Gram staining
|
WikiDoc Resources for Gram staining |
|
Articles |
|---|
|
Most recent articles on Gram staining Most cited articles on Gram staining |
|
Media |
|
Powerpoint slides on Gram staining |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Gram staining at Clinical Trials.gov Trial results on Gram staining Clinical Trials on Gram staining at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Gram staining NICE Guidance on Gram staining
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Gram staining Discussion groups on Gram staining Patient Handouts on Gram staining Directions to Hospitals Treating Gram staining Risk calculators and risk factors for Gram staining
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Gram staining |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
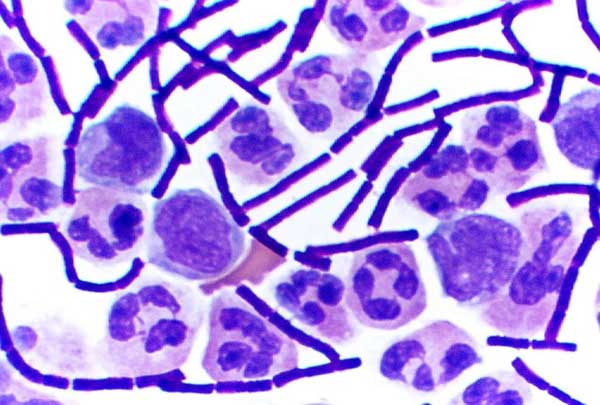
Gram staining (or Gram's method) is an empirical method of differentiating bacterial species into two large groups (Gram-positive and Gram-negative) based on the chemical and physical properties of their cell walls.[1]
The method is named after its inventor, the Danish scientist Hans Christian Gram (1853–1938), who developed the technique in 1884 to discriminate between pneumococci and Klebsiella pneumoniae bacteria.[2]
Uses
Research
Gram staining is one of the most useful staining procedures in the traditional bacteriological laboratory.[3] The technique is used as a tool for the differentiation of Gram-positive and Gram-negative bacteria, as a first step to determine the identity of a particular bacterial sample.[4]
The Gram stain is not an infallible tool for diagnosis, identification, or phylogeny, however. It is of extremely limited use in environmental microbiology, and has been largely superseded by molecular techniques even in the medical microbiology lab. Given that some organisms are gram-variable (i.e. they may stain either negative or positive), and that some organisms are not susceptible to either stain used by the Gram technique, its true utility to researchers should be considered limited and specific. In a modern environmental or molecular microbiology lab, most identification is done using genetic sequences and other molecular techniques, which are far more specific and information-rich than differential staining.
Medical
Gram stains are performed on body fluid or biopsy when infection is suspected. It yields results much more quickly than culture, and is especially important when infection would make an important difference in the patient's treatment and prognosis; examples are cerebrospinal fluid for meningitis and synovial fluid for septic arthritis.[3][5]
As a general rule of thumb (which has exceptions), Gram-negative bacteria are more pathogenic due to their outer membrane structure. The presence of a capsule will often increase the virulence of a pathogen. Additionally, Gram-negative bacteria have lipopolysaccharide in their outer membrane, an endotoxin which increases the severity of inflammation. This inflammation may be so severe that septic shock may occur. Gram-positive infections are generally less severe because the human body does not contain peptidoglycan; in fact, humans produce an enzyme (lysozyme) that attacks the open peptidoglycan layer of Gram-positive bacteria. Gram-positive bacteria are also frequently much more susceptible to beta-lactam antibiotics, such as penicillin.
Exceptions to the rule include branching and filamentous Gram-positive bacteria such as Mycobacterium tuberculosis and other agents of tuberculosis, or Nocardia species, the agents of nocardiosis and some types of actinomycetoma. These organisms present unique problems in diagnosis and treatment, and special stains such as the Ziehl-Neelsen stain and the Kinyoun stain are used in their laboratory workup.
Staining mechanism
Gram-positive bacteria have a thick mesh-like cell wall made of peptidoglycan (90% of cell wall), which stain purple and Gram-negative bacteria have a thinner layer (10% of cell wall), which stain pink. Gram-negative bacteria also have an additional outer membrane which contains lipids, and is separated from the cell wall by the periplasmic space. There are four basic steps of the Gram stain, which include applying a primary stain (crystal violet) to a heat-fixed smear of a bacterial culture, followed by the addition of a mordant (Gram's iodine), rapid decolorization with alcohol or acetone, and counterstaining with safranin or basic fuchsin.
Crystal violet (CV) dissociates in aqueous solutions into CV+ and chloride (Cl–) ions. These ions penetrate through the cell wall and cell membrane of both Gram-positive and Gram-negative cells. The CV+ ion interacts with negatively charged components of bacterial cells and stains the cells purple. Iodine (I– or I3–) interacts with CV+ and forms large complexes of crystal violet and iodine (CV–I) within the inner and outer layers of the cell. When a decolorizer such as alcohol or acetone is added, it interacts with the lipids of the cell membrane. A Gram-negative cell will lose its outer membrane and the peptidoglycan layer is left exposed. The CV–I complexes are washed from the Gram-negative cell along with the outer membrane. In contrast, a Gram-positive cell becomes dehydrated from an ethanol treatment. The large CV–I complexes become trapped within the Gram-positive cell due to the multilayered nature of its peptidoglycan. The decolorization step is critical and must be timed correctly; the crystal violet stain will be removed from both Gram-positive and negative cells if the decolorizing agent is left on too long (a matter of seconds).
After decolorization, the Gram-positive cell remains purple and the Gram-negative cell loses its purple color. Counterstain, which is usually positively-charged safranin or basic fuchsin, is applied last to give decolorized Gram-negative bacteria a pink or red color.[6][7]
Some bacteria, after staining with the Gram stain, yield a Gram-variable pattern: a mix of pink and purple cells are seen. The genera Actinomyces, Arthobacter, Corynebacterium, Mycobacterium, and Propionibacterium have cell walls particularly sensitive to breakage during cell division, resulting in Gram-negative staining of these Gram-positive cells. In cultures of Bacillus, Butyrivibrio, and Clostridium a decrease in peptidoglycan thickness during growth coincides with an increase in the number of cells that stain Gram-negative[8] In addition, in all bacteria stained using the Gram stain, the age of the culture may influence the results of the stain.
Gram staining protocol
- Make a slide of tissue or body fluid that is to be stained. Heat the slide for few seconds until it becomes hot to the touch so that bacteria are firmly mounted to the slide.
- Add the primary stain crystal violet and incubate 1 minute. This step colors all cells violet.
- Add Gram's iodine, for 30 seconds. It is not a stain; it is a mordant. It doesn't give color directly to the bacteria but it fixes the crystal violet to the bacterial cell wall. All cells remain violet.
- Wash with ethanol and acetone, the Decolorizer. If the bacteria is Gram-positive it will retain the primary stain. If it is Gram-negative it will lose the primary stain and appear colorless.
- Add the secondary stain, safranin, and incubate 1 min, then wash with water for a maximum of 5 seconds. If the bacteria is Gram-positive then the cell will retain the primary stain, will not take the secondary stain, and will appear black-violet. If the bacteria is Gram-negative then the cell will lose the primary stain, take secondary stain, and will appear red-pink.
Gram Stain is 2 g of 90% crystal violet dissolved in 20 ml of 95% ethyl alcohol.
Gram's iodine is 1 g of iodine, 2 g of potassium iodide, dissolved in 300 ml of distilled water.
Decolorizer is 50% ethyl alcohol, 50% acetone.[2]
In addition it is now common to use basic fuchsin instead of safranin.
See also
References
- ↑ Bergey, David H. (1994). Bergey's Manual of Determinative Bacteriology (9th ed. ed.). Lippincott Williams & Wilkins. ISBN 0-683-00603-7. Unknown parameter
|coauthors=ignored (help) - ↑ Gram, HC (1884). "Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten". Fortschritte der Medizin. 2: 185–89.
- ↑ 3.0 3.1 Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. pp. 232 &ndash, 3. ISBN 0838585299.
- ↑ Madigan, MT (2004). Brock Biology of Microorganisms (10th Edition ed.). Lippincott Williams & Wilkins. ISBN 0-13-066271-2. Unknown parameter
|coauthors=ignored (help) - ↑ Søgaard M, Nørgaard M, Schønheyder H (2007). "First notification of positive blood cultures: high accuracy of the Gram stain report (Epub ahead of publication)". J Clin Microbiol. PMID 17301283.
- ↑
Beveridge, T.J. "Cellular responses of Bacillus subtilis and Escherichia coli to the Gram stain" (PDF). J. Bacteriol. 156 (2): 846–858. PMID 6195148. Retrieved 2007-02-17. Unknown parameter
|coauthors=ignored (help) - ↑
Davies, J.A. "Chemical mechanism of the Gram stain and synthesis of a new electron-opaque marker for electron microscopy which replaces the iodine mordant of the stain" (PDF). J. Bacteriol. 156 (2): 837–845. PMID 6195147. Retrieved 2007-02-17. Unknown parameter
|coauthors=ignored (help) - ↑ Beveridge, T.J. "Mechanism of gram variability in select bacteria" (PDF). J. Bacteriol. 172 (3): 1609–1620. PMID 1689718. Retrieved 2007-02-17.
External links
ar:صبغة جرام zh-min-nan:Gram ní-sek-hoat ca:Tinció de Gram cs:Gramovo barvení da:Gramfarvning de:Gram-Färbung eo:Gram-kolorigo fa:رنگآمیزی گرم ko:그람 염색 id:Pewarnaan Gram it:Colorazione di Gram he:צביעת גרם lt:Gramo dažymas nl:Gram-kleuring no:Gramfarging sk:Farbenie podľa Grama sl:Barvanje po Gramu fi:Gramvärjäys sv:Gramfärgning