Nitroglycerin (Sublingual tablet): Difference between revisions
No edit summary |
m (Protected "Nitroglycerin (Sublingual tablet)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (26 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Main|Nitroglycerin}} | |||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|genericName=Nitroglycerin | |||
|aOrAn=an | |||
|drugClass=[[angina|anti-anginal]] [[vasodilator]] | |||
|indicationType=treatment | |||
|indication=[[angina pectoris]] due to [[coronary artery disease]] | |||
|adverseReactions=[[hypotension]], [[flushing]], [[dizziness]], [[headache]], and [[lightheadedness]] | |||
|genericName= | |fdaLIADAdult======Acute Relief of Angina Pectoris===== | ||
Nitroglycerin | |||
|aOrAn= | |||
an | |||
|drugClass= | |||
[[angina|anti-anginal]] [[vasodilator]] | |||
|indication= | |||
[[angina pectoris]] due to [[coronary artery disease]] | |||
|adverseReactions= | |||
[[hypotension]], [[flushing]], [[dizziness]], [[headache]], and [[lightheadedness]] | |||
|fdaLIADAdult= | |||
=====Acute Relief of Angina Pectoris===== | |||
* Dosing Information | * Dosing Information | ||
| Line 51: | Line 14: | ||
:* The dose may be repeated approximately every 5 minutes until relief is obtained. If the pain persists after a total of 3 tablets in a 15-minute period, or if the pain is different than is typically experienced, prompt medical attention is recommended. Nitrostat may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack. | :* The dose may be repeated approximately every 5 minutes until relief is obtained. If the pain persists after a total of 3 tablets in a 15-minute period, or if the pain is different than is typically experienced, prompt medical attention is recommended. Nitrostat may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack. | ||
:* During administration the patient should rest, preferably in the sitting position. | :* During administration the patient should rest, preferably in the sitting position. | ||
:* No dosage adjustment is required in patients with renal failure. | :* No dosage adjustment is required in patients with [[renal failure]]. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport======Congestive Heart Failure, Myocardial Infarction with Complication===== | |||
|offLabelAdultGuideSupport= | |||
=====Congestive Heart Failure, Myocardial Infarction with Complication===== | |||
* Developed by: ACC/AHA | * Developed by: ACC/AHA | ||
| Line 69: | Line 29: | ||
* Dosing Information | * Dosing Information | ||
:* Following [[acute myocardial infarction]], early (less than 10 hours after onset) nitrate therapy resulted in limitations of infarct progression and | :* Following [[acute myocardial infarction]], early (less than 10 hours after onset) nitrate therapy resulted in limitations of [[infarct]] progression and [[arrhythmia]]s and lowered the incidences of new [[congestive heart failure]] and early death. | ||
:* Conversion to oral | :* Conversion to oral [[nitrate]]s ([[isosorbide]] is best studied) or alternative routes of nitroglycerin administration should generally be accomplished within 24 to 48 hours.<ref>{{Cite journal | doi = 10.1016/j.jacc.2012.11.019 | issn = 1558-3597 | volume = 61 | issue = 4 | pages = –78-140 | last = American College of Emergency Physicians | coauthors = Society for Cardiovascular Angiography and Interventions, Patrick T. O'Gara, Frederick G. Kushner, Deborah D. Ascheim, Donald E. Casey, Mina K. Chung, James A. de Lemos, Steven M. Ettinger, James C. Fang, Francis M. Fesmire, Barry A. Franklin, Christopher B. Granger, Harlan M. Krumholz, Jane A. Linderbaum, David A. Morrow, L. Kristin Newby, Joseph P. Ornato, Narith Ou, Martha J. Radford, Jacqueline E. Tamis-Holland, Carl L. Tommaso, Cynthia M. Tracy, Y. Joseph Woo, David X. Zhao, Jeffrey L. Anderson, Alice K. Jacobs, Jonathan L. Halperin, Nancy M. Albert, Ralph G. Brindis, Mark A. Creager, David DeMets, Robert A. Guyton, Judith S. Hochman, Richard J. Kovacs, Frederick G. Kushner, E. Magnus Ohman, William G. Stevenson, Clyde W. Yancy | title = 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines | journal = Journal of the American College of Cardiology | date = 2013-01-29 | pmid = 23256914 }}</ref><ref>{{Cite journal | issn = 0002-9149 | volume = 70 | issue = 8 | pages = 82–87B | last = Jugdutt | first = B. I. | title = Role of nitrates after acute myocardial infarction | journal = The American Journal of Cardiology | date = 1992-09-24 | pmid = 1529930 }}</ref><ref>{{Cite journal | issn = 0008-6312 | volume = 79 Suppl 2 | pages = 5–13 | last = Schneider | first = W. | coauthors = W. D. Bussmann, A. Hartmann, M. Kaltenbach | title = Nitrate therapy in heart failure | journal = Cardiology | date = 1991 | pmid = 1760830 }}</ref><ref>{{Cite journal | issn = 0009-7322 | volume = 78 | issue = 4 | pages = 906–919 | last = Jugdutt | first = B. I. | coauthors = J. W. Warnica | title = Intravenous nitroglycerin therapy to limit myocardial infarct size, expansion, and complications. Effect of timing, dosage, and infarct location | journal = Circulation | date = 1988-10 | pmid = 3139326 }}</ref> | ||
:* In the immediate treatment of severe left ventricular failure following [[acute myocardial infarction]], nitroglycerin combined with dobutamine lowered abnormally elevated left ventricular filling pressure and augmented left ventricular pump function resulting in optimal hemodynamics more beneficially than either therapy alone.<ref>{{Cite journal | issn = 0002-8703 | volume = 106 | issue = 1 Pt 1 | pages = 35–40 | last = Awan | first = N. A. | coauthors = M. K. Evenson, K. E. Needham, J. M. Beattie, D. T. Mason | title = Effect of combined nitroglycerin and dobutamine infusion in left ventricular dysfunction | journal = American Heart Journal | date = 1983-07 | pmid = 6408917 }}</ref><ref>{{Cite journal | issn = 0009-7322 | volume = 68 | issue = 4 | pages = 813–820 | last = Loeb | first = H. S. | coauthors = J. P. Ostrenga, W. Gaul, J. Witt, G. Freeman, P. Scanlon, R. M. Gunnar | title = Beneficial effects of dopamine combined with intravenous nitroglycerin on hemodynamics in patients with severe left ventricular failure | journal = Circulation | date = 1983-10 | pmid = 6413087 }}</ref> | :* In the immediate treatment of severe left ventricular [[heart failure|failure]] following [[acute myocardial infarction]], nitroglycerin combined with [[dobutamine]] lowered abnormally elevated left ventricular filling pressure and augmented left ventricular pump function resulting in optimal [[hemodynamics]] more beneficially than either therapy alone.<ref>{{Cite journal | issn = 0002-8703 | volume = 106 | issue = 1 Pt 1 | pages = 35–40 | last = Awan | first = N. A. | coauthors = M. K. Evenson, K. E. Needham, J. M. Beattie, D. T. Mason | title = Effect of combined nitroglycerin and dobutamine infusion in left ventricular dysfunction | journal = American Heart Journal | date = 1983-07 | pmid = 6408917 }}</ref><ref>{{Cite journal | issn = 0009-7322 | volume = 68 | issue = 4 | pages = 813–820 | last = Loeb | first = H. S. | coauthors = J. P. Ostrenga, W. Gaul, J. Witt, G. Freeman, P. Scanlon, R. M. Gunnar | title = Beneficial effects of dopamine combined with intravenous nitroglycerin on hemodynamics in patients with severe left ventricular failure | journal = Circulation | date = 1983-10 | pmid = 6413087 }}</ref> | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport======Postoperative Pain===== | |||
|offLabelAdultNoGuideSupport= | |||
=====Postoperative Pain===== | |||
* Dosing Information | * Dosing Information | ||
:* Addition of transdermal nitroglycerin 5 mg/24 hours to intrathecal sufentanil in patients receiving knee surgery decreases the 24-hour analgesic requirement. However, transdermal nitroglycerin alone did not demonstrate a significant analgesic effect.<ref>{{Cite journal | issn = 0003-3022 | volume = 90 | issue = 3 | pages = 734–739 | last = Lauretti | first = G. R. | coauthors = R. de Oliveira, M. P. Reis, A. L. Mattos, N. L. Pereira | title = Transdermal nitroglycerine enhances spinal sufentanil postoperative analgesia following orthopedic surgery | journal = Anesthesiology | date = 1999-03 | pmid = 10078674 }}</ref> | :* Addition of transdermal nitroglycerin 5 mg/24 hours to intrathecal [[sufentanil]] in patients receiving knee surgery decreases the 24-hour analgesic requirement. However, transdermal nitroglycerin alone did not demonstrate a significant analgesic effect.<ref>{{Cite journal | issn = 0003-3022 | volume = 90 | issue = 3 | pages = 734–739 | last = Lauretti | first = G. R. | coauthors = R. de Oliveira, M. P. Reis, A. L. Mattos, N. L. Pereira | title = Transdermal nitroglycerine enhances spinal sufentanil postoperative analgesia following orthopedic surgery | journal = Anesthesiology | date = 1999-03 | pmid = 10078674 }}</ref> | ||
=====Chronic Anal Fissure===== | =====Chronic Anal Fissure===== | ||
| Line 87: | Line 44: | ||
* Dosing Information | * Dosing Information | ||
:* Nitroglycerin 0.2% ointment was effective for healing chronic anal | :* Nitroglycerin 0.2% ointment was effective for healing chronic [[anal fissure]]s compared with placebo with no significant difference in fissure recurrence after 9 months.<ref>{{Cite journal | issn = 0017-5749 | volume = 44 | issue = 5 | pages = 727–730 | last = Carapeti | first = E. A. | coauthors = M. A. Kamm, P. J. McDonald, S. J. Chadwick, D. Melville, R. K. Phillips | title = Randomised controlled trial shows that glyceryl trinitrate heals anal fissures, higher doses are not more effective, and there is a high recurrence rate | journal = Gut | date = 1999-05 | pmid = 10205213 | pmc = PMC1727506 }}</ref> | ||
=====Biliary Tract Disorder===== | =====Biliary Tract Disorder===== | ||
| Line 93: | Line 50: | ||
* Dosing Information | * Dosing Information | ||
:* Sublingual doses of 0.3 to 0.6 mg administered at the beginning of the procedure aided successful cannulation of the common bile duct, insertion of the Dormia basket, and removal of stones 4 to 11 mm in diameter.<ref>{{Cite journal | issn = 0002-9270 | volume = 92 | issue = 9 | pages = 1440–1443 | last = Uchida | first = N. | coauthors = T. Ezaki, S. Hirabayashi, A. Minami, H. Fukuma, H. Matsuoka, M. Yachida, K. Kurokohchi, S. A. Morshed, M. Nishioka, M. Matsuoka, T. Nakatsu | title = Endoscopic lithotomy of common bile duct stones with sublingual nitroglycerin and guidewire | journal = The American Journal of Gastroenterology | date = 1997-09 | pmid = 9317059 }}</ref> | :* Sublingual doses of 0.3 to 0.6 mg administered at the beginning of the procedure aided successful cannulation of the [[common bile duct]], insertion of the Dormia basket, and removal of stones 4 to 11 mm in diameter.<ref>{{Cite journal | issn = 0002-9270 | volume = 92 | issue = 9 | pages = 1440–1443 | last = Uchida | first = N. | coauthors = T. Ezaki, S. Hirabayashi, A. Minami, H. Fukuma, H. Matsuoka, M. Yachida, K. Kurokohchi, S. A. Morshed, M. Nishioka, M. Matsuoka, T. Nakatsu | title = Endoscopic lithotomy of common bile duct stones with sublingual nitroglycerin and guidewire | journal = The American Journal of Gastroenterology | date = 1997-09 | pmid = 9317059 }}</ref> | ||
=====Dysmenorrhea===== | =====Dysmenorrhea===== | ||
| Line 99: | Line 56: | ||
* Dosing Information | * Dosing Information | ||
:* Pain intensity was reduced and fewer | :* Pain intensity was reduced and fewer [[analgesic]]s were used compared with [[placebo]], but [[headache]] incidence was increased in women with primary [[dysmenorrhea]] who received nitroglycerin patch 0.1 mg/hr for 24 hours on days 1, 2, and 3 of each cycle.<ref>{{Cite journal | issn = 0020-7292 | volume = 69 | issue = 2 | pages = 113–118 | last = Moya | first = R. A. | coauthors = C. F. Moisa, F. Morales, H. Wynter, A. Ali, E. Narancio | title = Transdermal glyceryl trinitrate in the management of primary dysmenorrhea | journal = International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics | date = 2000-05 | pmid = 10802078 }}</ref> | ||
=====External Cephalic Version with Tocolysis===== | =====External Cephalic Version with Tocolysis===== | ||
| Line 111: | Line 68: | ||
* Dosing Information | * Dosing Information | ||
:* Combined therapy with vasopressin and nitroglycerin was more effective than vasopressin alone in controlling acute variceal hemorrhage, and nitroglycerin prevented cardiotoxic effects of vasopressin infusions.<ref>{{Cite journal | issn = 0270-9139 | volume = 6 | issue = 3 | pages = 410–413 | last = Gimson | first = A. E. | coauthors = D. Westaby, J. Hegarty, A. Watson, R. Williams | title = A randomized trial of vasopressin and vasopressin plus nitroglycerin in the control of acute variceal hemorrhage | journal = Hepatology (Baltimore, Md.) | date = 1986-06 | pmid = 3086204 }}</ref> | :* Combined therapy with [[vasopressin]] and nitroglycerin was more effective than [[vasopressin]] alone in controlling acute variceal hemorrhage, and nitroglycerin prevented cardiotoxic effects of [[vasopressin]] infusions.<ref>{{Cite journal | issn = 0270-9139 | volume = 6 | issue = 3 | pages = 410–413 | last = Gimson | first = A. E. | coauthors = D. Westaby, J. Hegarty, A. Watson, R. Williams | title = A randomized trial of vasopressin and vasopressin plus nitroglycerin in the control of acute variceal hemorrhage | journal = Hepatology (Baltimore, Md.) | date = 1986-06 | pmid = 3086204 }}</ref> | ||
=====Prophylaxis of Post-ERCP Pancreatitis===== | =====Prophylaxis of Post-ERCP Pancreatitis===== | ||
| Line 117: | Line 74: | ||
* Dosing Information | * Dosing Information | ||
:* Nitroglycerin prophylaxis reduced the incidence of | :* Nitroglycerin prophylaxis reduced the incidence of post-[[ERCP|endoscopic retrograde cholangiopancreatography]] [[pancreatitis]] in patients with primary biliary disease.<ref>{{Cite journal | doi = 10.1055/s-0029-1214951 | issn = 1438-8812 | volume = 41 | issue = 8 | pages = 690–695 | last = Bai | first = Y. | coauthors = C. Xu, X. Yang, J. Gao, D.-W. Zou, Z.-S. Li | title = Glyceryl trinitrate for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography: a meta-analysis of randomized, double-blind, placebo-controlled trials | journal = Endoscopy | date = 2009-08 | pmid = 19670137 }}</ref> | ||
=====Preeclampsia===== | =====Preeclampsia===== | ||
| Line 123: | Line 80: | ||
* Dosing Information | * Dosing Information | ||
:* Transdermal nitroglycerin 5 mg/24 hours initiated at 24 to 26 weeks gestation and continued for an average of 60 days increased the likelihood of a complication-free outcome with no significant effect on maternal blood pressure, uterine artery resistance index, or umbilical artery and middle cerebral artery pulsatility indices.<ref>{{Cite journal | doi = 10.1046/j.1469-0705.1998.12050334.x | issn = 0960-7692 | volume = 12 | issue = 5 | pages = 334–338 | last = Lees | first = C. | coauthors = H. Valensise, R. Black, K. Harrington, S. Byiers, C. Romanini, S. Campbell | title = The efficacy and fetal-maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre-eclampsia and its complications: a randomized double-blind placebo-controlled trial | journal = Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology | date = 1998-11 | pmid = 9819872 }}</ref> | :* Transdermal nitroglycerin 5 mg/24 hours initiated at 24 to 26 weeks [[gestation]] and continued for an average of 60 days increased the likelihood of a complication-free outcome with no significant effect on maternal [[blood pressure]], uterine artery resistance index, or umbilical artery and middle cerebral artery pulsatility indices.<ref>{{Cite journal | doi = 10.1046/j.1469-0705.1998.12050334.x | issn = 0960-7692 | volume = 12 | issue = 5 | pages = 334–338 | last = Lees | first = C. | coauthors = H. Valensise, R. Black, K. Harrington, S. Byiers, C. Romanini, S. Campbell | title = The efficacy and fetal-maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre-eclampsia and its complications: a randomized double-blind placebo-controlled trial | journal = Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology | date = 1998-11 | pmid = 9819872 }}</ref> | ||
=====Pulmonary Edema===== | =====Pulmonary Edema===== | ||
| Line 129: | Line 86: | ||
* Dosing Information | * Dosing Information | ||
:* Sublingual nitroglycerin (0.4 to 2.4 mg at 5- to 10-minute intervals) was beneficial in the emergency treatment of pulmonary edema.<ref>{{Cite journal | issn = 0002-9149 | volume = 41 | issue = 5 | pages = 931–936 | last = Bussmann | first = W. D. | coauthors = D. Schupp | title = Effect of sublingual nitroglycerin in emergency treatment of severe pulmonary edema | journal = The American Journal of Cardiology | date = 1978-05-01 | pmid = 417614 }}</ref> | :* Sublingual nitroglycerin (0.4 to 2.4 mg at 5- to 10-minute intervals) was beneficial in the emergency treatment of [[pulmonary edema]].<ref>{{Cite journal | issn = 0002-9149 | volume = 41 | issue = 5 | pages = 931–936 | last = Bussmann | first = W. D. | coauthors = D. Schupp | title = Effect of sublingual nitroglycerin in emergency treatment of severe pulmonary edema | journal = The American Journal of Cardiology | date = 1978-05-01 | pmid = 417614 }}</ref> | ||
=====Retained Placenta===== | =====Retained Placenta===== | ||
| Line 140: | Line 97: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=* The safety and effectiveness of nitroglycerin in pediatric patients have not been established. | |||
|fdaLIADPed= | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport======Chronic Anal Fissure===== | |||
* The safety and effectiveness of nitroglycerin in pediatric patients have not been established. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
=====Chronic Anal Fissure===== | |||
* Dosing Information | * Dosing Information | ||
:* Nitroglycerin ointment applied topically healed chronic anal | :* Nitroglycerin ointment applied topically healed chronic [[anal fissure]]s in 8 weeks without evidence of fissure recurrence on follow-up and was more effective in healing of the fissure than [[lidocaine]] or [[placebo]].<ref>{{Cite journal | issn = 1234-1010 | volume = 9 | issue = 10 | pages = –123-126 | last = Simpson | first = John | coauthors = Jonathan N. Lund, Richard J. Thompson, Leela Kapila, John H. Scholefield | title = The use of glyceryl trinitrate (GTN) in the treatment of chronic anal fissure in children | journal = Medical Science Monitor: International Medical Journal of Experimental and Clinical Research | date = 2003-10 | pmid = 14523338 }}</ref><ref>{{Cite journal | issn = 0022-3468 | volume = 34 | issue = 12 | pages = 1810–1812 | last = Tander | first = B. | coauthors = A. Güven, S. Demirbağ, Y. Ozkan, H. Oztürk, S. Cetinkurşun | title = A prospective, randomized, double-blind, placebo-controlled trial of glyceryl-trinitrate ointment in the treatment of children with anal fissure | journal = Journal of Pediatric Surgery | date = 1999-12 | pmid = 10626860 }}</ref> | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* [[Allergic]] reactions to organic nitrates are extremely rare, but they do occur. Nitroglycerin is contraindicated in patients who are allergic to it. | |||
|contraindications= | * Sublingual nitroglycerin therapy is contraindicated in patients with early [[myocardial infarction]], severe [[anemia]], [[IICP|increased intracranial pressure]], and those with a known [[hypersensitivity]] to nitroglycerin. | ||
* Administration of Nitrostat is contraindicated in patients who are using a [[phosphodiesterase]]-5 (PDE-5) inhibitor (e.g., [[sildenafil]] [[citrate]], [[tadalafil]], [[vardenafil]] hydrochloride) since these compounds have been shown to potentiate the [[hypotensive]] effects of organic [[nitrate]]s. | |||
* Allergic reactions to organic nitrates are extremely rare, but they do occur. Nitroglycerin is contraindicated in patients who are allergic to it. | |||
* Sublingual nitroglycerin therapy is contraindicated in patients with early myocardial infarction, severe anemia, increased intracranial pressure, and those with a known hypersensitivity to nitroglycerin. | |||
* Administration of Nitrostat is contraindicated in patients who are using a phosphodiesterase-5 (PDE-5) inhibitor (e.g., sildenafil citrate, tadalafil, vardenafil hydrochloride) since these compounds have been shown to potentiate the hypotensive effects of organic | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=* The benefits of sublingual nitroglycerin in patients with [[acute myocardial infarction]] or [[congestive heart failure]] have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or [[hemodynamic]] monitoring must be used because of the possibility of [[hypotension]] and [[tachycardia]]. | |||
|warnings= | |||
* The benefits of sublingual nitroglycerin in patients with acute myocardial infarction or [[congestive heart failure]] have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or hemodynamic monitoring must be used because of the possibility of hypotension and tachycardia. | |||
====Precautions==== | ====Precautions==== | ||
* Only the smallest dose required for effective relief of the acute [[angina|anginal attack]] should be used. Excessive use may lead to the development of tolerance. Nitrostat tablets are intended for sublingual or buccal administration and should not be swallowed. | * Only the smallest dose required for effective relief of the acute [[angina|anginal attack]] should be used. Excessive use may lead to the development of tolerance. Nitrostat tablets are intended for sublingual or buccal administration and should not be swallowed. | ||
* Severe hypotension, particularly with upright posture, may occur with small doses of nitroglycerin. This drug should therefore be used with caution in patients who may be volume-depleted or who, for whatever reason, are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris. | * Severe [[hypotension]], particularly with upright posture, may occur with small doses of nitroglycerin. This drug should therefore be used with caution in patients who may be volume-depleted or who, for whatever reason, are already [[hypotensive]]. [[Hypotension]] induced by nitroglycerin may be accompanied by paradoxical [[bradycardia]] and increased [[angina pectoris]]. | ||
* Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy. | * [[Nitrate]] therapy may aggravate the [[angina]] caused by [[hypertrophic cardiomyopathy]]. | ||
* As tolerance to other forms of nitroglycerin develops, the effects of sublingual nitroglycerin on exercise tolerance, although still observable, is blunted. | * As tolerance to other forms of nitroglycerin develops, the effects of sublingual nitroglycerin on exercise tolerance, although still observable, is blunted. | ||
* In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic | * In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic [[nitrate]]s, tolerance rarely occurs. [[Chest pain]], [[acute myocardial infarction]] and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence. | ||
* Several clinical trials of nitroglycerin patches or infusions in patients with angina pectoris have evaluated regimens that incorporated a 10- to 12-hour nitrate free interval. In some of these trials, an increase in the frequency of [[angina|anginal attack]] during the nitrate free interval was observed in a small number of patients. In one trial, patients had decreased exercise tolerance at the end of the nitrate interval. Hemodynamic rebound has been observed only rarely; on the other hand, few studies were so designed that rebound, if it had occurred, would have been detected. | * Several clinical trials of nitroglycerin patches or infusions in patients with [[angina pectoris]] have evaluated regimens that incorporated a 10- to 12-hour nitrate free interval. In some of these trials, an increase in the frequency of [[angina|anginal attack]] during the nitrate free interval was observed in a small number of patients. In one trial, patients had decreased exercise tolerance at the end of the nitrate interval. [[Hemodynamic]] rebound has been observed only rarely; on the other hand, few studies were so designed that rebound, if it had occurred, would have been detected. | ||
* Nitrate tolerance as a result of sublingual nitroglycerin administration is probably possible, but only in patients who maintain high continuous nitrate levels for more than 10 or 12 hours daily. Such use of sublingual nitroglycerin would entail administration of scores of tablets daily and is not recommended. | * [[Nitrate]] tolerance as a result of sublingual nitroglycerin administration is probably possible, but only in patients who maintain high continuous nitrate levels for more than 10 or 12 hours daily. Such use of sublingual nitroglycerin would entail administration of scores of tablets daily and is not recommended. | ||
* The drug should be discontinued if blurring of vision or drying of the mouth occurs. Excessive dosage of nitroglycerin may produce severe | * The drug should be discontinued if blurring of vision or drying of the mouth occurs. Excessive dosage of nitroglycerin may produce severe [[headache]]s. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
|clinicalTrials= | |||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=* [[Headache]] that may be severe and persistent may occur immediately after use. [[Vertigo]], [[dizziness]], [[weakness]], [[palpitation]], and other manifestations of postural [[hypotension]] may develop occasionally, particularly in erect, immobile patients. Marked sensitivity to the hypotensive effects of [[nitrate]]s (manifested by [[nausea]], [[vomiting]], [[weakness]], [[diaphoresis]], [[pallor]], and [[collapse]]) may occur at therapeutic doses. [[Syncope]] due to [[nitrate]] [[vasodilatation]] has been reported. [[Flushing]], drug [[rash]], and [[exfoliative dermatitis]] have been reported in patients receiving [[nitrate]] therapy. | |||
|postmarketing= | |||
* Headache that may be severe and persistent may occur immediately after use. Vertigo, dizziness, weakness, palpitation, and other manifestations of postural hypotension may develop occasionally, particularly in erect, immobile patients. Marked sensitivity to the hypotensive effects of | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=* Concomitant use of [[nitrate]]s and [[alcohol]] may cause [[hypotension]]. | |||
|drugInteractions= | * The vasodilatory and [[hemodynamic]] effects of nitroglycerin may be enhanced by concomitant administration of [[aspirin]]. | ||
* Intravenous administration of nitroglycerin decreases the [[thrombolytic]] effect of [[alteplase]]. Therefore, caution should be observed in patients receiving sublingual nitroglycerin during [[alteplase]] therapy. | |||
* Concomitant use of | * Intravenous nitroglycerin reduces the [[anticoagulant]] effect of [[heparin]] and [[aPTT|activated partial thromboplastin times (APTT)]] should be monitored in patients receiving [[heparin]] and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses. | ||
* The vasodilatory and hemodynamic effects of nitroglycerin may be enhanced by concomitant administration of aspirin. | * [[TCA|Tricyclic antidepressants]] ([[amitriptyline]], [[desipramine]], [[doxepin]], others) and [[anticholinergic]] drugs may cause [[dry mouth]] and diminished salivary secretions. This may make dissolution of sublingual nitroglycerin difficult. Increasing salivation with chewing gum or artificial [[saliva]] products may prove useful in aiding dissolution of sublingual nitroglycerin. | ||
* Intravenous administration of nitroglycerin decreases the thrombolytic effect of alteplase. Therefore, caution should be observed in patients receiving sublingual nitroglycerin during alteplase therapy. | * Oral administration of nitroglycerin markedly decreases the first-pass metabolism of [[dihydroergotamine]] and subsequently increases its oral [[bioavailability]]. [[Ergotamine]] is known to precipitate [[angina pectoris]]. Therefore, patients receiving sublingual nitroglycerin should avoid [[ergotamine]] and related drugs or be monitored for symptoms of [[ergotism]] if this is not possible. | ||
* Intravenous nitroglycerin reduces the anticoagulant effect of heparin and activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses. | * Administration of nitroglycerin is contraindicated in patients who are using [[PDE]]-5 inhibitors (e.g., [[sildenafil]] [[citrate]], [[tadalafil]], [[vardenafil]] hydrochloride). These compounds have been shown to potentiate the [[hypotensive]] effects of organic [[nitrate]]s. | ||
* Tricyclic antidepressants (amitriptyline, desipramine, doxepin, others) and anticholinergic drugs may cause dry mouth and diminished salivary secretions. This may make dissolution of sublingual nitroglycerin difficult. Increasing salivation with chewing gum or artificial saliva products may prove useful in aiding dissolution of sublingual nitroglycerin. | * A decrease in therapeutic effect of sublingual nitroglycerin may result from use of long-acting [[nitrate]]s. | ||
* Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible. | |||
* Administration of nitroglycerin is contraindicated in patients who are using PDE-5 inhibitors (e.g., sildenafil citrate, tadalafil, vardenafil hydrochloride). These compounds have been shown to potentiate the hypotensive effects of organic | |||
* A decrease in therapeutic effect of sublingual nitroglycerin may result from use of long-acting | |||
* Drug/Laboratory Test Interactions | * Drug/Laboratory Test Interactions | ||
:* Nitrates may interfere with the Zlatkis-Zak color reaction, causing a false report of decreased serum cholesterol. | :* Nitrates may interfere with the Zlatkis-Zak color reaction, causing a false report of decreased serum [[cholesterol]]. | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* '''Pregnancy Category B''' | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category B''' | |||
:* Animal reproduction and teratogenicity studies have not been conducted with nitroglycerin sublingual tablets. However, teratology studies conducted in rats and rabbits with topically applied nitroglycerin ointment at dosages up to 80 mg/kg/day and 240 mg/kg/day, respectively revealed no toxic effects on dams or fetuses. | :* Animal reproduction and teratogenicity studies have not been conducted with nitroglycerin sublingual tablets. However, teratology studies conducted in rats and rabbits with topically applied nitroglycerin ointment at dosages up to 80 mg/kg/day and 240 mg/kg/day, respectively revealed no toxic effects on dams or fetuses. | ||
:* There are no adequate and well-controlled studies in pregnant women. Nitroglycerin should be given to a pregnant woman only if clearly needed. | :* There are no adequate and well-controlled studies in pregnant women. Nitroglycerin should be given to a pregnant woman only if clearly needed. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nitroglycerin is administered to a nursing woman. | |||
|useInPed=* The safety and effectiveness of nitroglycerin in pediatric patients have not been established. | |||
|useInGeri=* Clinical studies of Nitrostat did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInLaborDelivery= | |useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInNursing= | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
* It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nitroglycerin is administered to a nursing woman. | |||
|useInPed= | |||
* The safety and effectiveness of nitroglycerin in pediatric patients have not been established. | |||
|useInGeri= | |||
* Clinical studies of Nitrostat did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |||
|administration= | |||
* Oral | |||
* Intravenous | * Intravenous | ||
* Transdermal patch | * Transdermal patch | ||
* Ointment | * Ointment | ||
|monitoring=* The benefits of sublingual nitroglycerin in patients with [[acute myocardial infarction]] or [[congestive heart failure]] have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or [[hemodynamic]] monitoring must be used because of the possibility of [[hypotension]] and [[tachycardia]]. | |||
|monitoring= | * Intravenous nitroglycerin reduces the [[anticoagulant]] effect of [[heparin]] and [[aPTT|activated partial thromboplastin times (APTT)]] should be monitored in patients receiving [[heparin]] and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses. | ||
* Oral administration of nitroglycerin markedly decreases the first-pass metabolism of [[dihydroergotamine]] and subsequently increases its oral [[bioavailability]]. [[Ergotamine]] is known to precipitate [[angina pectoris]]. Therefore, patients receiving sublingual nitroglycerin should avoid [[ergotamine]] and related drugs or be monitored for symptoms of [[ergotism]] if this is not possible. | |||
* The benefits of sublingual nitroglycerin in patients with acute myocardial infarction or [[congestive heart failure]] have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or hemodynamic monitoring must be used because of the possibility of hypotension and tachycardia. | |||
* Intravenous nitroglycerin reduces the anticoagulant effect of heparin and activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses. | |||
* Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|overdose====Acute Overdose=== | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
* Hemodynamic Effects | * Hemodynamic Effects | ||
:* The effects of nitroglycerin overdose are generally the results of nitroglycerin's capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; | :* The effects of nitroglycerin overdose are generally the results of nitroglycerin's capacity to induce [[vasodilatation]], venous pooling, reduced [[cardiac output]], and [[hypotension]]. These [[hemodynamic]] changes may have protean manifestations, including [[IICP|increased intracranial pressure]], with any or all of persistent throbbing [[headache]], [[confusion]], and moderate [[fever]]; [[vertigo]]; [[palpitation]]s; [[tachycardia]]; [[visual disturbance]]s; [[nausea]] and [[vomiting]] (possibly with colic and even [[bloody diarrhea]]); [[syncope]] (especially in the upright posture); [[dyspnea]], later followed by reduced ventilatory effort; [[diaphoresis]], with the skin either flushed or cold and clammy; [[heart block]] and [[bradycardia]]; [[paralysis]]; [[coma]]; [[seizure]]s; and death. | ||
:* No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary. | :* No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the [[hypotension]] associated with nitroglycerin overdose is the result of venodilatation and arterial [[hypovolemia]], prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary. | ||
:* The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good. | :* The use of [[epinephrine]] or other arterial vasoconstrictors in this setting is likely to do more harm than good. | ||
:* In patients with renal disease or [[congestive heart failure]] therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required. | :* In patients with [[renal disease]] or [[congestive heart failure]] therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required. | ||
* Methemoglobinemia | * [[Methemoglobinemia]] | ||
:* Methemoglobinemia has been rarely reported in association with organic | :* [[Methemoglobinemia]] has been rarely reported in association with organic [[nitrate]]s. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate [[cardiac output]] and adequate arterial PO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air. | ||
:* If methemoglobinemia is present, intravenous administration of methylene blue, 1 to 2 mg/kg of body weight, may be required. | :* If [[methemoglobinemia]] is present, intravenous administration of [[methylene blue]], 1 to 2 mg/kg of body weight, may be required. | ||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 306: | Line 190: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox=<!--Mechanism of Action--> | |||
|mechAction=* The principal pharmacological action of nitroglycerin is relaxation of vascular [[smooth muscle]]. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of both arterial and venous beds. Dilation of postcapillary vessels, including large veins, promotes peripheral pooling of blood, decreases venous return to the heart, and reduces left ventricular end-diastolic pressure (preload). Nitroglycerin also produces arteriolar relaxation, thereby reducing [[peripheral vascular resistance]] and arterial pressure (afterload), and dilates large epicardial coronary arteries; however, the extent to which this latter effect contributes to the relief of exertional [[angina]] is unclear. | |||
* Therapeutic doses of nitroglycerin may reduce systolic, diastolic, and mean arterial [[blood pressure]]. Effective coronary perfusion pressure is usually maintained, but can be compromised if blood pressure falls excessively, or increased heart rate decreases diastolic filling time. | |||
* Elevated [[CVP|central venous]] and [[PCWP|pulmonary capillary wedge pressure]]s, and pulmonary and systemic vascular resistance are also reduced by nitroglycerin therapy. [[Heart rate]] is usually slightly increased, presumably due to a compensatory response to the fall in [[blood pressure]]. [[Cardiac index]] may be increased, decreased, or unchanged. Myocardial oxygen consumption or demand (as measured by the pressure-rate product, tension-time index, and stroke-work index) is decreased and a more favorable supply-demand ratio can be achieved. Patients with elevated left ventricular filling pressures and increased [[systemic vascular resistance]] in association with a depressed [[cardiac index]] are likely to experience an improvement in [[cardiac index]]. In contrast, when filling pressures and [[cardiac index]] are normal, [[cardiac index]] may be slightly reduced following nitroglycerin administration. | |||
* Nitroglycerin forms free radical [[nitric oxide]] (NO) which activates [[guanylate cyclase]], resulting in an increase of guanosine 3'5' monophosphate ([[cGMP|cyclic GMP]]) in [[smooth muscle]] and other tissues. These events lead to dephosphorylation of myosin light chains, which regulate the contractile state in [[smooth muscle]], and result in [[vasodilatation]]. | |||
* Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3'5' monophosphate (cyclic GMP) in smooth muscle and other tissues. These events lead to dephosphorylation of myosin light chains, which regulate the contractile state in smooth muscle, and result in vasodilatation. | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* Nitrostat is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg , or 0.6 mg nitroglycerin; as well as lactose monohydrate, NF; glyceryl monostearate, NF; pregelatinized starch, NF; calcium stearate, NF powder; and silicon dioxide, colloidal, NF. | |||
|structure= | |||
* Nitrostat is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg , or 0.6 mg nitroglycerin; as well as lactose monohydrate, NF; glyceryl monostearate, NF; pregelatinized starch, NF; calcium stearate, NF powder; and silicon dioxide, colloidal, NF. | |||
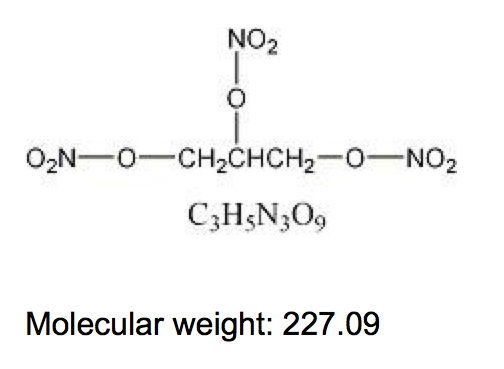
* Nitroglycerin, an organic nitrate, is a vasodilating agent. The chemical name for nitroglycerin is 1, 2, 3 propanetriol trinitrate and the chemical structure is: | * Nitroglycerin, an organic nitrate, is a vasodilating agent. The chemical name for nitroglycerin is 1, 2, 3 propanetriol trinitrate and the chemical structure is: | ||
: [[File: | : [[File:Nitroglycerin01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=* Consistent with the symptomatic relief of angina, digital plethysmography indicates that onset of the vasodilatory effect occurs approximately 1 to 3 minutes after sublingual nitroglycerin administration and reaches a maximum by 5 minutes postdose. Effects persist for at least 25 minutes following Nitrostat administration. | |||
|PD= | |||
* Consistent with the symptomatic relief of angina, digital plethysmography indicates that onset of the vasodilatory effect occurs approximately 1 to 3 minutes after sublingual nitroglycerin administration and reaches a maximum by 5 minutes postdose. Effects persist for at least 25 minutes following Nitrostat administration. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
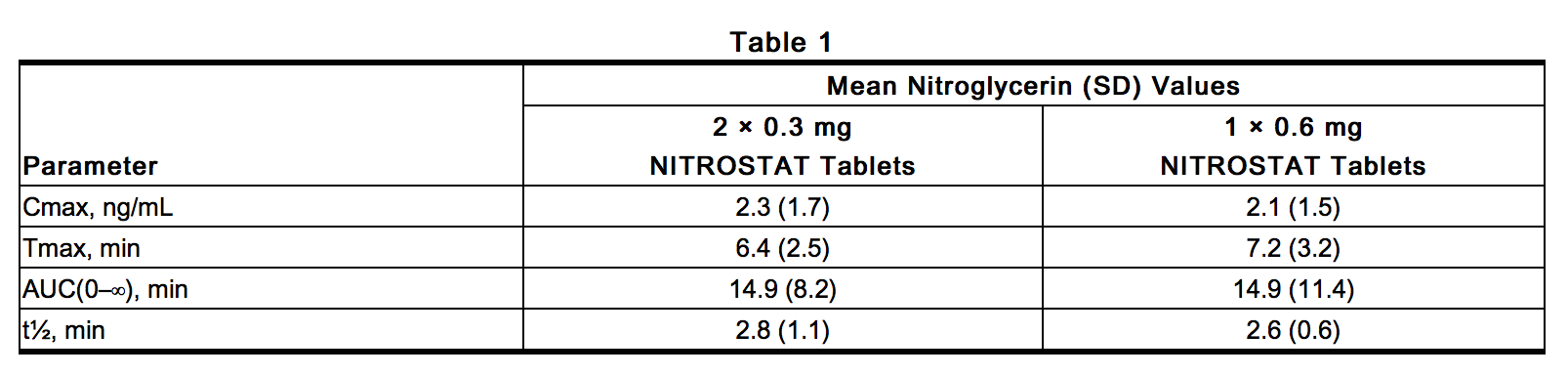
|PK=* Nitroglycerin is rapidly absorbed following sublingual administration of Nitrostat tablets. Mean peak nitroglycerin plasma concentrations occur at a mean time of approximately 6 to 7 minutes postdose (Table 1). Maximum plasma nitroglycerin concentrations (Cmax) and area under the plasma concentration-time curves (AUC) increase dose-proportionally following 0.3 to 0.6 mg Nitrostat. The absolute bioavailability of nitroglycerin from Nitrostat tablets is approximately 40% but tends to be variable due to factors influencing drug absorption, such as sublingual hydration and mucosal metabolism. | |||
: [[File:Nitroglycerin02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File: | |||
* Distribution | * Distribution | ||
| Line 355: | Line 224: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=* Carcinogenesis, Mutagenesis, Impairment of Fertility | |||
|nonClinToxic= | |||
* Carcinogenesis, Mutagenesis, Impairment of Fertility | |||
:* Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed. | :* Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed. | ||
:* Carcinogenicity potential of nitroglycerin was evaluated in rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years. Rats developed dose-related fibrotic and neoplastic changes in liver, including | :* Carcinogenicity potential of nitroglycerin was evaluated in rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years. Rats developed dose-related fibrotic and neoplastic changes in liver, including [[carcinoma]]s, and interstitial cell tumors in testes. At high dose, the incidences of [[hepatocellular carcinoma]]s in males was 48% and in females was 33%, compared to 0% in untreated controls. Incidences of [[testicular tumor]]s were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice. | ||
:* Nitroglycerin was mutagenic in Ames tests performed in 2 different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, PO, or in ex vivo cytogenetic tests in rat and dog cells. | :* Nitroglycerin was mutagenic in Ames tests performed in 2 different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, PO, or in ex vivo [[cytogenetic]] tests in rat and dog cells. | ||
:* In a 3-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for 6 months prior to mating of the F0 generation, with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this 3-generation study, there was no clear evidence of teratogenicity. | :* In a 3-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for 6 months prior to mating of the F0 generation, with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this 3-generation study, there was no clear evidence of teratogenicity. | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* Nitrostat is supplied as white, round, flat-faced tablets in 3 strengths (0.3 mg, 0.4 mg, and 0.6 mg) in bottles containing 100 tablets each, with color-coded labels, and in color-coded Patient Convenience Packages of 4 bottles of 25 tablets each. | |||
: [[File:Nitroglycerin03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File: | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=* Nitrostat is a sublingual tablet and should not be chewed, crushed, or swallowed. | |||
|fdaPatientInfo= | |||
* Nitrostat is a sublingual tablet and should not be chewed, crushed, or swallowed. | |||
* If possible, patients should sit down when taking Nitrostat tablets and should use caution when returning to a standing position. This eliminates the possibility of falling due to lightheadedness or dizziness. | * If possible, patients should sit down when taking Nitrostat tablets and should use caution when returning to a standing position. This eliminates the possibility of falling due to lightheadedness or dizziness. | ||
| Line 394: | Line 251: | ||
* Nitroglycerin may produce a burning or tingling sensation when administered sublingually; however, the ability to produce a burning or tingling sensation should not be considered a reliable method for determining the potency of the tablets. | * Nitroglycerin may produce a burning or tingling sensation when administered sublingually; however, the ability to produce a burning or tingling sensation should not be considered a reliable method for determining the potency of the tablets. | ||
* | * [[Headache]]s can sometimes accompany treatment with nitroglycerin. In patients who get these [[headache]]s, the headaches may be a marker of the activity of the drug. | ||
* Treatment with nitroglycerin may be associated with lightheadedness upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol. | * Treatment with nitroglycerin may be associated with [[lightheadedness]] upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol. | ||
* Nitroglycerin should be kept in the original glass container and must be tightly capped after each use to prevent loss of tablet potency. | * Nitroglycerin should be kept in the original glass container and must be tightly capped after each use to prevent loss of tablet potency. | ||
: [[File: | : [[File:Nitroglycerin11.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Treatment with nitroglycerin may be associated with [[lightheadedness]] upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed [[alcohol]]. | |||
|alcohol= | * Concomitant use of nitrates and [[alcohol]] may cause [[hypotension]]. | ||
* Treatment with nitroglycerin may be associated with lightheadedness upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol. | |||
* Concomitant use of nitrates and alcohol may cause hypotension. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* Minitran® | |||
|brandNames= | |||
* Minitran® | |||
* Nitro-Bid® | * Nitro-Bid® | ||
* Nitro-Dur® | * Nitro-Dur® | ||
| Line 424: | Line 275: | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=* N/A<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
|packLabel=: [[File:Nitroglycerin04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Nitroglycerin05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Nitroglycerin06.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Nitroglycerin07.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Nitroglycerin08.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Nitroglycerin09.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Nitroglycerin10.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
< | |||
<!--Overdosage--> | |||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage}} | |||
{{PillImage | |||
}} | |||
<!--Category--> | <!--Category--> | ||
| Line 485: | Line 297: | ||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Antianginals]] | |||
Latest revision as of 16:49, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nitroglycerin (Sublingual tablet) is an anti-anginal vasodilator that is FDA approved for the treatment of angina pectoris due to coronary artery disease. Common adverse reactions include hypotension, flushing, dizziness, headache, and lightheadedness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Relief of Angina Pectoris
- Dosing Information
- One tablet should be dissolved under the tongue or in the buccal pouch at the first sign of an acute anginal attack.
- The dose may be repeated approximately every 5 minutes until relief is obtained. If the pain persists after a total of 3 tablets in a 15-minute period, or if the pain is different than is typically experienced, prompt medical attention is recommended. Nitrostat may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack.
- During administration the patient should rest, preferably in the sitting position.
- No dosage adjustment is required in patients with renal failure.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Congestive Heart Failure, Myocardial Infarction with Complication
- Developed by: ACC/AHA
- Class of Recommendation: Class IIa
- Strength of Evidence: Category B
- Dosing Information
- Following acute myocardial infarction, early (less than 10 hours after onset) nitrate therapy resulted in limitations of infarct progression and arrhythmias and lowered the incidences of new congestive heart failure and early death.
- Conversion to oral nitrates (isosorbide is best studied) or alternative routes of nitroglycerin administration should generally be accomplished within 24 to 48 hours.[1][2][3][4]
- In the immediate treatment of severe left ventricular failure following acute myocardial infarction, nitroglycerin combined with dobutamine lowered abnormally elevated left ventricular filling pressure and augmented left ventricular pump function resulting in optimal hemodynamics more beneficially than either therapy alone.[5][6]
Non–Guideline-Supported Use
Postoperative Pain
- Dosing Information
- Addition of transdermal nitroglycerin 5 mg/24 hours to intrathecal sufentanil in patients receiving knee surgery decreases the 24-hour analgesic requirement. However, transdermal nitroglycerin alone did not demonstrate a significant analgesic effect.[7]
Chronic Anal Fissure
- Dosing Information
- Nitroglycerin 0.2% ointment was effective for healing chronic anal fissures compared with placebo with no significant difference in fissure recurrence after 9 months.[8]
Biliary Tract Disorder
- Dosing Information
- Sublingual doses of 0.3 to 0.6 mg administered at the beginning of the procedure aided successful cannulation of the common bile duct, insertion of the Dormia basket, and removal of stones 4 to 11 mm in diameter.[9]
Dysmenorrhea
- Dosing Information
- Pain intensity was reduced and fewer analgesics were used compared with placebo, but headache incidence was increased in women with primary dysmenorrhea who received nitroglycerin patch 0.1 mg/hr for 24 hours on days 1, 2, and 3 of each cycle.[10]
External Cephalic Version with Tocolysis
- Dosing Information
- IV nitroglycerin improved the success rate of external cephalic version in nulliparous and multiparous women.[11]
Gastrointestinal Hemorrhage
- Dosing Information
- Combined therapy with vasopressin and nitroglycerin was more effective than vasopressin alone in controlling acute variceal hemorrhage, and nitroglycerin prevented cardiotoxic effects of vasopressin infusions.[12]
Prophylaxis of Post-ERCP Pancreatitis
- Dosing Information
- Nitroglycerin prophylaxis reduced the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in patients with primary biliary disease.[13]
Preeclampsia
- Dosing Information
- Transdermal nitroglycerin 5 mg/24 hours initiated at 24 to 26 weeks gestation and continued for an average of 60 days increased the likelihood of a complication-free outcome with no significant effect on maternal blood pressure, uterine artery resistance index, or umbilical artery and middle cerebral artery pulsatility indices.[14]
Pulmonary Edema
- Dosing Information
- Sublingual nitroglycerin (0.4 to 2.4 mg at 5- to 10-minute intervals) was beneficial in the emergency treatment of pulmonary edema.[15]
Retained Placenta
- Dosing Information
- IV bolus nitroglycerin 500 mcg resulted in spontaneously uterine relaxation and placentas were extracted without complications.[16]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of nitroglycerin in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Sublingual tablet) in pediatric patients.
Non–Guideline-Supported Use
Chronic Anal Fissure
- Dosing Information
- Nitroglycerin ointment applied topically healed chronic anal fissures in 8 weeks without evidence of fissure recurrence on follow-up and was more effective in healing of the fissure than lidocaine or placebo.[17][18]
Contraindications
- Allergic reactions to organic nitrates are extremely rare, but they do occur. Nitroglycerin is contraindicated in patients who are allergic to it.
- Sublingual nitroglycerin therapy is contraindicated in patients with early myocardial infarction, severe anemia, increased intracranial pressure, and those with a known hypersensitivity to nitroglycerin.
- Administration of Nitrostat is contraindicated in patients who are using a phosphodiesterase-5 (PDE-5) inhibitor (e.g., sildenafil citrate, tadalafil, vardenafil hydrochloride) since these compounds have been shown to potentiate the hypotensive effects of organic nitrates.
Warnings
- The benefits of sublingual nitroglycerin in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or hemodynamic monitoring must be used because of the possibility of hypotension and tachycardia.
Precautions
- Only the smallest dose required for effective relief of the acute anginal attack should be used. Excessive use may lead to the development of tolerance. Nitrostat tablets are intended for sublingual or buccal administration and should not be swallowed.
- Severe hypotension, particularly with upright posture, may occur with small doses of nitroglycerin. This drug should therefore be used with caution in patients who may be volume-depleted or who, for whatever reason, are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris.
- Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
- As tolerance to other forms of nitroglycerin develops, the effects of sublingual nitroglycerin on exercise tolerance, although still observable, is blunted.
- In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance rarely occurs. Chest pain, acute myocardial infarction and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence.
- Several clinical trials of nitroglycerin patches or infusions in patients with angina pectoris have evaluated regimens that incorporated a 10- to 12-hour nitrate free interval. In some of these trials, an increase in the frequency of anginal attack during the nitrate free interval was observed in a small number of patients. In one trial, patients had decreased exercise tolerance at the end of the nitrate interval. Hemodynamic rebound has been observed only rarely; on the other hand, few studies were so designed that rebound, if it had occurred, would have been detected.
- Nitrate tolerance as a result of sublingual nitroglycerin administration is probably possible, but only in patients who maintain high continuous nitrate levels for more than 10 or 12 hours daily. Such use of sublingual nitroglycerin would entail administration of scores of tablets daily and is not recommended.
- The drug should be discontinued if blurring of vision or drying of the mouth occurs. Excessive dosage of nitroglycerin may produce severe headaches.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Nitroglycerin (Sublingual tablet) in the drug label.
Postmarketing Experience
- Headache that may be severe and persistent may occur immediately after use. Vertigo, dizziness, weakness, palpitation, and other manifestations of postural hypotension may develop occasionally, particularly in erect, immobile patients. Marked sensitivity to the hypotensive effects of nitrates (manifested by nausea, vomiting, weakness, diaphoresis, pallor, and collapse) may occur at therapeutic doses. Syncope due to nitrate vasodilatation has been reported. Flushing, drug rash, and exfoliative dermatitis have been reported in patients receiving nitrate therapy.
Drug Interactions
- Concomitant use of nitrates and alcohol may cause hypotension.
- The vasodilatory and hemodynamic effects of nitroglycerin may be enhanced by concomitant administration of aspirin.
- Intravenous administration of nitroglycerin decreases the thrombolytic effect of alteplase. Therefore, caution should be observed in patients receiving sublingual nitroglycerin during alteplase therapy.
- Intravenous nitroglycerin reduces the anticoagulant effect of heparin and activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses.
- Tricyclic antidepressants (amitriptyline, desipramine, doxepin, others) and anticholinergic drugs may cause dry mouth and diminished salivary secretions. This may make dissolution of sublingual nitroglycerin difficult. Increasing salivation with chewing gum or artificial saliva products may prove useful in aiding dissolution of sublingual nitroglycerin.
- Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
- Administration of nitroglycerin is contraindicated in patients who are using PDE-5 inhibitors (e.g., sildenafil citrate, tadalafil, vardenafil hydrochloride). These compounds have been shown to potentiate the hypotensive effects of organic nitrates.
- A decrease in therapeutic effect of sublingual nitroglycerin may result from use of long-acting nitrates.
- Drug/Laboratory Test Interactions
- Nitrates may interfere with the Zlatkis-Zak color reaction, causing a false report of decreased serum cholesterol.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Animal reproduction and teratogenicity studies have not been conducted with nitroglycerin sublingual tablets. However, teratology studies conducted in rats and rabbits with topically applied nitroglycerin ointment at dosages up to 80 mg/kg/day and 240 mg/kg/day, respectively revealed no toxic effects on dams or fetuses.
- There are no adequate and well-controlled studies in pregnant women. Nitroglycerin should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitroglycerin (Sublingual tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nitroglycerin (Sublingual tablet) during labor and delivery.
Nursing Mothers
- It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nitroglycerin is administered to a nursing woman.
Pediatric Use
- The safety and effectiveness of nitroglycerin in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Nitrostat did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Nitroglycerin (Sublingual tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nitroglycerin (Sublingual tablet) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nitroglycerin (Sublingual tablet) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nitroglycerin (Sublingual tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nitroglycerin (Sublingual tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nitroglycerin (Sublingual tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
- Transdermal patch
- Ointment
Monitoring
- The benefits of sublingual nitroglycerin in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or hemodynamic monitoring must be used because of the possibility of hypotension and tachycardia.
- Intravenous nitroglycerin reduces the anticoagulant effect of heparin and activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses.
- Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
IV Compatibility
There is limited information regarding IV Compatibility of Nitroglycerin (Sublingual tablet) in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Hemodynamic Effects
- The effects of nitroglycerin overdose are generally the results of nitroglycerin's capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; tachycardia; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures; and death.
- No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
- The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
- In patients with renal disease or congestive heart failure therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
- Methemoglobinemia has been rarely reported in association with organic nitrates. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial PO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
- If methemoglobinemia is present, intravenous administration of methylene blue, 1 to 2 mg/kg of body weight, may be required.
Chronic Overdose
There is limited information regarding Chronic Overdose of Nitroglycerin (Sublingual tablet) in the drug label.
Pharmacology
There is limited information regarding Nitroglycerin (Sublingual tablet) Pharmacology in the drug label.
Mechanism of Action
- The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of both arterial and venous beds. Dilation of postcapillary vessels, including large veins, promotes peripheral pooling of blood, decreases venous return to the heart, and reduces left ventricular end-diastolic pressure (preload). Nitroglycerin also produces arteriolar relaxation, thereby reducing peripheral vascular resistance and arterial pressure (afterload), and dilates large epicardial coronary arteries; however, the extent to which this latter effect contributes to the relief of exertional angina is unclear.
- Therapeutic doses of nitroglycerin may reduce systolic, diastolic, and mean arterial blood pressure. Effective coronary perfusion pressure is usually maintained, but can be compromised if blood pressure falls excessively, or increased heart rate decreases diastolic filling time.
- Elevated central venous and pulmonary capillary wedge pressures, and pulmonary and systemic vascular resistance are also reduced by nitroglycerin therapy. Heart rate is usually slightly increased, presumably due to a compensatory response to the fall in blood pressure. Cardiac index may be increased, decreased, or unchanged. Myocardial oxygen consumption or demand (as measured by the pressure-rate product, tension-time index, and stroke-work index) is decreased and a more favorable supply-demand ratio can be achieved. Patients with elevated left ventricular filling pressures and increased systemic vascular resistance in association with a depressed cardiac index are likely to experience an improvement in cardiac index. In contrast, when filling pressures and cardiac index are normal, cardiac index may be slightly reduced following nitroglycerin administration.
- Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3'5' monophosphate (cyclic GMP) in smooth muscle and other tissues. These events lead to dephosphorylation of myosin light chains, which regulate the contractile state in smooth muscle, and result in vasodilatation.
Structure
- Nitrostat is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg , or 0.6 mg nitroglycerin; as well as lactose monohydrate, NF; glyceryl monostearate, NF; pregelatinized starch, NF; calcium stearate, NF powder; and silicon dioxide, colloidal, NF.
- Nitroglycerin, an organic nitrate, is a vasodilating agent. The chemical name for nitroglycerin is 1, 2, 3 propanetriol trinitrate and the chemical structure is:
Pharmacodynamics
- Consistent with the symptomatic relief of angina, digital plethysmography indicates that onset of the vasodilatory effect occurs approximately 1 to 3 minutes after sublingual nitroglycerin administration and reaches a maximum by 5 minutes postdose. Effects persist for at least 25 minutes following Nitrostat administration.
Pharmacokinetics
- Nitroglycerin is rapidly absorbed following sublingual administration of Nitrostat tablets. Mean peak nitroglycerin plasma concentrations occur at a mean time of approximately 6 to 7 minutes postdose (Table 1). Maximum plasma nitroglycerin concentrations (Cmax) and area under the plasma concentration-time curves (AUC) increase dose-proportionally following 0.3 to 0.6 mg Nitrostat. The absolute bioavailability of nitroglycerin from Nitrostat tablets is approximately 40% but tends to be variable due to factors influencing drug absorption, such as sublingual hydration and mucosal metabolism.
- Distribution
- The volume of distribution (VArea) of nitroglycerin following intravenous administration is 3.3 L/kg. At plasma concentrations between 50 and 500 ng/mL, the binding of nitroglycerin to plasma proteins is approximately 60%, while that of 1,2- and 1,3-dinitroglycerin is 60% and 30%, respectively.
- Metabolism
- A liver reductase enzyme is of primary importance in the metabolism of nitroglycerin to glycerol di- and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, 2 major metabolites 1,2- and 1,3-dinitroglycerin, are found in plasma. Mean peak 1,2- and 1,3-dinitroglycerin plasma concentrations occur at approximately 15 minutes postdose. The elimination half-life of 1,2- and 1,3-dinitroglycerin is 36 and 32 minutes, respectively. The 1,2- and 1,3-dinitroglycerin metabolites have been reported to possess approximately 2% and 10%, respectively, of the pharmacological activity of nitroglycerin. Higher plasma concentrations of the dinitro metabolites, along with their nearly 10-fold longer elimination half-lives, may contribute significantly to the duration of pharmacologic effect. Glycerol mononitrate metabolites of nitroglycerin are biologically inactive.
- Elimination
- Nitroglycerin plasma concentrations decrease rapidly, with a mean elimination half-life of 2 to 3 minutes. Half-life values range from 1.5 to 7.5 minutes. Clearance (13.6 L/min) greatly exceeds hepatic blood flow. Metabolism is the primary route of drug elimination.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed.
- Carcinogenicity potential of nitroglycerin was evaluated in rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years. Rats developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At high dose, the incidences of hepatocellular carcinomas in males was 48% and in females was 33%, compared to 0% in untreated controls. Incidences of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
- Nitroglycerin was mutagenic in Ames tests performed in 2 different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, PO, or in ex vivo cytogenetic tests in rat and dog cells.
- In a 3-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for 6 months prior to mating of the F0 generation, with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this 3-generation study, there was no clear evidence of teratogenicity.
Clinical Studies
There is limited information regarding Clinical Studies of Nitroglycerin (Sublingual tablet) in the drug label.
How Supplied
- Nitrostat is supplied as white, round, flat-faced tablets in 3 strengths (0.3 mg, 0.4 mg, and 0.6 mg) in bottles containing 100 tablets each, with color-coded labels, and in color-coded Patient Convenience Packages of 4 bottles of 25 tablets each.
Storage
There is limited information regarding Nitroglycerin (Sublingual tablet) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nitroglycerin (Sublingual tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine. 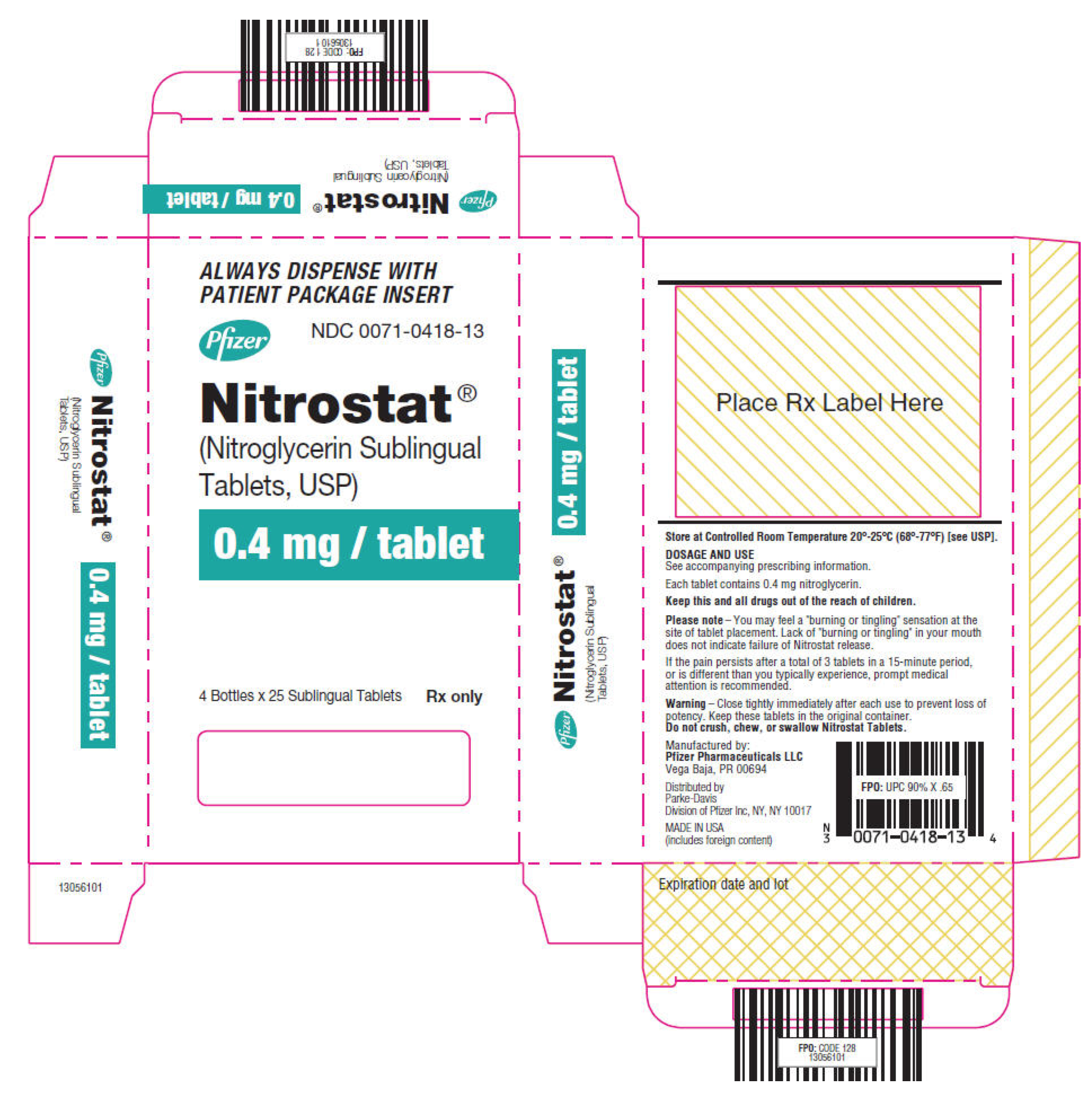
This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine. 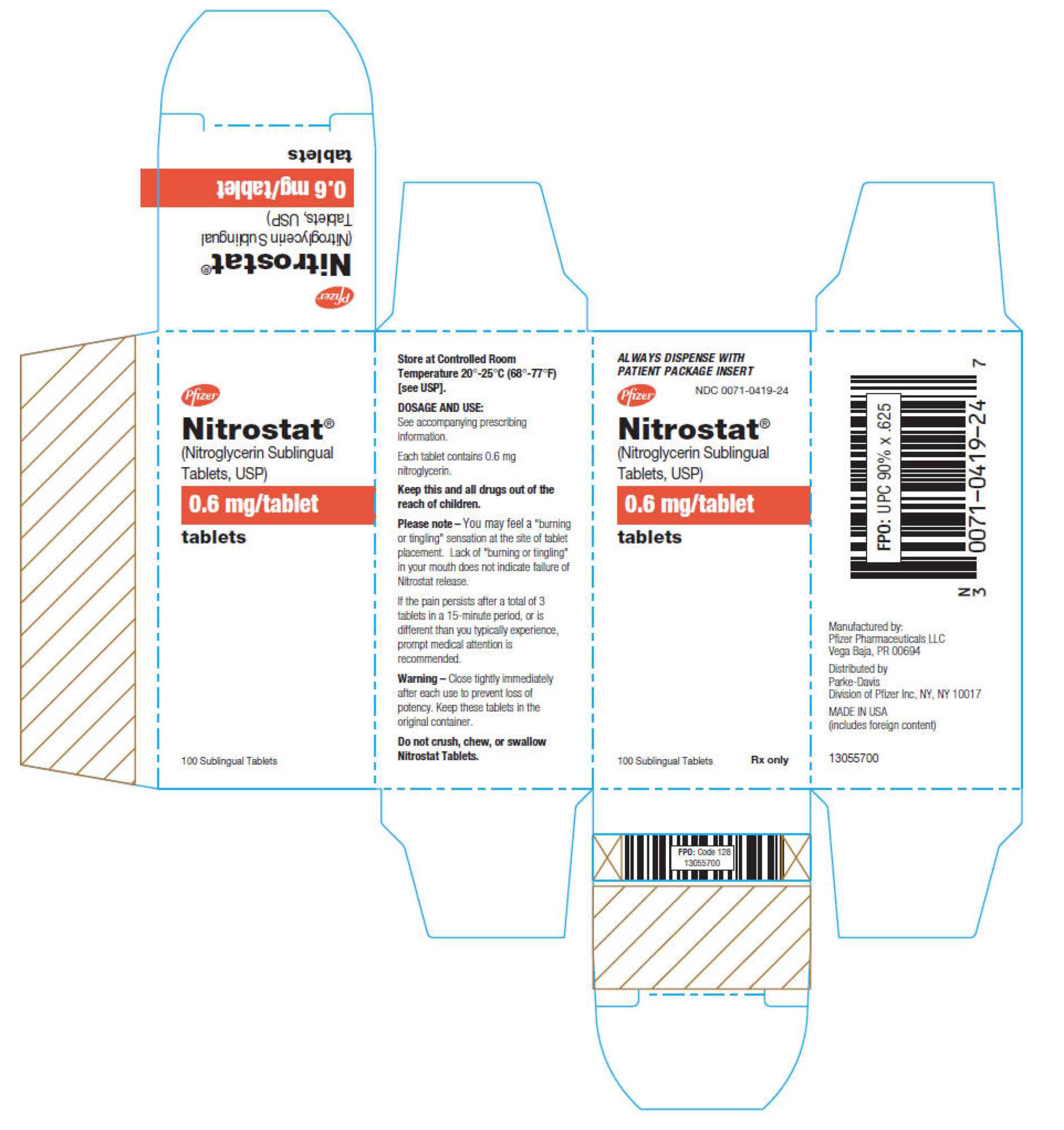
This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
{{#ask: Label Page::Nitroglycerin (Sublingual tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Nitrostat is a sublingual tablet and should not be chewed, crushed, or swallowed.
- If possible, patients should sit down when taking Nitrostat tablets and should use caution when returning to a standing position. This eliminates the possibility of falling due to lightheadedness or dizziness.
- One tablet should be dissolved under the tongue or in the buccal pouch at the first sign of an acute anginal attack. The dose may be repeated approximately every 5 minutes until relief is obtained.
- If chest pain persists after a total of 3 tablets in a 15-minute period, or if the pain is different than is typically experienced, prompt medical attention is recommended.
- Nitrostat may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack.
- Nitroglycerin may produce a burning or tingling sensation when administered sublingually; however, the ability to produce a burning or tingling sensation should not be considered a reliable method for determining the potency of the tablets.
- Headaches can sometimes accompany treatment with nitroglycerin. In patients who get these headaches, the headaches may be a marker of the activity of the drug.
- Treatment with nitroglycerin may be associated with lightheadedness upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
- Nitroglycerin should be kept in the original glass container and must be tightly capped after each use to prevent loss of tablet potency.
Precautions with Alcohol
- Treatment with nitroglycerin may be associated with lightheadedness upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
- Concomitant use of nitrates and alcohol may cause hypotension.
Brand Names
- Minitran®
- Nitro-Bid®
- Nitro-Dur®
- Nitrolingual®
- Nitrostat®
- Nitrotab®
- Nitrek®
- NitroMist®
- Nitrostat®[19]
Look-Alike Drug Names
- N/A[20]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ American College of Emergency Physicians (2013-01-29). "2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Journal of the American College of Cardiology. 61 (4): –78-140. doi:10.1016/j.jacc.2012.11.019. ISSN 1558-3597. PMID 23256914. Unknown parameter
|coauthors=ignored (help) - ↑ Jugdutt, B. I. (1992-09-24). "Role of nitrates after acute myocardial infarction". The American Journal of Cardiology. 70 (8): 82–87B. ISSN 0002-9149. PMID 1529930.
- ↑ Schneider, W. (1991). "Nitrate therapy in heart failure". Cardiology. 79 Suppl 2: 5–13. ISSN 0008-6312. PMID 1760830. Unknown parameter
|coauthors=ignored (help) - ↑ Jugdutt, B. I. (1988-10). "Intravenous nitroglycerin therapy to limit myocardial infarct size, expansion, and complications. Effect of timing, dosage, and infarct location". Circulation. 78 (4): 906–919. ISSN 0009-7322. PMID 3139326. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Awan, N. A. (1983-07). "Effect of combined nitroglycerin and dobutamine infusion in left ventricular dysfunction". American Heart Journal. 106 (1 Pt 1): 35–40. ISSN 0002-8703. PMID 6408917. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Loeb, H. S. (1983-10). "Beneficial effects of dopamine combined with intravenous nitroglycerin on hemodynamics in patients with severe left ventricular failure". Circulation. 68 (4): 813–820. ISSN 0009-7322. PMID 6413087. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Lauretti, G. R. (1999-03). "Transdermal nitroglycerine enhances spinal sufentanil postoperative analgesia following orthopedic surgery". Anesthesiology. 90 (3): 734–739. ISSN 0003-3022. PMID 10078674. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Carapeti, E. A. (1999-05). "Randomised controlled trial shows that glyceryl trinitrate heals anal fissures, higher doses are not more effective, and there is a high recurrence rate". Gut. 44 (5): 727–730. ISSN 0017-5749. PMC 1727506. PMID 10205213. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Uchida, N. (1997-09). "Endoscopic lithotomy of common bile duct stones with sublingual nitroglycerin and guidewire". The American Journal of Gastroenterology. 92 (9): 1440–1443. ISSN 0002-9270. PMID 9317059. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Moya, R. A. (2000-05). "Transdermal glyceryl trinitrate in the management of primary dysmenorrhea". International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics. 69 (2): 113–118. ISSN 0020-7292. PMID 10802078. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Hilton, Jennifer (2009-09). "Intravenous nitroglycerin for external cephalic version: a randomized controlled trial". Obstetrics and Gynecology. 114 (3): 560–567. doi:10.1097/AOG.0b013e3181b05a19. ISSN 0029-7844. PMID 19701035. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Gimson, A. E. (1986-06). "A randomized trial of vasopressin and vasopressin plus nitroglycerin in the control of acute variceal hemorrhage". Hepatology (Baltimore, Md.). 6 (3): 410–413. ISSN 0270-9139. PMID 3086204. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Bai, Y. (2009-08). "Glyceryl trinitrate for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography: a meta-analysis of randomized, double-blind, placebo-controlled trials". Endoscopy. 41 (8): 690–695. doi:10.1055/s-0029-1214951. ISSN 1438-8812. PMID 19670137. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Lees, C. (1998-11). "The efficacy and fetal-maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre-eclampsia and its complications: a randomized double-blind placebo-controlled trial". Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology. 12 (5): 334–338. doi:10.1046/j.1469-0705.1998.12050334.x. ISSN 0960-7692. PMID 9819872. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Bussmann, W. D. (1978-05-01). "Effect of sublingual nitroglycerin in emergency treatment of severe pulmonary edema". The American Journal of Cardiology. 41 (5): 931–936. ISSN 0002-9149. PMID 417614. Unknown parameter
|coauthors=ignored (help) - ↑ Peng, A. T. (1989-07). "Intravenous nitroglycerin for uterine relaxation in the postpartum patient with retained placenta". Anesthesiology. 71 (1): 172–173. ISSN 0003-3022. PMID 2502047. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Simpson, John (2003-10). "The use of glyceryl trinitrate (GTN) in the treatment of chronic anal fissure in children". Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 9 (10): –123-126. ISSN 1234-1010. PMID 14523338. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Tander, B. (1999-12). "A prospective, randomized, double-blind, placebo-controlled trial of glyceryl-trinitrate ointment in the treatment of children with anal fissure". Journal of Pediatric Surgery. 34 (12): 1810–1812. ISSN 0022-3468. PMID 10626860. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "Nitrostat (nitroglycerin) tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Nitroglycerin (Sublingual tablet)
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}