Niacin (extended-release tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Alberto Plate [5]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Niacin (extended-release tablet) is an antihyperlipidemic, nutriceutical, nutritive agent, Vitamin B that is FDA approved for the treatment of hyperlipidemia, prevention of recurrence of myocardial infarction, reduce atheroesclerotic plaque in CAD and hypertriglyceridemia in patients with risk for pancreatitis. Common adverse reactions include flushing, diarrhea, nausea, vomiting, increased cough, and pruritus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Niacin extended-release tablets USP should be taken at bedtime, after a low-fat snack, and doses should be individualized according to patient response. Therapy with niacin extended-release tablets USP must be initiated at 500 mg at bedtime in order to reduce the incidence and severity of side effects which may occur during early therapy. The recommended dose escalation is shown in Table 1 below.
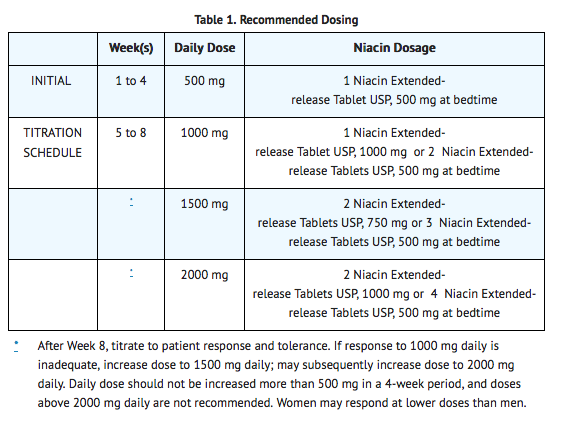
Maintenance Dose
- The daily dosage of niacin extended-release tablets USP should not be increased by more than 500 mg in any 4-week period. The recommended maintenance dose is 1000 mg (two 500 mg tablets or one 1000 mg tablet) to 2000 mg (two 1000 mg tablets or four 500 mg tablets) once daily at bedtime. Doses greater than 2000 mg daily are not recommended. Women may respond at lower niacin extended-release tablets USP doses than men.
- Single-dose bioavailability studies have demonstrated that two of the 500 mg and one of the 1000 mg tablet strengths are interchangeable but three of the 500 mg and two of the 750 mg tablet strengths are not interchangeable.
- If lipid response to niacin extended-release tablets USP alone is insufficient or if higher doses of niacin extended-release tablets USP are not well tolerated, some patients may benefit from combination therapy with a bile acid binding resin or statin.
- Tolerance to flushing of skin develops rapidly over the course of several weeks. Flushing, pruritus, and gastrointestinal distress are also greatly reduced by slowly increasing the dose of niacin and avoiding administration on an empty stomach. Concomitant alcoholic, hot drinks or spicy foods may increase the side effects of flushing and pruritus and should be avoided around the time of niacin extended-release tablets USP ingestion.
- Equivalent doses of niacin extended-release tablets USP should not be substituted for sustained-release (modified-release, timed-release) niacin preparations or immediate-release (crystalline) niacin. Patients previously receiving other niacin products should be started with the recommended niacin extended-release tablets USP titration schedule (see Table 1), and the dose should subsequently be individualized based on patient response.
- If niacin extended-release tablets USP therapy is discontinued for an extended period, reinstitution of therapy should include a titration phase (see Table 1).
- Niacin extended-release tablets USP should be taken whole and should not be broken, crushed or chewed before swallowing.
Concomitant Therapy
Concomitant Therapy with Lovastatin or Simvastatin
Patients already receiving a stable dose of lovastatin or simvastatin who require further TG-lowering or HDL-raising (e.g., to achieve NCEP non-HDL-C goals), may receive concomitant dosage titration with niacin extended-release tablets USP per niacin extended-release tablets USP recommended initial titration schedule. For patients already receiving a stable dose of niacin extended-release tablets USP who require further LDL-lowering (e.g., to achieve NCEP LDL-C goals), the usual recommended starting dose of lovastatin and simvastatin is 20 mg once a day. Dose adjustments should be made at intervals of 4 weeks or more. Combination therapy with niacin extended-release tablets USP and lovastatin or niacin extended-release tablets USP and simvastatin should not exceed doses of 2000 mg niacin extended-release tablets USP and 40 mg lovastatin or simvastatin daily.
Dosage in Patients with Renal or Hepatic Impairment
Use of niacin extended-release tablets USP in patients with renal or hepatic impairment has not been studied. Niacin extended-release tablet USP is contraindicated in patients with significant or unexplained hepatic dysfunction. Niacin extended-release tablets USP should be used with caution in patients with renal impairment
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Niacin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Niacin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Niacin (extended-release tablet) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Niacin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Niacin in pediatric patients.
Contraindications
Niacin extended-release tablet is contraindicated in the following conditions:
- Active liver disease or unexplained persistent elevations in hepatic transaminases.
- Patients with active peptic ulcer disease
- Patients with arterial bleeding
- Hypersensitivity to niacin or any component of this medication.
Warnings
Niacin extended-release tablet preparations should not be substituted for equivalent doses of immediate-release (crystalline) niacin. For patients switching from immediate-release niacin to niacin extended-release tablets, therapy with niacin extended-release tablets should be initiated with low doses (i.e., 500 mg at bedtime) and the niacin extended-release tablets dose should then be titrated to the desired therapeutic response.
Caution should also be used when niacin extended-release tablet is used in patients with unstable angina or in the acute phase of an MI, particularly when such patients are also receiving vasoactive drugs such as nitrates, calcium channel blockers, or adrenergic blocking agents.
Niacin is rapidly metabolized by the liver, and excreted through the kidneys. Niacin extended-release tablet is contraindicated in patients with significant or unexplained hepatic impairment and should be used with caution in patients with renal impairment. Patients with a past history of jaundice, hepatobiliary disease, or peptic ulcer should be observed closely during niacin extended-release tablets therapy.
Mortality and Coronary Heart Disease Morbidity
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial was a randomized placebo-controlled trial of 3414 patients with stable, previously diagnosed cardiovascular disease. Mean baseline lipid levels were LDL-C 74 mg/dL, HDL-C 35 mg/dL, non-HDL-C 111 mg/dL and median triglyceride level of 163 to 177 mg/dL. Ninety-four percent of patients were on background statin therapy prior to entering the trial. All participants received simvastatin, 40 to 80 mg per day, plus ezetimibe 10 mg per day if needed, to maintain an LDL-C level of 40 to 80 mg/dL, and were randomized to receive niacin extended-release tablets 1500 to 2000 mg/day (n=1718) or matching placebo (IR Niacin, 100 - 150 mg, n=1696). On-treatment lipid changes at two years for LDL-C were -12.0% for the simvastatin plus niacin extended-release tablets group and -5.5% for the simvastatin plus placebo group. HDL-C increased by 25.0% to 42 mg/dL in the simvastatin plus niacin extended-release tablets group and by 9.8% to 38 mg/dL in the simvastatin plus placebo group (P<0.001). Triglyceride levels decreased by 28.6% in the simvastatin plus niacin extended-release tablets group and by 8.1% in the simvastatin plus placebo group. The primary outcome was an ITT composite of the first study occurrence of coronary heart disease death, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome or symptom-driven coronary or cerebral revascularization procedures. The trial was stopped after a mean follow-up period of 3 years owing to a lack of efficacy. The primary outcome occurred in 282 patients in the simvastatin plus niacin extended-release tablets group (16.4%) and in 274 patients in the simvastatin plus placebo group (16.2%) (HR 1.02 [95% CI, 0.87 to 1.21], P=0.79. In an ITT analysis, there were 42 cases of first occurrence of ischemic stroke reported, 27 (1.6%) in the simvastatin plus niacin extended-release tablets group and 15 (0.9%) in the simvastatin plus placebo group, a non-statistically significant result (HR 1.79, [95%CI = 0.95 to 3.36], p=0.071). The on-treatment ischemic stroke events were 19 for the simvastatin plus niacin extended-release tablets group and 15 for the simvastatin plus placebo group.
Skeletal Muscle
Cases of rhabdomyolysis have been associated with concomitant administration of lipid-altering doses (≥1 g/day) of niacin and statins. Physicians contemplating combined therapy with statins and niacin extended-release tablets should carefully weigh the potential benefits and risks and should carefully monitor patients for any signs and symptoms of muscle pain, tenderness, or weakness, particularly during the initial months of therapy and during any periods of upward dosage titration of either drug. Periodic serum creatine phosphokinase (CPK) and potassium determinations should be considered in such situations, but there is no assurance that such monitoring will prevent the occurrence of severe myopathy.
The risk for myopathy and rhabdomyolysis are increased when lovastatin or simvastatin are coadministered with niacin extended-release tablets, particularly in elderly patients and patients with diabetes, renal failure, or uncontrolled hypothyroidism.
Liver Dysfunction
Cases of severe hepatic toxicity, including fulminant hepatic necrosis, have occurred in patients who have substituted sustained-release (modified-release, timed-release) niacin products for immediate-release (crystalline) niacin at equivalent doses.
Niacin extended-release tablets should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained transaminase elevations are contraindications to the use of niacin extended-release tablets.
Niacin preparations have been associated with abnormal liver tests. In three placebo-controlled clinical trials involving titration to final daily niacin extended-release tablets doses ranging from 500 to 3000 mg, 245 patients received niacin extended-release tablets for a mean duration of 17 weeks. No patient with normal serum transaminase levels (AST, ALT) at baseline experienced elevations to more than 3 times the upper limit of normal (ULN) during treatment with niacin extended-release tablets. In these studies, fewer than 1% (2/245) of niacin extended-release tablets patients discontinued due to transaminase elevations greater than 2 times the ULN.
In three safety and efficacy studies with a combination tablet of niacin extended-release tablets and lovastatin involving titration to final daily doses (expressed as mg of niacin/ mg of lovastatin) 500 mg/10 mg to 2500 mg/40 mg, ten of 1028 patients (1.0%) experienced reversible elevations in AST/ALT] to more than 3 times the ULN. Three of ten elevations occurred at doses outside the recommended dosing limit of 2000 mg/40 mg; no patient receiving 1000 mg/20 mg had 3-fold elevations in AST/ALT.
Niacin extended-release and simvastatin can cause abnormal liver tests. In a simvastatin- controlled, 24 week study with a fixed dose combination of niacin extended-release tablets and simvastatin in 641 patients, there were no persistent increases (more than 3x the ULN) in serum transaminases. In three placebo-controlled clinical studies of extended-release niacin there were no patients with normal serum transaminase levels at baseline who experienced elevations to more than 3x the ULN. Persistent increases (more than 3x the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies. When drug treatment was interrupted or discontinued in these patients, the transaminases levels usually fell slowly to pretreatment levels. The increases were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity.
In the placebo-controlled clinical trials and the long-term extension study, elevations in transaminases did not appear to be related to treatment duration; elevations in AST levels did appear to be dose related. Transaminase elevations were reversible upon discontinuation of niacin extended-release tablets.
Liver function tests should be performed on all patients during therapy with niacin extended-release tablets. Serum transaminase levels, including AST and ALT (SGOT and SGPT), should be monitored before treatment begins, every 6 to 12 weeks for the first year, and periodically thereafter (e.g., at approximately 6-month intervals). Special attention should be paid to patients who develop elevated serum transaminase levels, and in these patients, measurements should be repeated promptly and then performed more frequently. If the transaminase levels show evidence of progression, particularly if they rise to 3 times ULN and are persistent, or if they are associated with symptoms of nausea, fever, and/or malaise, the drug should be discontinued.
Laboratory Abnormalities
- Increase in Blood Glucose: Niacin treatment can increase fasting blood glucose. Frequent monitoring of blood glucose should be performed to ascertain that the drug is producing no adverse effects. Diabetic patients may experience a dose-related increase in glucose intolerance. Diabetic or potentially diabetic patients should be observed closely during treatment with niacin extended-release tablets, particularly during the first few months of use or dose adjustment; adjustment of diet and/or hypoglycemic therapy may be necessary.
- Reduction in platelet count: Niacin extended-release tablet has been associated with small but statistically significant dose-related reductions in platelet count (mean of -11% with 2000 mg). Caution should be observed when niacin extended-release tablet is administered concomitantly with anticoagulants; platelet counts should be monitored closely in such patients.
- Increase in Prothrombin Time (PT): Niacin extended-release tablet has been associated with small but statistically significant increases in prothrombin time (mean of approximately +4%); accordingly, patients undergoing surgery should be carefully evaluated. Caution should be observed when niacin extended-release tablet is administered concomitantly with anticoagulants; prothrombin time should be monitored closely in such patients.
- Increase in Uric Acid: Elevated uric acid levels have occurred with niacin therapy, therefore use with caution in patients predisposed to gout.
- Decrease in Phosphorus: In placebo-controlled trials, niacin extended-release tablet has been associated with small but statistically significant, dose-related reductions in phosphorus levels (mean of -13% with 2000 mg). Although these reductions were transient, phosphorus levels should be monitored periodically in patients at risk for hypophosphatemia.
Adverse Reactions
Clinical Trials Experience
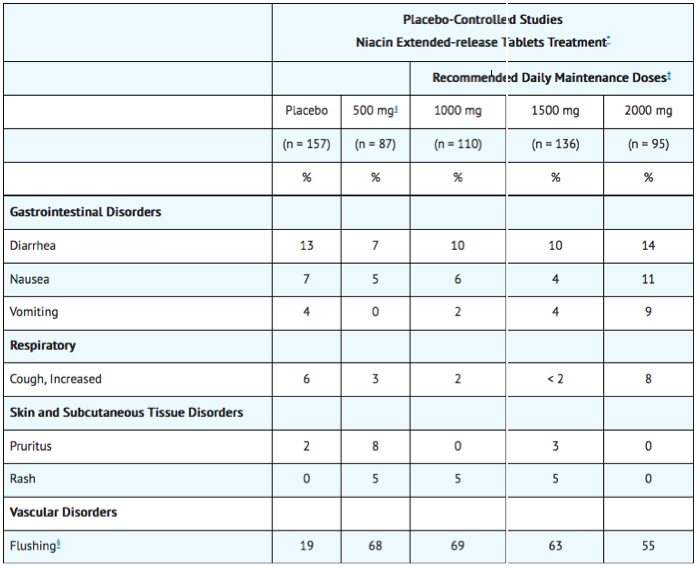
In the placebo-controlled clinical trials database of 402 patients (age range 21 to 75 years, 33% women, 89% Caucasians, 7% Blacks, 3% Hispanics, 1% Asians) with a median treatment duration of 16 weeks. 16% of patients on niacin extended-release tablets and 4% of patients on placebo discontinued due to adverse reactions. The most common adverse reactions in the group of patients treated with niacin extended-release tablets that led to treatment discontinuation and occurred at a rate greater than placebo were flushing (6% vs. 0%), rash (2% vs. 0%), diarrhea (2% vs. 0%), nausea (1% vs. 0%), and vomiting (1% vs. 0%). The most commonly reported adverse reactions (incidence >5% and greater than placebo) in the niacin extended-release tablets controlled clinical trial database of 402 patients were flushing, diarrhea, nausea, vomiting, increased cough and pruritus.
In the placebo-controlled clinical trials, flushing episodes (i.e., warmth, redness, itching and/or tingling) were the most common treatment-emergent adverse reactions (reported by as many as 88% of patients) for niacin extended-release tablets. Spontaneous reports suggest that flushing may also be accompanied by symptoms of dizziness, tachycardia, palpitations, shortness of breath, sweating, burning sensation/skin burning sensation, chills, and/or edema, which in rare cases may lead to syncope. In pivotal studies, 6% (14/245) of niacin extended-release tablets patients discontinued due to flushing. In comparisons of immediate-release (IR) niacin and niacin extended-release tablets, although the proportion of patients who flushed was similar, fewer flushing episodes were reported by patients who received niacin extended-release tablets. Following 4 weeks of maintenance therapy at daily doses of 1500 mg, the incidence of flushing over the 4-week period averaged 8.6 events per patient for IR niacin versus 1.9 following niacin extended-release tablets.
Other adverse reactions occurring in ≥5% of patients treated with niacin extended-release tablets and at an incidence greater than placebo are shown in Table 2 below.
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH)
In AIM-HIGH involving 3414 patients (mean age of 64 years, 15% women, 92% Caucasians, 34% with diabetes mellitus) with stable, previously diagnosed cardiovascular disease, all patients received simvastatin, 40 to 80 mg per day, plus ezetimibe 10 mg per day if needed, to maintain an LDL-C level of 40 to 80 mg/dL, and were randomized to receive niacin extended-release tablets 1500 to 2000 mg/day (n=1718) or matching placebo (IR Niacin, 100 to 150 mg, n=1696). The incidence of the adverse reactions of "blood glucose increased" (6.4% vs. 4.5%) and "diabetes mellitus" (3.6% vs. 2.2%) was significantly higher in the simvastatin plus niacin extended-release tablets group as compared to the simvastatin plus placebo group. There were 5 cases of rhabdomyolysis reported, 4 (0.2%) in the simvastatin plus niacin extended-release tablets group and one (<0.1%) in the simvastatin plus placebo group.
Postmarketing Experience
Because the below reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following additional adverse reactions have been identified during post-approval use of niacin extended-release tablets:
Hypersensitivity Reactions
Respiratory System
General Effects
Cardiovascular System
Special Senses
Gastrointestinal System
Musculoeskeletal System
Neuropsychiatric System
Clinical Laboratory Abnormalities
- Chemistry: Elevations in serum transaminases [see WARNINGS AND PRECAUTIONS (5.3)], LDH, fasting glucose, uric acid, total bilirubin, amylase and creatine kinase, and reduction in phosphorus.
- Hematology: Slight reductions in platelet counts and prolongation in prothrombin time.
Drug Interactions
Statins
Caution should be used when prescribing niacin (≥ 1 gm/day) with statins as these drugs can increase risk of myopathy/rhabdomyolysis. Combination therapy with niacin extended-release tablets and lovastatin or niacin extended-release tablets and simvastatin should not exceed doses of 2000 mg niacin extended-release tablets and 40 mg lovastatin or simvastatin daily.
Bile Acid Sequestrants
An in vitro study results suggest that the bile acid-binding resins have high niacin binding capacity. Therefore, 4 to 6 hours, or as great an interval as possible, should elapse between the ingestion of bile acid-binding resins and the administration of niacin extended-release tablets.
Aspirin
Concomitant aspirin may decrease the metabolic clearance of nicotinic acid. The clinical relevance of this finding is unclear.
Antihypertensive Therapy
Niacin may potentiate the effects of ganglionic blocking agents and vasoactive drugs resulting in postural hypotension.
Other
Vitamins or other nutritional supplements containing large doses of niacin or related compounds such as nicotinamide may potentiate the adverse effects of niacin extended-release tablets.
Laboratory Test Interactions
Niacin may produce false elevations in some fluorometric determinations of plasma or urinary catecholamines. Niacin may also give false-positive reactions with cupric sulfate solution (Benedict's reagent) in urine glucose tests.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Animal reproduction studies have not been conducted with niacin or with niacin extended-release tablets. It is also not known whether niacin at doses typically used for lipid disorders can cause fetal harm when administered to pregnant women or whether it can affect reproductive capacity. If a woman receiving niacin for primary hyperlipidemia becomes pregnant, the drug should be discontinued. If a woman being treated with niacin for hypertriglyceridemia conceives, the benefits and risks of continued therapy should be assessed on an individual basis.
All statins are contraindicated in pregnant and nursing women. When niacin extended-release tablet is administered with a statin in a woman of childbearing potential, refer to the pregnancy category and product labeling for the statin.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Niacin (extended-release tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Niacin (extended-release tablet) during labor and delivery.
Nursing Mothers
Niacin is excreted into human milk but the actual infant dose or infant dose as a percent of the maternal dose is not known. Because of the potential for serious adverse reactions in nursing infants from lipid-altering doses of nicotinic acid, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. No studies have been conducted with niacin extended-release tablets in nursing mothers.
Pediatric Use
Safety and effectiveness of niacin therapy in pediatric patients (≤16 years) have not been established.
Geriatic Use
Of 979 patients in clinical studies of niacin extended-release tablets, 21% of the patients were age 65 and over. No overall differences in safety and effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
Data from the clinical trials suggest that women have a greater hypolipidemic response than men at equivalent doses of niacin extended-release tablets.
Race
There is no FDA guidance on the use of Niacin (extended-release tablet) with respect to specific racial populations.
Renal Impairment
No studies have been performed in this population. Niacin extended-release tablets should be used with caution in patients with renal impairment.
Hepatic Impairment
No studies have been performed in this population. Niacin extended-release tablets should be used with caution in patients with a past history of liver disease and/or who consume substantial quantities of alcohol. Active liver disease, unexplained transaminase elevations and significant or unexplained hepatic dysfunction are contraindications to the use of niacin extended-release tablets
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Niacin (extended-release tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Niacin (extended-release tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Niacin (extended-release tablet) Administration in the drug label.
Monitoring
There is limited information regarding Niacin (extended-release tablet) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Niacin (extended-release tablet) and IV administrations.
Overdosage
Supportive measures should be undertaken in the event of an overdose.
Pharmacology
Mechanism of Action
The mechanism by which niacin alters lipid profiles has not been well defined. It may involve several actions including partial inhibition of release of free fatty acids from adipose tissue, and increased lipoprotein lipase activity, which may increase the rate of chylomicron triglyceride removal from plasma. Niacin decreases the rate of hepatic synthesis of VLDL and LDL, and does not appear to affect fecal excretion of fats, sterols, or bile acids.
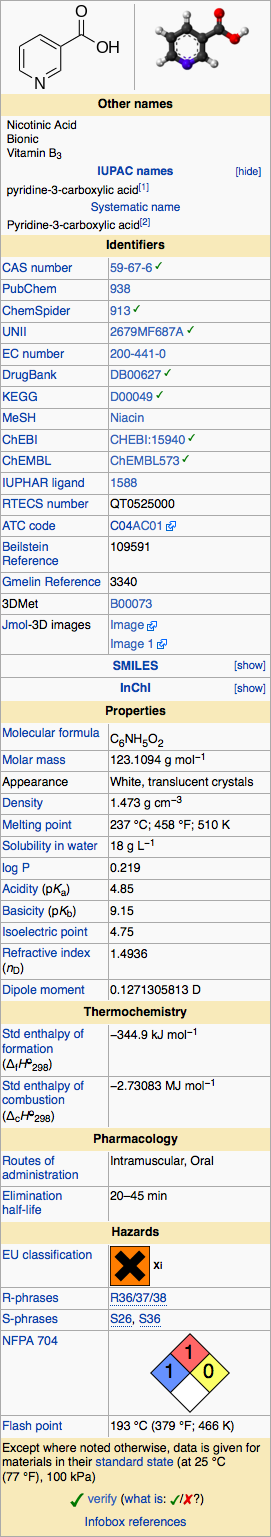
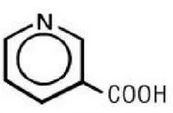
Structure
Niacin extended-release tablets USP (film-coated), contain niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the following structural formula:
Pharmacodynamics
Niacin functions in the body after conversion to nicotinamide adenine dinucleotide (NAD) in the NAD coenzyme system. Niacin (but not nicotinamide) in gram doses reduces total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), and triglycerides (TG), and increases high-density lipoprotein cholesterol (HDL-C). The magnitude of individual lipid and lipoprotein responses may be influenced by the severity and type of underlying lipid abnormality. The increase in HDL-C is associated with an increase in apolipoprotein A-I (Apo A-I]]) and a shift in the distribution of HDL subfractions. These shifts include an increase in the HDL2:HDL3 ratio, and an elevation in lipoprotein A-I (Lp A-I, an HDL-C particle containing only Apo A-I). Niacin treatment also decreases serum levels of apolipoprotein B-100 (Apo B), the major protein component of the very low-density lipoprotein (VLDL) and LDL fractions, and of Lp(a), a variant form of LDL independently associated with coronary risk. In addition, preliminary reports suggest that niacin causes favorable LDL particle size transformations, although the clinical relevance of this effect requires further investigation. The effect of niacin-induced changes in lipids/proteins on cardiovascular morbidity or mortality in individuals without preexisting coronary disease has not been established.
A variety of clinical studies have demonstrated that elevated levels of TC, LDL-C, and Apo B promote human atherosclerosis. Similarly, decreased levels of HDL-C are associated with the development of atherosclerosis. Epidemiological investigations have established that cardiovascular morbidity and mortality vary directly with the level of Total-C and LDL-C, and inversely with the level of HDL-C.
Like LDL, cholesterol-enriched triglyceride-rich lipoproteins, including VLDL, intermediate-density lipoprotein (IDL), and their remnants, can also promote atherosclerosis. Elevated plasma TG are frequently found in a triad with low HDL-C levels and small LDL particles, as well as in association with non-lipid metabolic risk factors for coronary heart disease (CHD). As such, total plasma TG has not consistently been shown to be an independent risk factor for CHD. Furthermore, the independent effect of raising HDL-C or lowering TG on the risk of coronary and cardiovascular morbidity and mortality has not been determined.
Pharmacokinetics
Absorption
Due to extensive and saturable first-pass metabolism, niacin concentrations in the general circulation are dose dependent and highly variable. Time to reach the maximum niacin plasma concentrations was about 5 hours following niacin extended-release tablets. To reduce the risk of gastrointestinal (GI) upset, administration of niacin extended-release tablets with a low-fat meal or snack is recommended.
Single-dose bioavailability studies have demonstrated that the 500 mg and 1000 mg tablet strengths are dosage form equivalent but the 500 mg and the 750 mg tablet strengths are not dosage form equivalent.
Metabolism
The pharmacokinetic profile of niacin is complicated due to extensive first-pass metabolism that is dose-rate specific and, at the doses used to treat dyslipidemia, saturable. In humans, one pathway is through a simple conjugation step with glycine to form nicotinuric acid (NUA). NUA is then excreted in the urine, although there may be a small amount of reversible metabolism back to niacin. The other pathway results in the formation of nicotinamide adenine dinucleotide (NAD). It is unclear whether nicotinamide is formed as a precursor to, or following the synthesis of, NAD. Nicotinamide is further metabolized to at least N-methylnicotinamide (MNA) and nicotinamide-N-oxide (NNO). MNA is further metabolized to two other compounds, N-methyl-2-pyridone-5-carboxamide (2PY) and N-methyl-4-pyridone-5-carboxamide (4PY). The formation of 2PY appears to predominate over 4PY in humans. At the doses used to treat hyperlipidemia, these metabolic pathways are saturable, which explains the nonlinear relationship between niacin dose and plasma concentrations following multiple-dose niacin extended-release tablets administration.
Nicotinamide does not have hypolipidemic activity; the activity of the other metabolites is unknown.
Elimination
Following single and multiple doses, approximately 60 to 76% of the niacin dose administered as niacin extended-release tablet was recovered in urine as niacin and metabolites; up to 12% was recovered as unchanged niacin after multiple dosing. The ratio of metabolites recovered in the urine was dependent on the dose administered.
Nonclinical Toxicology
There is limited information regarding Niacin (extended-release tablet) Nonclinical Toxicology in the drug label.
Clinical Studies
Niacin Clinical Studies
The role of LDL-C in atherogenesis is supported by pathological observations, clinical studies, and many animal experiments. Observational epidemiological studies have clearly established that high total cholesterol or LDL-C and low HDL-C are risk factors for CHD. Additionally, elevated levels of Lp(a) have been shown to be independently associated with CHD risk.
Niacin's ability to reduce mortality and the risk of definite, nonfatal myocardial infarction (MI) has been assessed in long-term studies. The Coronary Drug Project, completed in 1975, was designed to assess the safety and efficacy of niacin and other lipid-altering drugs in men 30 to 64 years old with a history of MI. Over an observation period of 5 years, niacin treatment was associated with a statistically significant reduction in nonfatal, recurrent MI. The incidence of definite, nonfatal MI was 8.9% for the 1,119 patients randomized to nicotinic acid versus 12.2% for the 2,789 patients who received placebo (p <0.004). Total mortality was similar in the two groups at 5 years (24.4% with nicotinic acid versus 25.4% with placebo; p =N.S.). At the time of a 15-year follow-up, there were 11% (69) fewer deaths in the niacin group compared to the placebo cohort (52.0% versus 58.2%; p =0.0004). However, mortality at 15 years was not an original endpoint of the Coronary Drug Project. In addition, patients had not received niacin for approximately 9 years, and confounding variables such as concomitant medication use and medical or surgical treatments were not controlled.
The Cholesterol-Lowering Atherosclerosis Study (CLAS) was a randomized, placebo-controlled, angiographic trial testing combined colestipol and niacin therapy in 162 non-smoking males with previous coronary bypass surgery. The primary, per-subject cardiac endpoint was global coronary artery change score. After 2 years, 61% of patients in the placebo cohort showed disease progression by global change score (n=82), compared with only 38.8% of drug-treated subjects (n=80), when both native arteries and grafts were considered (p <0.005); disease regression also occurred more frequently in the drug-treated group (16.2% versus 2.4%; p =0.002). In a follow-up to this trial in a subgroup of 103 patients treated for 4 years, again, significantly fewer patients in the drug-treated group demonstrated progression than in the placebo cohort (48% versus 85%, respectively; p <0.0001).
The Familial Atherosclerosis Treatment Study (FATS) in 146 men ages 62 and younger with Apo B levels ≥125 mg/dL, established coronary artery disease, and family histories of vascular disease, assessed change in severity of disease in the proximal coronary arteries by quantitative arteriography. Patients were given dietary counseling and randomized to treatment with either conventional therapy with double placebo (or placebo plus colestipol if the LDL-C was elevated); lovastatin plus colestipol; or niacin plus colestipol. In the conventional therapy group, 46% of patients had disease progression (and no regression) in at least one of nine proximal coronary segments; regression was the only change in 11%. In contrast, progression (as the only change) was seen in only 25% in the niacin plus colestipol group, while regression was observed in 39%. Though not an original endpoint of the trial, clinical events (death, MI, or revascularization for worsening angina) occurred in 10 of 52 patients who received conventional therapy, compared with 2 of 48 who received niacin plus colestipol.
The Harvard Atherosclerosis Reversibility Project (HARP) was a randomized placebo-controlled, 2.5-year study of the effect of a stepped-care antihyperlipidemic drug regimen on 91 patients (80 men and 11 women) with CHD and average baseline TC levels less than 250 mg/dL and ratios of TC to HDL-C greater than 4.0. Drug treatment consisted of an HMG-CoA reductase inhibitor administered alone as initial therapy followed by addition of varying dosages of either a slow-release nicotinic acid, cholestyramine, or gemfibrozil. Addition of nicotinic acid to the HMG-CoA reductase inhibitor resulted in further statistically significant mean reductions in TC, LDL-C, and TG, as well as a further increase in HDL-C in a majority of patients (40 of 44 patients). The ratios of TC to HDL-C and LDL-C to HDL-C were also significantly reduced by this combination drug regimen.
Niacin Extended-release Tablets Clinical Studies
Placebo-Controlled Clinical Studies in Patients with Primary Hyperlipidemia and Mixed Dyslipidemia
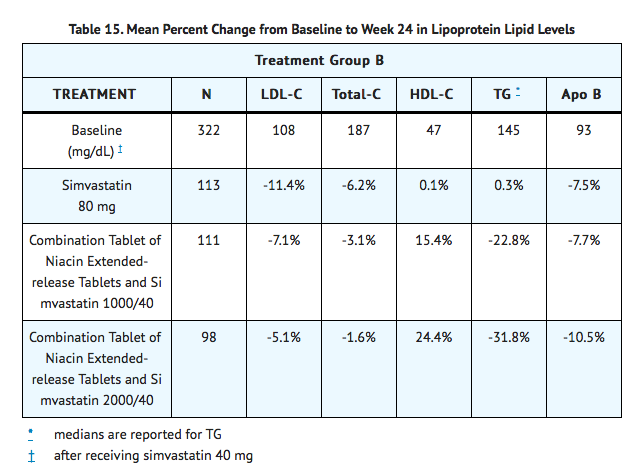
In two randomized, double-blind, parallel, multi-center, placebo-controlled trials, niacin extended-release tablets dosed at 1000, 1500 or 2000 mg daily at bedtime with a low-fat snack for 16 weeks (including 4 weeks of dose escalation) favorably altered lipid profiles compared to placebo (Table 3). Women appeared to have a greater response than men at each niacin extended-release tablet dose level (see Gender Effect, below).
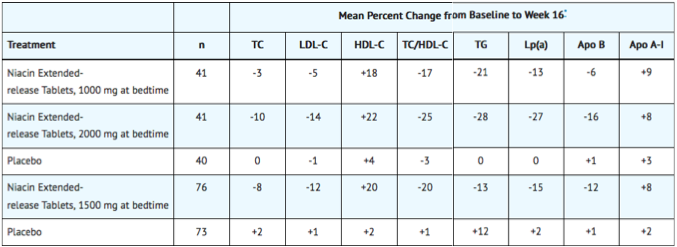
In a double-blind, multi-center, forced dose-escalation study, monthly 500 mg increases in niacin extended-release tablet dose resulted in incremental reductions of approximately 5% in LDL-C and Apo B levels in the daily dose range of 500 mg through 2000 mg (TABLE 4). Women again tended to have a greater response to niacin extended-release tablets than men.
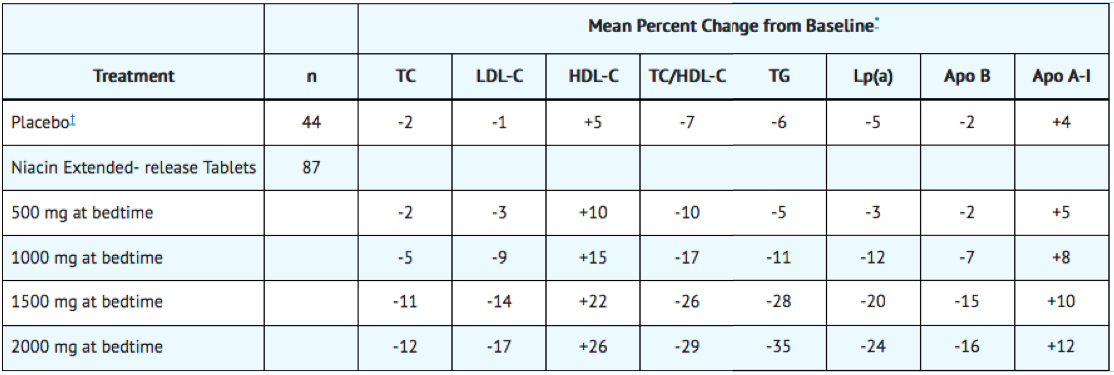
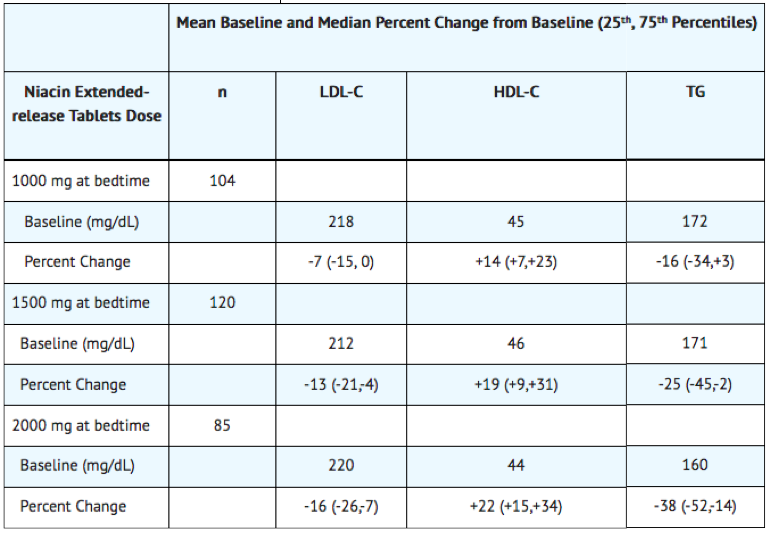
Pooled results for major lipids from these three placebo-controlled studies are shown below (Table 5).
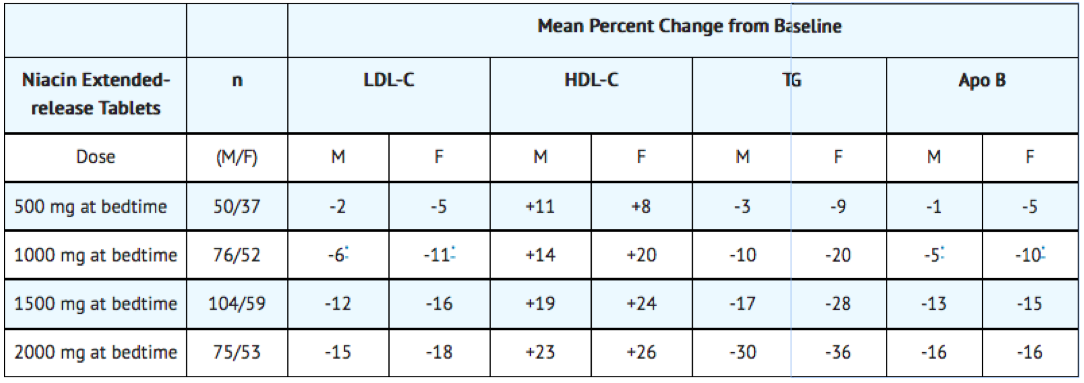
Gender Effect
Combined data from the three placebo-controlled niacin extended-release tablets studies in patients with primary hyperlipidemia and mixed dyslipidemia suggest that, at each niacin extended-release tablets dose level studied, changes in lipid concentrations are greater for women than for men.
Other Patient Populations
In a double-blind, multi-center, 19-week study the lipid-altering effects of niacin extended-release tablet (forced titration to 2000 mg at bedtime) were compared to baseline in patients whose primary lipid abnormality was a low level of HDL-C (HDL-C ≤40 mg/dL, TG ≤400 mg/dL, and LDL-C ≤160, or <130 mg/dL in the presence of CHD). Results are shown below
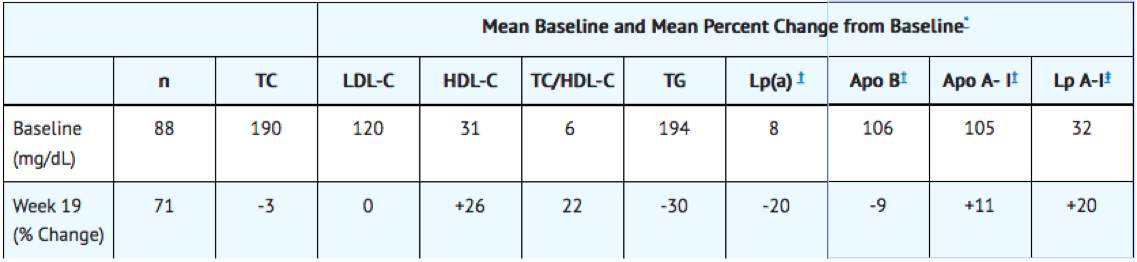
At niacin extended-release tablets 2000 mg/day, median changes from baseline (25th, 75th percentiles) for LDL-C, HDL-C, and TG were -3% (-14, +12%), +27% (+13, +38%), and -33% (-50, -19%), respectively.
Niacin Extended-release Tablets and Lovastatin Clinical Studies
Combination niacin extended-release tablets and Lovastatin Study
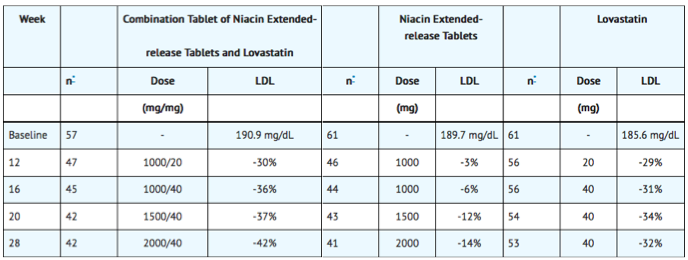
In a multi-center, randomized, double-blind, parallel, 28-week study, a combination tablet of niacin extended-release tablets and lovastatin was compared to each individual component in patients with Type IIa and IIb hyperlipidemia. Using a forced dose-escalation study design, patients received each dose for at least 4 weeks. Patients randomized to treatment with the combination tablet of niacin extended-release tablets and lovastatin initially received 500 mg/20 mg (expressed as mg of niacin/mg of lovastatin) once daily before bedtime. The dose was increased by 500 mg at 4-week intervals (based on the niacin extended-release tablets component) to a maximum dose of 1000 mg/20 mg in one-half of the patients and 2000 mg/40 mg in the other half. The niacin extended-release tablets monotherapy group underwent a similar titration from 500 mg to 2000 mg. The patients randomized to lovastatin monotherapy received 20 mg for 12 weeks titrated to 40 mg for up to 16 weeks. Up to a third of the patients randomized to the combination tablet of niacin extended-release tablets and lovastatin or niacin extended-release tablets monotherapy discontinued prior to Week 28. Results from this study showed that combination therapy decreased LDL-C, TG and Lp(a), and increased HDL-C in a dose-dependent fashion (Tables 8, 9, 10, and 11). Results from this study for LDL-C mean percent change from baseline (the primary efficacy variable) showed that:
- LDL-lowering with the combination tablet of niacin extended-release tablets and lovastatin was significantly greater than that achieved with lovastatin 40 mg only after 28 weeks of titration to a dose of 2000 mg/40 mg (p <0.
- The combination tablet of niacin extended-release tablets and lovastatin at doses of 1000 mg/20 mg or higher achieved greater LDL-lowering than niacin extended-release tablets (p<0.0001).
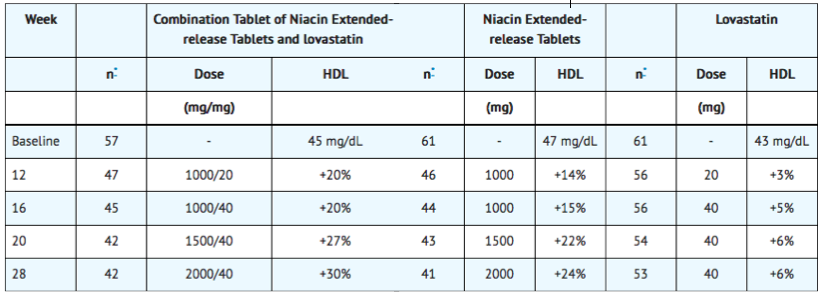
Combination therapy achieved significantly greater HDL-raising compared to lovastatin and niacin extended-release tablets monotherapy at all doses.
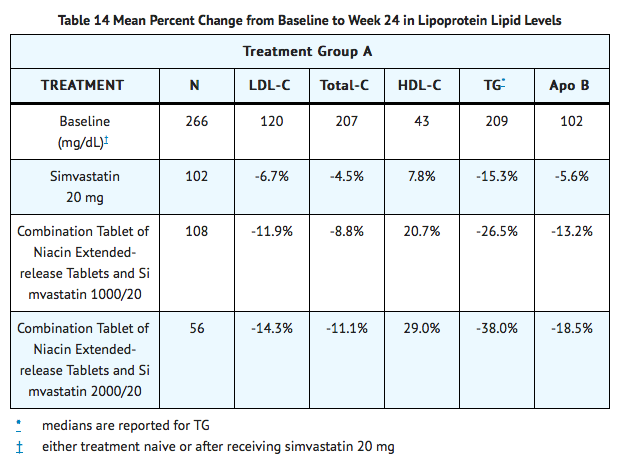
In addition, combination therapy achieved significantly greater TG-lowering at doses of 1000 mg/ 20 mg or greater compared to lovastatin and niacin extended-release tablets monotherapy
How Supplied
Niacin extended-release tablets USP are supplied as orange coloured, film-coated, capsule-shaped tablets containing 500 mg of niacin in an extended-release formulation. Tablets are debossed "LU" on one side and "D11" on the other side. Tablets are supplied in bottles of 100 and 1000s as shown below.
- 500 mg tablets: bottles of 100 - NDC# 68180-221-01
- 500 mg tablets: bottles of 1000 - NDC# 68180-221-03
Niacin extended-release tablets USP are supplied as orange coloured, film-coated, capsule-shaped tablets containing 750 mg of niacin in an extended-release formulation. Tablets are debossed "LU" on one side and "D12" on the other side. Tablets are supplied in bottles of 100 and 500s as shown below.
- 750 mg tablets: bottles of 100 - NDC# 68180-222-01
- 750 mg tablets: bottles of 500 - NDC# 68180-222-02
Niacin extended-release tablets USP are supplied as orange coloured, film-coated, oval-shaped tablets containing 1000 mg of niacin in an extended-release formulation. Tablets are debossed "LU" on one side and "D13" on the other side. Tablets are supplied in bottles of 100 and 1000s as shown below.
- 1000 mg tablets: bottles of 100 - NDC# 68180-223-01
- 1000 mg tablets: bottles of 1000 - NDC# 68180-223-03
Storage
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F)
Images
Drug Images
{{#ask: Page Name::Niacin (extended-release tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Niacin (extended-release tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Niacin (extended-release tablet) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Niacin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Niacin (extended-release tablet) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Niacin (extended-release tablet) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [6]
Overview
Niacin, also known as nicotinic acid or vitamin B3, is a water-soluble vitamin discovered by Conrad Elvehjem in 1937. Its derivatives, NADH, NAD, NAD+, and NADP play essential roles in energy metabolism in the living cell and DNA repair (an enzymatic process in a living cell). [1] The designation vitamin B3 also includes the corresponding amide nicotinamide (or "niacinamide"), whose chemical formula is C6H6N2O.
Other functions of niacin include removing toxic chemicals from the body,[2] and assisting in the production of steroid hormones made by the adrenal gland, such as sex hormones and stress-related hormones.
History
Niacin was first discovered from the oxidation of nicotine to form nicotinic acid. When the properties of nicotinic acid were discovered, it was thought prudent to choose a name to dissociate it from nicotine, in order to avoid the perception that vitamins or niacin-rich food contains nicotine. The resulting name 'niacin' was derived from nicotinic acid + vitamin.
Niacin is also referred to as Vitamin B3 because it was the third of the B vitamins to be discovered. It has historically been referred to as "vitamin PP", a name derived from the term "pellagra-preventing factor".
Dietary needs
The recommended daily allowance of niacin is 2-12 mg a day for children, 14 mg a day for women, 16 mg a day for men, and 18 mg a day for pregnant or breast-feeding women.[3]
Severe deficiency of niacin in the diet causes the disease pellagra, whereas mild deficiency slows down the metabolism, causing decreased tolerance to cold.
Dietary niacin deficiency tends to occur only in areas where people eat corn (maize), the only grain low in niacin, as a staple food, and that do not use lime during meal/flour production. Alkali lime releases the tryptophan from the corn in a process called nixtamalization so that it can be absorbed in the intestine, and converted to niacin.[2]
Pharmacological uses
Niacin, when taken in large doses, blocks the breakdown of fats in adipose tissue, thus altering blood lipid levels. Niacin is used in the treatment of hyperlipidemia because it reduces very-low-density lipoprotein (VLDL), a precursor of low-density lipoprotein (LDL) or "bad" cholesterol. Because niacin blocks breakdown of fats, it causes a decrease in free fatty acids in the blood and, as a consequence, decreased secretion of VLDL and cholesterol by the liver.[4]
By lowering VLDL levels, niacin also increases the level of high-density lipoprotein (HDL) or "good" cholesterol in blood, and therefore it is sometimes prescribed for patients with low HDL, who are also at high risk of a heart attack.[5][6] An extended release formulation of niacin for this indication is marketed by Abbott Laboratories under the trade name Niaspan.
Niacin is sometimes consumed in large quantities by people who wish to fool drug screening tests, particularly for lipid-soluble drugs such as marijuana.[7] It is believed to "promote metabolism" of the drug and cause it to be "flushed out." Scientific studies have shown it does not affect drug screenings, but can pose a risk of overdose, causing arrhythmias, metabolic acidosis, hyperglycemia, and other serious problems (see below).
In October 2008, Merck expanded the THRIVE Trial from 20,000 to 25,000 patients in order to expedite a second bid for FDA approval of its experimental cholesterol drug, MK-0524A. MK-0524A is a combination of niacin and laropiprant, which is aimed at limiting facial flushing associated with niacin. In April 2008 the FDA decided to withhold approval for the experimental drug, deciding to wait until the results of the THRIVE Trial could be analyzed.[8]
Toxicity
People taking pharmacological doses of niacin (1.5 - 6 g per day) often experience a syndrome of side-effects that can include one or more of the following:[9]
- dermatological complaints
- facial flushing and itching
- dry skin
- skin rashes including acanthosis nigricans
- gastrointestinal complaints
- dyspepsia (indigestion)
- liver toxicity
- hyperglycemia
- cardiac arrhythmias
- birth defects
Facial flushing is the most commonly-reported side-effect.[10] It lasts for about 15 to 30 minutes, and is sometimes accompanied by a prickly or itching sensation. This effect is mediated by prostaglandins and can be blocked by taking 300 mg of aspirin half an hour before taking niacin, or by taking one tablet of ibuprofen per day. Taking the niacin with meals also helps reduce this side-effect. After 1 to 2 weeks of a stable dose, most patients no longer flush. Slow- or "sustained"-release forms of niacin have been developed to lessen these side-effects.[11][12] [13] One study showed the incidence of flushing was 4.5x lower (1.9 vs. 8.6 episodes in the first month) with a sustained-release formulation.[14]
Doses above 2 g per day have been associated with liver damage, particularly with slow-release formulations. [15]
High-dose niacin may also elevate blood sugar, thereby worsening diabetes mellitus.[16] Hyperuricemia is another side-effect of taking high-dose niacin; thus niacin may worsen gout.
Niacin at doses used in lowering cholesterol has been associated with birth defects in laboratory animals and should not be taken by pregnant women.[17]
Niacin at extremely high doses can have life-threatening acute toxic reactions. One patient suffered vomiting after taking eleven 500-milligram niacin tablets over 36 hours, and another was unresponsive for several minutes after taking five 500-milligram tablets over two days.[18][19] Extremely high doses of niacin can also cause niacin maculopathy, a thickening of the macula and retina which leads to blurred vision and blindness.[20]
Inositol hexanicotinate
One popular form of dietary supplement is inositol hexanicotinate, usually sold as "flush-free" or "no-flush" niacin (although those terms are also used for regular sustained-release.) While this form of niacin does not cause the flushing associated with the nicotinic acid form, it is not clear whether it is pharmacologically equivalent in its positive effect.[21]
Biosynthesis
The liver can synthesize niacin from the essential amino acid tryptophan (see below), but the synthesis is extremely inefficient; 60 mg of tryptophan are required to make one milligram of niacin.[22]
The 5-membered aromatic heterocycle of the essential amino acid, tryptophan, is cleaved and rearranged with the alpha amino group of tryptophan into the 6-membered aromatic heterocycle of niacin by the following reaction:
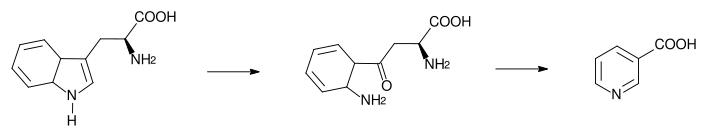
Biosynthesis: Tryptophan → kynurenine → niacin
Receptor
The receptor for niacin is a G-protein coupled receptor called HM74A.[23] It couples to Gi[24].
Food sources
| Animal products: | Fruits and vegetables: | Seeds: | Fungi: |
|---|---|---|---|
|
References
- ↑ Northwestern University Nutrition
- ↑ 2.0 2.1 Vitamin B3 University of Maryland Medical Center.
- ↑ Template:Pauling
- ↑ T. Katzung, Basic and Clinical Pharmacology, 9th ed. p. 570.
- ↑ Postgraduate Medicine
- ↑ Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8(6):1245-1255.
- ↑ Niacin abuse in the attempt to alter urine drug tests. Pharmacist's Letter/Prescriber's Letter 2007;23(6):230606.
- ↑ http://biz.yahoo.com/ap/081017/merck_cholesterol_study.html?.v=2
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.991.
- ↑ NIH Medline Plus: Niacin. http://www.nlm.nih.gov/medlineplus/ency/article/002409.htm.
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.991.
- ↑ T. Katzung, Basic and Clinical Pharmacology, 9th ed. p. 570.
- ↑ Options for therapeutic intervention: How effective are the different agents? European Heart Journal Supplements Vol 8 Suppl F Pp. F47-F53 [1]
- ↑ Chapman M, Assmann G, Fruchart J, Sheperd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid - a position paper developed by the European Consensus Panel on HDL-C. Cur Med Res Opin. 2004 Aug;20(8):1253-68. PMID 15324528
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.992.
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.991.
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.992.
- ↑ Hazards: Niacin to Pass a Drug Test Can Have Dangerous Results, By ERIC NAGOURNEY, New York Times, April 17, 2007[2]
- ↑ Mittal MK, Florin T, Perrone J, Delgado JH, Osterhoudt KC. Toxicity From the Use of Niacin to Beat Urine Drug Screening. Ann Emerg Med. 2007 Apr 4. PMID 17418450[3]
- ↑ JD Gass, Nictonic Acid Maculopathy, Am. J. Opthamology, 1973;76:500-10
- ↑ No-Flush Niacin for the Treatment of Hyperlipidemia
- ↑ Oxidization Reactions of Niacin from the Linus Pauling Institute at Oregon State University Linus Pauling Institute.
- ↑ medscape.com - The Metabolic Syndrome: Etiology, Controversies, and Emerging ...
- ↑ Variations in human HM74 (GPR109B) and HM74A (GPR109A) niacin receptors Christian Zellner 1 *, Clive R. Pullinger 1, Bradley E. Aouizerat 2, Philip H. Frost 1, Pui-Yan Kwok 1, Mary J. Malloy 1, John P. Kane
External links
Template:Peripheral vasodilators Template:Lipid modifying agents