamydial conjunctivitis presents with mild hyperemia, watery discharge, eyelid swelling, papillary reaction, and pseudomembrane formation. If left untreated, it can progress to copious and purulent discharge. This may lead to central corneal opacification and blindness. Herpetic conjunctivitis is a rare cause of neonatal conjunctivitis. It usually occurs within the first 2 weeks after birth and has an incubation period of approximately 6-14 days. If left untreated, HSV conjunctivitis can cause corneal scarring and ulceration. Additionally, disseminated HSV infection can cause central nervous system (CNS) involvement. Ophthalmia neonatorum caused by pseudomonas is rare but can present with eyelid edema, erythema, and purulent discharge. If left untreated, can progress to corneal perforation, endophthalmitis, blindness, and possibly death.[2][3][22]
Complications
Complications to neonatal conjunctivitis include:[3][4]
- Ocular complications
- Pseudomembrane formation
- Corneal edema
- Thickened palpebral conjunctiva
- Peripheral pannus formation
- Corneal opacification
- Staphyloma
- Corneal perforation
- Endophthalmitis
- Loss of eye and blindness
- Systemic complications of chlamydia conjunctivitis
- Systemic complications of gonococcal conjunctivitis
- Arthritis
- Meningitis
- Anorectal infection
- Septicemia
- Systemic complications of herpes simplex keratoconjunctivitis
Prognosis
Early detection and early treatment of neonatal conjunctivitis is associated with a good prognosis.[3][4]
Diagnosis
Prompt diagnosis is necessary in order to establish proper treatment and minimizing potential serious complications of ophthalmia neonatorum.
History
A detailed and thorough history from the mother will help determine the prompt diagnosis and therapy. tSpecific areas of focus when obtaining a history from the mother include:[2][3][4]
- History of sexually transmitted disease (STD) in mother
- History of ocular prophylaxis with silver nitrate at the birth
- Time frame of signs and symptoms following birth
Symptoms and Physical examination
Symptoms of neonatal conjunctivitis (ophthalmia neonatorum), based on casualty, may include the following:[2][3][4][23][24]
| Causally | Symptomes | Physical Examination |
|---|---|---|
| Gonococcal |
|
|
| Chlamydial |
|
|
| Herpetic |
|
|
| Chemical |
|
|
Diagnosis
Prompt diagnosis is necessary in order to establish proper treatment and minimizing potential serious complications of ophthalmia neonatorum
History
A detailed and thorough history will help determine the prompt diagnosis and treatment. It includes:[2][3][4]
- History of sexually transmitted disease (STD) in mother
- History of ocular prophylaxis with silver nitrate
- Correct time frame of signs/symptoms from the mother
Symptoms & Physical examination
Symptoms of neonatal conjunctivitis (ophthalmia neonatorum), based on casualty, may include the following:[2][3][4][23][24]
| Causality | Symptomes | Physical Examination |
|---|---|---|
| Gonococcal |
|
|
| Chlamydial |
|
|
| Herpetic |
|
|
| Chemical |
|
|
Laboratory findings
Common laboratory studies for neonatal conjunctivitis may include the following:[2][5][25][26][27][28]
- Conjunctival scrapings
- Gram stain
- Cultures (blood agar, chocolate agar or Thayer-Martin)
- Giemsa stain
- Polymerase chain reaction (PCR)
Chlamydial
- Culture of epithelial cells for obligate intracellular organisms
- Gram staining of conjunctival scrapings (neutrophils, lymphocytes, plasma cells)
- Giemsa staining of conjunctival scrapings (basophilic intracytoplasmic inclusions)
- Polymerase chain reaction (PCR) of conjunctival scrapings
- DNA hybridization assays
- Enzyme immuno-assays for chlamydia organisms
Gonococcal
- Gram staining of conjunctival scrapings for (Gram-negative intra-cellular diplococci and neutrophils)
- Chocolate agar or Thayer-Martincultures for Neisseria species
Viral
- Viral culture of corneal epithelial cells for HSV
- Direct florescent antibody (rapid detection)
- Conjunctival scrapings for polymerase chain reaction (PCR) (more sensitive than viral culture)
Imaging Findings
There are no imaging findings associated with neonatal conjunctivitis. However, CT scan and MRI may be helpful in the diagnosis of other related complication such as orbital cellulitis, meningitis, encephalitis, and pneumonia.
Treatment
Medical Treatment
- Empric antibiotic treatment for neonatal conjunctivitis includes Erythromycin ophthalmic ointment and Ceftriaxone IV or IM, the treatment is altered when culture results are available
- Systemic treatment is necessary in cases of chlamydial conjunctivitis to eradicate the bacteria in the nasopharynx
- Prompt treatment of gonococcal conjunctivitis is essential in order to prevent the corneal ulceration
- Systemic Acyclovir is necessary in a case of HSV conjunctivitis to reduce the risk of a systemic infection
- Neonates with HSV infection require prompt consultation with the pediatrician, because systemic HSV infection can result in life-threatening condition
Infectious conjunctivitis[29][30]
- 1. Common causative pathogens
- 2. Conjunctivitis, neonatal prophylaxis
- Preferred regimen (1): 0.5% Erythromycin ophthalmic ointment, single dose
- Alternative regimen: 2.5% Providone-iodine solution ophthalmic ointment, single dose
- 3. Empiric antimicrobial therapy
- Preferred regimen (1): 0.5% Erythromycin ophthalmic ointment and Ceftriaxone 25-50 mg/kg (maximum dose 125 mg) IV or IM single dose
- 4. Pathogen-directed antimicrobial therapy
- 4.1 Chlamydia trachomatis
- Neonatal regimen: Erythromycin 50 mg/kg/day PO qid for 2 weeks OR Ethylsuccinate 50 mg/kg/day PO qid for 2 weeks
- Note (1): Neonates administered Erythromycin should be followed for signs and symptoms of infantile hypertrophic pyloric stenosis
- 4.2 Neisseria gonorrhoeae
- Neonatal dose: Ceftriaxone 25-50 mg/kg (maximum dose 125 mg) IV or IM, single dose
- 4.3 Herpes Simplex Virus
- Preferred regimen: Acyclovir 60 mg/kg IV tid for 2 weeks
- Alternative regimen (1): Trifluridine 1% solution q4h for 1 week
- Alternative regimen (2): Acyclovir 200 mg to 400 mg PO q5h per day for 1 week
- Alternative regimen (5): Ganciclovir 0.15% ophthlamic gel qid for 1 week
- Note (1): Prompt consultation with the pediatrician because systemic HSV infection is a life-threatening condition
- Note (2): Corticosteroids should be avoided
Chemical conjunctivitis
- Treatment of neonatal chemical conjunctivitis is not require
- Disappears spontaneously with 2 to 3 days
Surgery
Surgical intervention is not recommended for the management of neonatal conjunctivitis (ophthalmia neonatorum).[2]
Primary Prevention
Effective measures for the primary prevention of ophthalmia neonatorum include:[22]
- Prophylaxis
- Ocular prophylaxis with 0.5% erythromycin ointment or 1% tetracycline hydrochloride be given to all newborn
- Systemic prophylaxis with ceftriaxone 50 mg/kg IM or IV, single dose is recommended in the neonate born to mothers with untreated or suspected gonococcal infection
- Educate pregnant women on the importance of regular examinations to detect and treat sexually transmitted infections (STD)
- Avoid vaginal delivery (HSV Transmission rates are high for women who acquire genital herpes in the last few weeks of pregnancy)
- Avoid ocular prophylaxis with silver nitrate
Secondary Prevention
Effective measures for the secondary prevention of ophthalmia neonatorum include:[22][31]
- Prompt diagnosis
- Initiate appropriate and aggressive treatment
References
- ↑ Centers for Disease Control and Prevention's Public Health Image Library[1]
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Mallika P, Asok T, Faisal H, Aziz S, Tan A, Intan G (2008). "Neonatal conjunctivitis - a review". Malays Fam Physician. 3 (2): 77–81. PMC 4170304. PMID 25606121.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Fransen L, Nsanze H, Klauss V, Van der Stuyft P, D'Costa L, Brunham RC; et al. (1986). "Ophthalmia neonatorum in Nairobi, Kenya: the roles of [[Neisseria gonorrhoeae]] and [[Chlamydia trachomatis]]". J Infect Dis. 153 (5): 862–9. PMID 3084664. URL–wikilink conflict (help)
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Centers for Disease Control and Prevention (2015)[2] Accessed on June 30, 2016
- ↑ 5.0 5.1 Pinto RD, Lira RP, Arieta CE, Castro RS, Bonon SH (2015). "The prevalence of adenoviral conjunctivitis at the Clinical Hospital of the State University of Campinas, Brazil". Clinics (Sao Paulo). 70 (11): 748–50. doi:10.6061/clinics/2015(11)06. PMC 4642493. PMID 26602522.
- ↑ "Red Book - Report of the Committee on Infectious Diseases, 29th Edition. The American Academy of Pediatrics". Retrieved 2007-07-12.
- ↑ "Reports from the obstetrical clinic in Leipzig. Prevention of eye inflammation in the newborn". Am J Dis Child. 121 (1): 3–4. 1971. PMID 5543850.
- ↑ Treadwell P (1994). "Sexually transmitted diseases in neonates and infants". Semin Dermatol. 13 (4): 256–61. PMID 7848819.
- ↑ American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel. Preferred Practice Pattern® Guidelines. Pediatric Eye Evaluations. San Francisco, CA: American Academy of Ophthalmology; 2012. Available at: www.aao.org/ppp.
- ↑ DermNet NZ (2015)[3] Accessed on June 26, 2016
- ↑ http://picasaweb.google.com/mcmumbi/USMLEIIImages
- ↑ 12.0 12.1 Woods, Charles R. "Gonococcal infections in neonates and young children." Seminars in pediatric infectious diseases. Vol. 16. No. 4. WB Saunders, 2005
- ↑ Isenberg SJ, Apt L, Wood M (1996). "The influence of perinatal infective factors on ophthalmia neonatorum". J Pediatr Ophthalmol Strabismus. 33 (3): 185–8. PMID 8771523.
- ↑ Moore DL, MacDonald NE, Canadian Paediatric Society, Infectious Diseases and Immunization Committee (2015). "Preventing ophthalmia neonatorum". Can J Infect Dis Med Microbiol. 26 (3): 122–5. PMC 4507834. PMID 26236350.
- ↑ Azari AA, Barney NP (2013). "Conjunctivitis: a systematic review of diagnosis and treatment". JAMA. 310 (16): 1721–9. doi:10.1001/jama.2013.280318. PMC 4049531. PMID 24150468.
- ↑ 16.0 16.1 Schaller UC, Klauss V (2001). "Is Credé's prophylaxis for ophthalmia neonatorum still valid?". Bull World Health Organ. 79 (3): 262–3. PMC 2566367. PMID 11285676.
- ↑ Gichuhi S, Bosire R, Mbori-Ngacha D, Gichuhi C, Wamalwa D, Maleche-Obimbo E; et al. (2009). "Risk factors for neonatal conjunctivitis in babies of HIV-1 infected mothers". Ophthalmic Epidemiol. 16 (6): 337–45. doi:10.3109/09286580903144746. PMC 3223245. PMID 19995198.
- ↑ Zar HJ (2005). "Neonatal chlamydial infections: prevention and treatment". Paediatr Drugs. 7 (2): 103–10. PMID 15871630.
- ↑ Nahmias AJ, Visintine AM, Caldwell DR, Wilson LA (1976). "Eye infections with herpes simplex viruses in neonates". Surv Ophthalmol. 21 (2): 100–5. PMID 982267.
- ↑ Centers for Disease Control and Prevention (2015) http://www.cdc.gov/std/tg2015/screening-recommendations.htm Accessed on June 29, 2016
- ↑ Centers for Disease Control and Prevention (2002) http://www.cdc.gov/mmwr/PDF/rr/rr5115.pdf Accessed on June 29, 2016
- ↑ 22.0 22.1 22.2 Matejcek A, Goldman RD (2013). "Treatment and prevention of ophthalmia neonatorum". Can Fam Physician. 59 (11): 1187–90. PMC 3828094. PMID 24235191.
- ↑ 23.0 23.1 Rours, Ingrid GIJG, et al. "Chlamydia trachomatis as a cause of neonatal conjunctivitis in Dutch infants." Pediatrics 121.2 (2008): e321-e326.
- ↑ 24.0 24.1 Pickering, Larry K. Red book®: 2003 report of the committee on infectious diseases. No. Ed. 26. American Academy of Pediatrics, 2003.
- ↑ Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB (2015). "Adenoviral keratoconjunctivitis". Surv Ophthalmol. 60 (5): 435–43. doi:10.1016/j.survophthal.2015.04.001. PMID 26077630.
- ↑ Høvding G (2004). "[Acute bacterial conjunctivitis]". Tidsskr Nor Laegeforen. 124 (11): 1518–20. PMID 15195156.
- ↑ Høvding G (2008). "Acute bacterial conjunctivitis". Acta Ophthalmol. 86 (1): 5–17. doi:10.1111/j.1600-0420.2007.01006.x. PMID 17970823.
- ↑ Pickering, Larry K. Red book®: 2003 report of the committee on infectious diseases. No. Ed. 26. American Academy of Pediatrics, 2003.
- ↑ Quinn, Christopher J.; Mathews, Dennis E. (Nov 8 2002). "Optometric clinical practice guideline care of the patient with conjunctivitis". Check date values in:
|date=(help) - ↑ McLeod, Stephen D.; Feder, Robert S. (2013). "Conjunctivitis: Preferred Practice Pattern - American Academy of Ophthalmology".
- ↑ Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb Mortal Wkly Rep 2010;59 (No. RR-12):55.