Niraparib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Niraparib is a poly(ADP-ribose) polymerase (PARP) inhibitor that is FDA approved for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy. Common adverse reactions include thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, constipation, vomiting, abdominal pain/distention, mucositis/stomatitis, diarrhea, dyspepsia, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, AST/ALT elevation, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, and hypertension.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Niraparib is indicated for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy.
Recommended Dosage
- The recommended dose of niraparib as monotherapy is 300 mg (three 100 mg capsules) taken orally once daily.
- Instruct patients to take their dose of niraparib at approximately the same time each day. Each capsule should be swallowed whole. Niraparib may be taken with or without food. Bedtime administration may be a potential method for managing nausea.
- Patients should start treatment with ZEJULA no later than 8 weeks after their most recent platinum-containing regimen.
- Niraparib treatment should be continued until disease progression or unacceptable toxicity.
- In the case of a missed dose of niraparib, instruct patients to take their next dose at its regularly scheduled time. If a patient vomits or misses a dose of niraparib, an additional dose should not be taken.
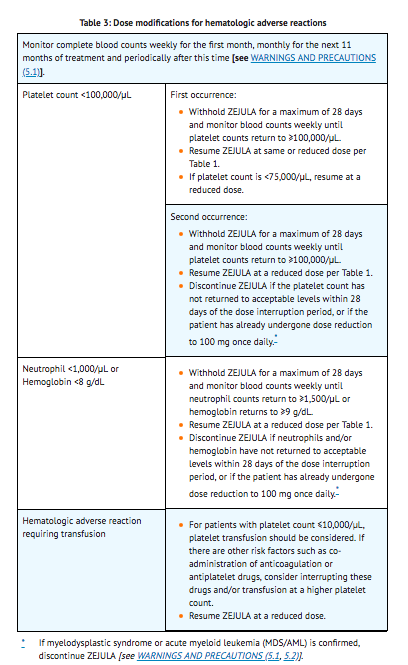
Dose Adjustments for Adverse Reactions
- To manage adverse reactions, consider interruption of treatment, dose reduction, or dose discontinuation. The recommended dose modifications for adverse reactions are listed in Tables 1, 2 and 3.


Dosage Forms and Strengths
- 100 mg capsule having a white body with "100 mg" printed in black ink, and a purple cap with "Niraparib" printed in white ink.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding niraparib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding niraparib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Niraparib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding niraparib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding niraparib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None.
Warnings
Myelodysplastic Syndrome/Acute Myeloid Leukemia
- Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML), including cases with fatal outcome, have been reported in patients who received niraparib. In Trial 1 (NOVA), MDS/AML occurred in 5 out of 367 (1.4%) of patients who received niraparib and in 2 out of 179 (1.1%) patients who received placebo. Overall, MDS/AML has been reported in 7 out of 751 (0.9%) patients treated with niraparib in clinical studies.
- The duration of niraparib treatment in patients prior to developing MDS/AML varied from <1 month to 2 years. All patients had received previous chemotherapy with platinum and some had also received other DNA damaging agents and radiotherapy. Discontinue niraparib if MDS/AML is confirmed.
Bone Marrow Suppression
- Hematologic adverse reactions (thrombocytopenia, anemia and neutropenia) have been reported in patients treated with niraparib. Grade ≥3 thrombocytopenia, anemia and neutropenia were reported, respectively, in 29%, 25%, and 20% of patients receiving niraparib. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 3%, 1%, and 2% of patients.
- Do not start niraparib until patients have recovered from hematological toxicity caused by previous chemotherapy (≤ Grade 1). Monitor complete blood counts weekly for the first month, monthly for the next 11 months of treatment, and periodically after this time. If hematological toxicities do not resolve within 28 days following interruption, discontinue niraparib, and refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics.
Cardiovascular Effects
- Hypertension and hypertensive crisis have been reported in patients treated with niraparib. Grade 3-4 hypertension occurred in 9% of niraparib treated patients compared to 2% of placebo treated patients in Trial 1. Discontinuation due to hypertension occurred in <1% of patients.
- Monitor blood pressure and heart rate monthly for the first year and periodically thereafter during treatment with niraparib. Closely monitor patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. Medically manage hypertension with antihypertensive medications and adjustment of the niraparib dose, if necessary.
Embryo-Fetal Toxicity
- Based on its mechanism of action, niraparib can cause fetal harm when administered to a pregnant woman. Niraparib has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow). Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib.
- Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of niraparib.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of niraparib monotherapy 300 mg once daily has been studied in 367 patients with platinum-sensitive recurrent ovarian, fallopian tube, and primary peritoneal cancer in Trial 1 (NOVA). Adverse reactions in Trial 1 led to dose reduction or interruption in 69% of patients, most frequently from thrombocytopenia (41%) and anemia (20%). The permanent discontinuation rate due to adverse reactions in Trial 1 was 15%. The median exposure to niraparib in these patients was 250 days.
- Table 4 and Table 5 summarize the common adverse reactions and abnormal laboratory findings, respectively, observed in patients treated with niraparib.
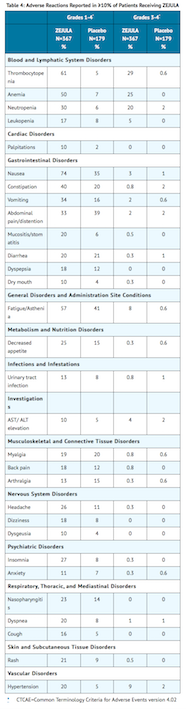

- The following adverse reactions and laboratory abnormalities have been identified in ≥1 to <10% of the 367 patients receiving niraparib in the NOVA trial and not included in the table: tachycardia, peripheral edema, hypokalemia, bronchitis, conjunctivitis, gamma-glutamyl transferase increased, blood creatinine increased, blood alkaline phosphatase increased, weight decreased, depression, epistaxis.
Postmarketing Experience
There is limited information regarding Niraparib Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Niraparib Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Risk Summary
- Based on its mechanism of action, niraparib can cause fetal harm when administered to pregnant women. There are no data regarding the use of niraparib in pregnant women to inform the drug-associated risk. Niraparib has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow). Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib. Apprise pregnant women of the potential risk to a fetus.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Niraparib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Niraparib during labor and delivery.
Nursing Mothers
Risk Summary
- No data are available regarding the presence of niraparib or its metabolites in human milk, or on its effects on the breastfed infant or milk production. Because of the potential for serious adverse reactions in breastfed infants from niraparib, advise a lactating woman not to breastfeed during treatment with niraparib and for 1 month after receiving the final dose.
Pediatric Use
- Safety and effectiveness of niraparib have not been established in pediatric patients.
Geriatic Use
- In Trial 1 (NOVA), 35% of patients were aged ≥65 years and 8% were aged ≥75 years. No overall differences in safety and effectiveness of niraparib were observed between these patients and younger patients but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Niraparib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Niraparib with respect to specific racial populations.
Renal Impairment
- No dose adjustment is necessary for patients with mild (CLcr:60 to 89 mL/min) to moderate (CLcr:30 to 59 mL/min) renal impairment. The degree of renal impairment was determined by creatinine clearance as estimated by the Cockcroft-Gault equation. The safety of niraparib in patients with severe renal impairment or end stage renal disease undergoing hemodialysis is unknown.
Hepatic Impairment
- No dose adjustment is needed in patients with mild hepatic impairment according to the National Cancer Institute – Organ Dysfunction Working Group (NCI-ODWG) criteria. The safety of niraparib in patients with moderate to severe hepatic impairment is unknown.
Females of Reproductive Potential and Males
Pregnancy Testing
- Niraparib can cause fetal harm when administered to a pregnant woman.
- A pregnancy test is recommended for females of reproductive potential prior to initiating niraparib treatment.
Contraception
Females
- Niraparib can cause fetal harm when administered to a pregnant woman.
- Advise females of reproductive potential to use effective contraception treatment with niraparib and for at least for 6 months following the last dose.
Infertility
Males
- Based on animal studies, niraparib may impair fertility in males of reproductive potential.
Immunocompromised Patients
There is no FDA guidance one the use of Niraparib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Evidence of disease response or stabilization is indicative of efficacy.
- Pregnancy test: Prior to therapy initiation.
- CBC: Weekly for the first month, monthly for the next 11 months, and periodically thereafter for clinically significant changes; if hematologic toxicity occurs, monitor weekly; include a differential.
- Blood pressure and heart rate: Monthly for the first year and periodically thereafter, especially in patients with cardiovascular disorders (eg, coronary insufficiency, cardiac arrhythmias, hypertension).
IV Compatibility
There is limited information regarding the compatibility of Niraparib and IV administrations.
Overdosage
- There is no specific treatment in the event of niraparib overdose, and symptoms of overdose are not established. In the event of an overdose, healthcare practitioners should follow general supportive measures and should treat symptomatically.
Pharmacology
| |
Niraparib
| |
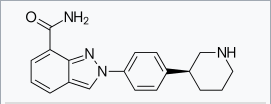
| Systematic (IUPAC) name | |
| 2-[4-[(3S)-3-Piperidyl]phenyl]indazole-7-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 320.394 g/mol |
| SMILES | & |
| Synonyms | MK-4827 |
| Physical data | |
| Solubility in water | 0.7–1.1 mg/mL (20 °C) |
| Pharmacokinetic data | |
| Bioavailability | 73% |
| Protein binding | 83% |
| Metabolism | Carboxylesterases |
| Half life | 36 hours |
| Excretion | 48% urine, 29% feces |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- Niraparib is an inhibitor of poly(ADP-ribose) polymerase (PARP) enzymes, PARP-1 and PARP-2, which play a role in DNA repair. In vitro studies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis and cell death. Increased niraparib-induced cytotoxicity was observed in tumor cell lines with or without deficiencies in BRCA1/2. Niraparib decreased tumor growth in mouse xenograft models of human cancer cell lines with deficiencies in BRCA1/2 and in human patient-derived xenograft tumor models with homologous recombination deficiency that had either mutated or wild type BRCA1/2.
Structure

Pharmacodynamics
- The pharmacodynamic response of niraparib has not been characterized.
Cardiovascular Effects
- Niraparib has the potential to cause effects on pulse rate and blood pressure in patients receiving the recommended dose, which may be related to pharmacological inhibition of the dopamine transporter (DAT), norepinephrine transporter (NET) and serotonin transporter (SERT).
- In the NOVA study, mean pulse rate and blood pressure increased over baseline in the niraparib arm relative to the placebo arm at all on-study assessments. Mean greatest increases from baseline in pulse rate on treatment were 24.1 and 15.8 beats/min in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in systolic blood pressure on treatment were 24.5 and 18.3 mmHg in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in diastolic blood pressure on treatment were 16.5 and 11.6 mmHg in the niraparib and placebo arms, respectively.
Cardiac Electrophysiology
- The potential for QTc prolongation with niraparib was evaluated in a randomized, placebo-controlled trial in cancer patients (367 patients on niraparib and 179 patients on placebo). No large changes in the mean QTc interval (>20 ms) were detected in the trial following the treatment of niraparib 300 mg once daily.
Pharmacokinetics
- Following a single-dose administration of 300 mg niraparib, the mean (±SD) peak plasma concentration (Cmax) was 804 (± 403) ng/mL. The systemic exposures (Cmax and AUC) of niraparib increased in a dose proportional manner with daily doses ranging from 30 mg (0.1 times the approved recommended dosage) to 400 mg (1.3 times the approved recommended dosage). The accumulation ratio of niraparib exposure following 21 days of repeated daily doses was approximately 2 fold for doses ranging from 30 mg to 400 mg.
Absorption
- The absolute bioavailability of niraparib is approximately 73%. Following oral administration of niraparib, peak plasma concentration, Cmax, is reached within 3 hours.
- Concomitant administration of a high fat meal (800-1,000 calories with approximately 50% of total caloric content of the meal from fat) did not significantly affect the pharmacokinetics of niraparib.
Distribution
- Niraparib is 83.0% bound to human plasma proteins. The average (±SD) apparent volume of distribution (Vd/F) was 1220 (±1114) L. In a population pharmacokinetic analysis, the Vd/F of niraparib was 1074 L in cancer patients.
Elimination
- Following multiple daily doses of 300 mg niraparib, the mean half-life (t1/2) is 36 hours. In a population pharmacokinetic analysis, the apparent total clearance (CL/F) of niraparib was 16.2 L/h in cancer patients.
Metabolism
- Niraparib is metabolized primarily by carboxylesterases (CEs) to form a major inactive metabolite, which subsequently undergoes glucuronidation.
Excretion
- Following administration of a single oral 300 mg dose of radio-labeled niraparib, the average percent recovery of the administered dose over 21 days was 47.5% (range 33.4% to 60.2%) in urine, and 38.8% (range 28.3% to 47.0%) in feces. In pooled samples collected over 6 days, unchanged niraparib accounted for 11% and 19% of the administered dose recovered in urine and feces, respectively.
Specific Populations
- Age (18 to 65 years old), race/ethnicity, and mild to moderate renal impairment had no clinically significant effect on the pharmacokinetics of niraparib.
- The effect of severe renal impairment or end-stage renal disease undergoing hemodialysis on the pharmacokinetics of niraparib is unknown.
- The effect of moderate or severe hepatic impairment on the pharmacokinetics of niraparib is unknown.
Drug Interaction Studies
- No formal drug interaction studies have been performed with niraparib.
- In Vitro Studies
- Inhibition of CYPs: Neither niraparib nor the major primary metabolite M1 is an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4.
- Induction of CYPs: Neither niraparib nor M1 is a CYP3A4 inducer. Niraparib weakly induces CYP1A2 in vitro.
- Substrate of CYPs: Niraparib is a substrate of carboxylesterases (CEs) and UDP-glucuronosyltransferases (UGTs) in vivo.
- Inhibition of transporter systems: Niraparib is a weak inhibitor of BCRP, but does not inhibit P-gp or BSEP. The M1 metabolite is not an inhibitor of P-gp, BCRP, or BSEP. Neither niraparib nor M1 is an inhibitor of organic anion transport polypeptide 1B1 (OATP1B1), 1B3 (OATP1B3), or organic cation transporter 1 (OCT1), organic anion transporter 1 (OAT1), 3 (OAT3), or organic cation transporter 2 (OCT2).
- Substrate of transporter systems: Niraparib is a substrate of P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP). Niraparib is not a substrate of bile salt export pump (BSEP). The M1 metabolite is not a substrate of P-gp, BCRP, or BSEP. Neither niraparib nor M1 is a substrate of organic anion transport polypeptide 1B1 (OATP1B1), 1B3 (OATP1B3), or organic cation transporter 1 (OCT1), organic anion transporter 1 (OAT1), 3 (OAT3), or organic cation transporter 2 (OCT2).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been conducted with niraparib.
- Niraparib was clastogenic in an in vitro mammalian chromosomal aberration assay and in an in vivo rat bone marrow micronucleus assay. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of niraparib and indicates potential for genotoxicity in humans. Niraparib was not mutagenic in a bacterial reverse mutation assay (Ames) test.
- Fertility studies in animals have not been conducted with niraparib. In repeat-dose oral toxicity studies, niraparib was administered daily for up to 3 months duration in rats and dogs. Reduced sperm, spermatids and germ cells in epididymides and testes were observed at doses ≥10 mg/kg and ≥1.5 mg/kg in rats and dogs, respectively. These dose levels resulted in systemic exposures approximately 0.3 and 0.012 times, respectively, the human exposure (AUC0-24hr) at the recommended dose of 300 mg daily. There was a trend toward reversibility of these findings 4 weeks after dosing was stopped.
Animal Toxicology and/or Pharmacology
- In vitro, niraparib bound to the dopamine transporter (DAT), norepinephrine transporter (NET) and serotonin transporter (SERT) and inhibited uptake of norepinephrine and dopamine in cells with IC50 values that were lower than the Cmin at steady-state in patients receiving the recommended dose. Niraparib has the potential to cause effects in patients related to inhibition of these transporters (e.g., cardiovascular or CNS).
- Intravenous administration of niraparib to vagotomized dogs over 30 minutes at 1, 3 and 10 mg/kg resulted in an increased range of arterial pressures of 13-20, 18-27 and 19-25% and increased range of heart rates of 2-11, 4-17 and 12-21% above pre-dose levels, respectively. The unbound plasma concentrations of niraparib in dogs at these dose levels were approximately 0.7, 2 and 8 times the unbound Cmax at steady-state in patients receiving the recommended dose.
- In addition, niraparib crossed the blood-brain barrier in rats and monkeys following oral administration. The cerebrospinal fluid (CSF):plasma Cmax ratios of niraparib administered at 10 mg/kg orally to two Rhesus monkeys were 0.10 and 0.52.
Clinical Studies
- Trial 1 (NOVA) was a double-blind, placebo-controlled trial in which patients (n=553) with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer were randomized 2:1 to niraparib 300 mg orally daily or matched placebo within 8 weeks of the last therapy. All patients had received at least two prior platinum-containing regimens and were in response (complete or partial) to their most recent platinum-based regimen.
- Randomization was stratified by time to progression after the penultimate platinum therapy (6 to <12 months and ≥12 months); use of bevacizumab in conjunction with the penultimate or last platinum regimen (yes/no); and best response during the most recent platinum regimen (complete response and partial response). Eligible patients were assigned to one of two cohorts based on the results of the BRACAnalysis CDx. Patients with deleterious or suspected deleterious germline BRCA mutations (gBRCAm) were assigned to the germline BRCA mutated (gBRCAmut) cohort (n=203), and those without germline BRCA mutations were assigned to the non-gBRCAmut cohort (n=350).
- The major efficacy outcome measure, PFS (progression-free survival), was determined primarily by central independent assessment per RECIST (Response Evaluation Criteria in Solid Tumors, version 1.1). In some cases, criteria other than RECIST, such as clinical signs and symptoms and increasing CA-125, were also applied.
- The median age of patients ranged from 57-64 years among patients treated with niraparib and 58-67 years among patients treated with placebo. Eighty-six percent of all patients were white. Sixty-seven percent of patients receiving niraparib and 69% of patients receiving placebo had an ECOG of 0 at study baseline. Approximately 40% of patients were enrolled in the U.S. or Canada and 51% of all patients were in complete response to most recent platinum-based regimen, with 39% on both arms with an interval of 6-12 months since the penultimate platinum regimen. Twenty-six percent of those treated with niraparib and 31% treated with placebo had received prior bevacizumab therapy. Approximately 40% of patients had 3 or more lines of treatment.
- The trial demonstrated a statistically significant improvement in PFS for patients randomized to niraparib as compared with placebo in the gBRCAmut cohort and the non-gBRCAmut cohort (Table 6, and Figures 1 and 2).



- At the time of the PFS analysis, limited overall survival data were available with 17% deaths across the two cohorts.
How Supplied
- Niraparib is available as capsules having a white body printed with "100 mg" in black ink, and a purple cap printed with "Niraparib" in white ink.
- Each capsule contains 100 mg of niraparib free base.
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Niraparib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Niraparib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling.
MDS/AML
- Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. This may be a sign of hematological toxicity or myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) which has been reported in patients treated with niraparib.
Bone Marrow Suppression
- Advise patients that periodic monitoring of their blood counts is required. Advise patients to contact their healthcare provider for new onset of bleeding, fever, or symptoms of infection.
Cardiovascular Effects
- Advise patients to undergo monthly blood pressure and heart rate monitoring for the first year of treatment and then periodically thereafter and to contact their healthcare provider if blood pressure is elevated.
Dosing Instructions
- Inform patients on how to take niraparib. Niraparib should be taken once daily. Instruct patients that if they miss a dose of niraparib, not to take an extra dose to make up for the one that they missed. They should take their next dose at the regularly scheduled time. Each capsule should be swallowed whole. Niraparib may be taken with or without food. Bedtime administration may be a potential method for managing nausea.
Embryo-Fetal Toxicity
- Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy.
Contraception
- Advise females of reproductive potential to use effective contraception during treatment with niraparib and for at least 6 months after receiving the last dose.
Lactation
- Advise patients not to breastfeed while taking niraparib and for 1 month after the last dose.

Precautions with Alcohol
Alcohol-Niraparib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Zejula
Look-Alike Drug Names
There is limited information regarding Niraparib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.