Nelarabine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Neurologic Adverse Reactions</span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">Neurologic Adverse Reactions</span></i> | ||
* Severe neurologic adverse reactions have been reported with the use of ARRANON®. These adverse reactions have included altered mental states including severe somnolence, central nervous system effects including convulsions, and peripheral neuropathy ranging from numbness and paresthesias to motor weakness and paralysis. There have also been reports of adverse reactions associated with demyelination, and ascending peripheral neuropathies similar in appearance to | * Severe neurologic adverse reactions have been reported with the use of ARRANON®. These adverse reactions have included altered mental states including severe somnolence, central nervous system effects including convulsions, and peripheral neuropathy ranging from numbness and paresthesias to motor weakness and paralysis. There have also been reports of adverse reactions associated with demyelination, and ascending peripheral neuropathies similar in appearance to Guillain-Barré syndrome. | ||
* Full recovery from these adverse reactions has not always occurred with cessation of therapy with ARRANON. | * Full recovery from these adverse reactions has not always occurred with cessation of therapy with ARRANON. | ||
* Close monitoring for neurologic adverse reactions is strongly recommended, and ARRANON should be discontinued for neurologic adverse reactions of NCI Common Toxicity Criteria Grade 2 or greater. | * Close monitoring for neurologic adverse reactions is strongly recommended, and ARRANON should be discontinued for neurologic adverse reactions of NCI Common Toxicity Criteria Grade 2 or greater. | ||
| Line 307: | Line 307: | ||
*NDC 0007-4401-06 (package of 6) | *NDC 0007-4401-06 (package of 6) | ||
|storage=* Store at 25° C (77° F); excursions permitted to 15° to 30° C (59° to 86° F) [see USP Controlled Room Temperature]. | |storage=* Store at 25° C (77° F); excursions permitted to 15° to 30° C (59° to 86° F) [see USP Controlled Room Temperature]. | ||
|packLabel=[[File:Nelarabine pdp.jpg|600px|thumbnail|left]] | |packLabel=[[File:Nelarabine pdp.jpg|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
Revision as of 20:24, 16 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: NEUROLOGIC ADVERSE REACTIONS
See full prescribing information for complete Boxed Warning.
Neurologic Adverse Reactions
|
Overview
Nelarabine is a prodrug of ara-G that is FDA approved for the {{{indicationType}}} of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
T-cell acute lymphoblastic leukemia, relapsed or refractory
- Dosing Information
- The recommended dose of nelarabine is 1500 mg/m(2) administered intravenously over 2 hours on days 1, 3, and 5 repeated every 21 days. Nelarabine is administered undiluted. The recommended duration of treatment in adults has not been established. In clinical trials, treatment was generally continued until there was evidence of disease progression, the patient experienced unacceptable toxicity, the patient became a candidate for bone marrow transplant, or the patient no longer continued to benefit from treatment.
- Appropriate measures must be taken to prevent hyperuricemia of tumor lysis syndrome (eg, hydration, urine alkalinization, and prophylaxis with allopurinol).
T-cell lymphoma, T-cell lymphoblastic lymphoma, relapsed or refractory
- Dosing Information
- The recommended dose of nelarabine is 1500 milligrams/square meter (mg/m(2)) administered intravenously over 2 hours on days 1, 3, and 5 repeated every 21 days. Nelarabine is administered undiluted. The recommended duration of treatment in adults has not been established. In clinical trials, treatment was generally continued until there was evidence of disease progression, the patient experienced unacceptable toxicity, the patient became a candidate for bone marrow transplant, or the patient no longer continued to benefit from treatment.
- Appropriate measures must be taken to prevent hyperuricemia of tumor lysis syndrome (eg, hydration, urine alkalinization, and prophylaxis with allopurinol).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Nelarabine in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Nelarabine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
T-cell acute lymphoblastic leukemia, relapsed or refractory
- Dosing Information
- The recommended pediatric dose of nelarabine is 650 milligrams/square meter (mg/m(2)) administered intravenously over 1 hour daily for 5 consecutive days and repeated every 21 days. Nelarabine is administered undiluted. The recommended duration of treatment in pediatric patients has not been established. In clinical trials, treatment was generally continued until there was evidence of disease progression, the patient experienced unacceptable toxicity, the patient became a candidate for bone marrow transplant, or the patient no longer continued to benefit from treatment.
- Appropriate measures must be taken to prevent hyperuricemia of tumor lysis syndrome (eg, hydration, urine alkalinization, and prophylaxis with allopurinol).
T-cell lymphoma, T-cell lymphoblastic lymphoma, relapsed or refractory
- The recommended pediatric dose of nelarabine is 650 milligrams/square meter (mg/m(2)) administered intravenously over 1 hour daily for 5 consecutive days and repeated every 21 days. Nelarabine is administered undiluted. The recommended duration of treatment in pediatric patients has not been established. In clinical trials, treatment was generally continued until there was evidence of disease progression, the patient experienced unacceptable toxicity, the patient became a candidate for bone marrow transplant, or the patient no longer continued to benefit from treatment.
- Appropriate measures must be taken to prevent hyperuricemia of tumor lysis syndrome (eg, hydration, urine alkalinization, and prophylaxis with allopurinol).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Nelarabine in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Nelarabine in pediatric patients.
Contraindications
- None.
Warnings
|
WARNING: NEUROLOGIC ADVERSE REACTIONS
See full prescribing information for complete Boxed Warning.
Neurologic Adverse Reactions
|
Neurologic Adverse Reactions
- Neurotoxicity is the dose-limiting toxicity of nelarabine. Patients undergoing therapy with ARRANON should be closely observed for signs and symptoms of neurologic toxicity. Common signs and symptoms of nelarabine-related neurotoxicity include somnolence, confusion, convulsions, ataxia, paresthesias, and hypoesthesia. Severe neurologic toxicity can manifest as coma, status epilepticus, craniospinal demyelination, or ascending neuropathy similar in presentation to Guillain-Barré syndrome.
Patients treated previously or concurrently with intrathecal chemotherapy or previously with craniospinal irradiation may be at increased risk for neurologic adverse events.
Hematologic Adverse Reactions
- Leukopenia, thrombocytopenia, anemia, and neutropenia, including febrile neutropenia, have been associated with nelarabine therapy. Complete blood counts including platelets should be monitored regularly.
Pregnancy
Pregnancy Category D
- ARRANON can cause fetal harm when administered to a pregnant woman.
- Nelarabine administered during the period of organogenesis caused increased incidences of fetal malformations, anomalies, and variations in rabbits.
- There are no adequate and well-controlled studies of ARRANON in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of child-bearing potential should be advised to avoid becoming pregnant while receiving treatment with ARRANON.
Hyperuricemia
- Patients receiving ARRANON should receive intravenous hydration according to standard medical practice for the management of hyperuricemia in patients at risk for tumor lysis syndrome. Consideration should be given to the use of allopurinol in patients at risk of hyperuricemia.
Vaccinations
- Administration of live vaccines to immunocompromised patients should be avoided.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Neurologic
- Hematologic
- Hyperuricemia
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- ARRANON was studied in 459 patients in Phase I and Phase II clinical trials.
- Adults: The safety profile of ARRANON is based on data from 103 adult patients treated with the recommended dose and schedule in 2 studies: an adult T-cell acute lymphoblastic leukemia (T-ALL)/T-cell lymphoblastic lymphoma (T-LBL) trial and an adult chronic lymphocytic leukemia trial.
- The most common adverse reactions in adults, regardless of causality, were fatigue; gastrointestinal (GI) disorders (nausea, diarrhea, vomiting, and constipation); hematologic disorders (anemia, neutropenia, and thrombocytopenia); respiratory disorders (cough and dyspnea); nervous system disorders (somnolence and dizziness); and pyrexia.
- The most common adverse reactions in adults, by System Organ Class, regardless of causality, including severe or life-threatening adverse reactions (NCI Common Toxicity Criteria Grade 3 or Grade 4) and fatal adverse reactions (Grade 5) are shown in Table 1.
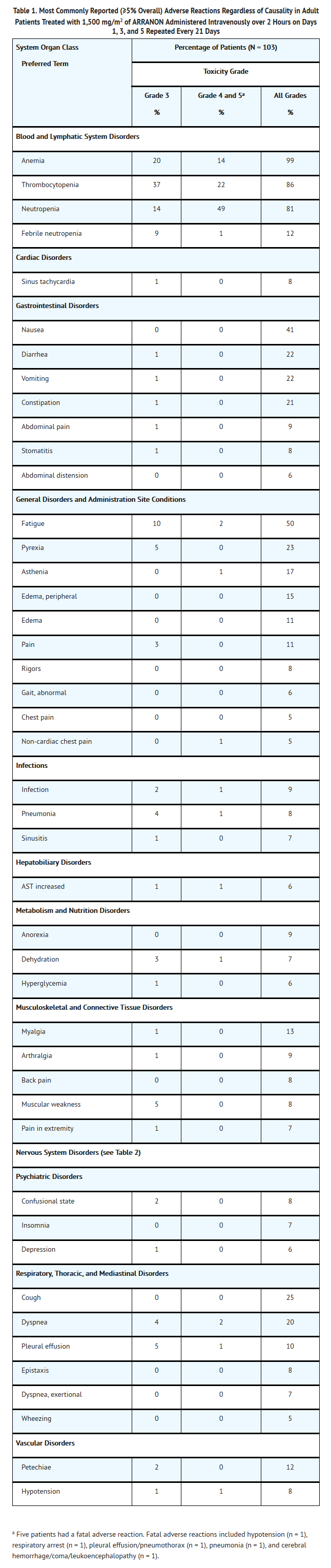
- Other Adverse Events: Blurred vision was also reported in 4% of adult patients.
- There was a single report of biopsy-confirmed progressive multifocal leukoencephalopathy in the adult patient population.
- Neurologic Adverse Reactions: Nervous system adverse reactions, regardless of drug relationship, were reported for 76% of adult patients across the Phase I and Phase II trials. The most common neurologic adverse reactions (≥2%) in adult patients, regardless of causality, including all grades (NCI Common Toxicity Criteria) are shown in Table 2.

- One patient had a fatal neurologic adverse reaction, cerebral hemorrhage/coma/leukoencephalopathy.
- Most nervous system adverse reactions in the adult patients were evaluated as Grade 1 or 2. The additional Grade 3 adverse reactions in adult patients, regardless of causality, were aphasia, convulsion, hemiparesis, and loss of consciousness, each reported in 1 patient (1%). The additional Grade 4 adverse reactions, regardless of causality, were cerebral hemorrhage, coma, intracranial hemorrhage, leukoencephalopathy, and metabolic encephalopathy, each reported in one patient (1%).
- The other neurologic adverse reactions, regardless of causality, reported as Grade 1, 2, or unknown in adult patients were abnormal coordination, burning sensation, disturbance in attention, dysarthria, hyporeflexia, neuropathic pain, nystagmus, peroneal nerve palsy, sciatica, sensory disturbance, sinus headache, and speech disorder, each reported in one patient (1%).
- Pediatrics: The safety profile for children is based on data from 84 pediatric patients treated with the recommended dose and schedule in a T-cell acute lymphoblastic leukemia (T-ALL)/T-cell lymphoblastic lymphoma (T-LBL) treatment trial.
- The most common adverse reactions in pediatric patients, regardless of causality, were hematologic disorders (anemia, leukopenia, neutropenia, and thrombocytopenia). Of the non-hematologic adverse reactions in pediatric patients, the most frequent adverse reactions reported were headache, increased transaminase levels, decreased blood potassium, decreased blood albumin, increased blood bilirubin, and vomiting.
- The most common adverse reactions in pediatric patients, by System Organ Class, regardless of causality, including severe or life threatening adverse reactions (NCI Common Toxicity Criteria Grade 3 or Grade 4) and fatal adverse reactions (Grade 5) are shown in Table 3.
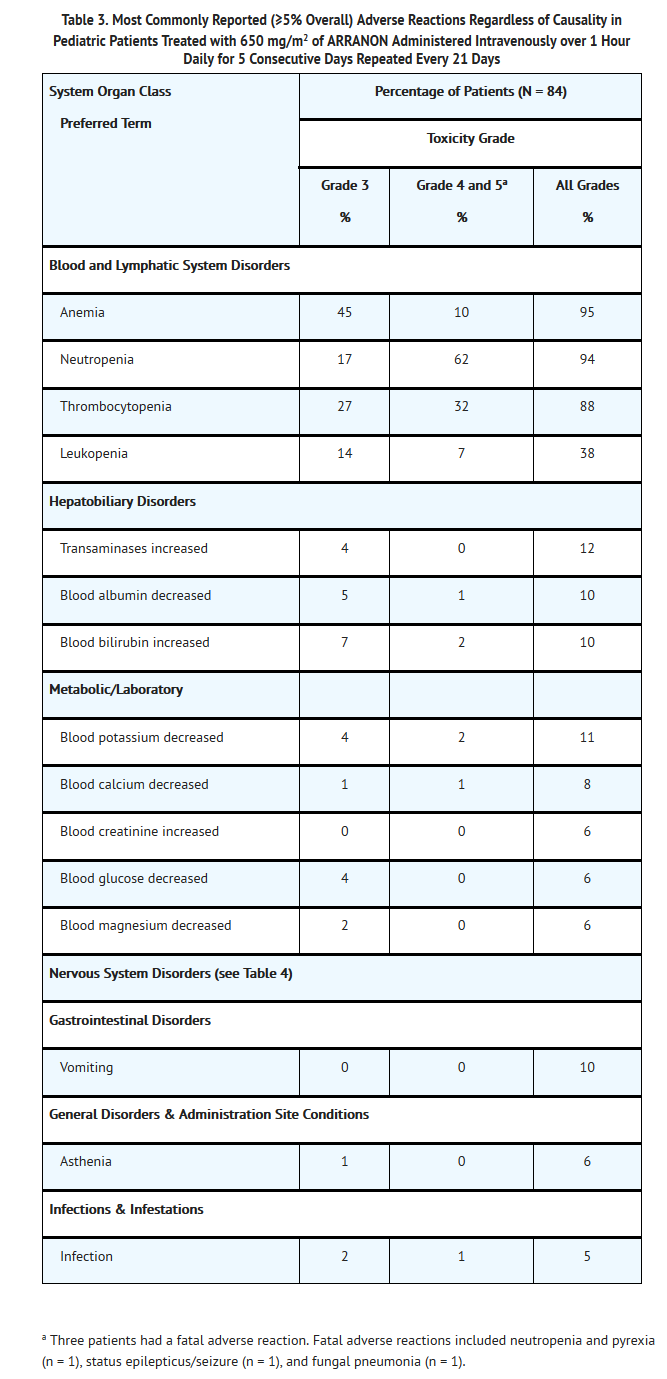
- Neurologic Adverse Reactions: Nervous system adverse reactions, regardless of drug relationship, were reported for 42% of pediatric patients across the Phase I and Phase II trials. The most common neurologic adverse reactions (≥2%) in pediatric patients, regardless of causality, including all grades (NCI Common Toxicity Criteria) are shown in Table 4.
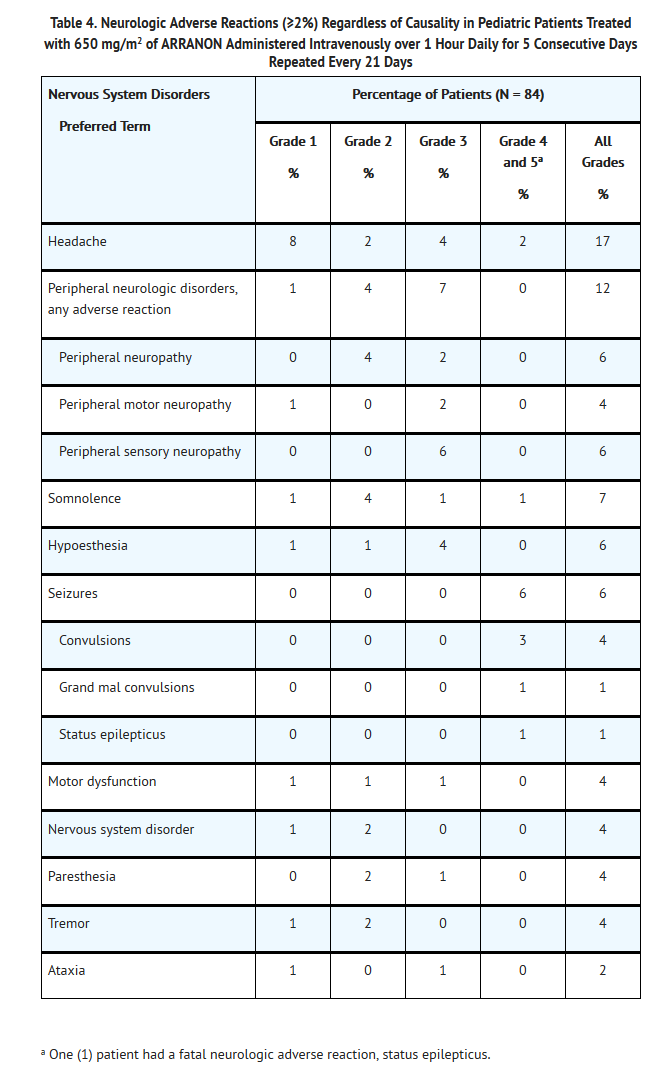
- The other Grade 3 neurologic adverse reaction in pediatric patients, regardless of causality, was hypertonia reported in 1 patient (1%). The additional Grade 4 neurologic adverse reactions, regardless of causality, were 3rd nerve paralysis, and 6th nerve paralysis, each reported in 1 patient (1%).
- The other neurologic adverse reactions, regardless of causality, reported as Grade 1, 2, or unknown in pediatric patients were dysarthria, encephalopathy, hydrocephalus, hyporeflexia, lethargy, mental impairment, paralysis, and sensory loss, each reported in 1 patient (1%).
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of ARRANON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infections and Infestations: Fatal opportunistic infections.
- Metabolism and Nutrition Disorders: Tumor lysis syndrome.
- Nervous System Disorders: Demyelination and ascending peripheral neuropathies similar in appearance to Guillain-Barré syndrome.
- Musculoskeletal and Connective Disorders: Rhabdomyolysis, blood creatine phosphokinase increased.
Drug Interactions
- Administration of nelarabine in combination with adenosine deaminase inhibitors, such as pentostatin, is not recommended.
Use in Specific Populations
Pregnancy
- ARRANON can cause fetal harm when administered to a pregnant woman. Nelarabine administered to rabbits during the period of organogenesis caused increased incidences of fetal malformations, anomalies, and variations at doses ≥360 mg/m2/day (8-hour IV infusion; approximately ¼ the adult dose compared on a mg/m2 basis), which was the lowest dose tested. Cleft palate was seen in rabbits given 3,600 mg/m2/day (approximately 2-fold the adult dose), absent pollices (digits) in rabbits given ≥1,200 mg/m2/day (approximately ¾ the adult dose), while absent gall bladder, absent accessory lung lobes, fused or extra sternebrae, and delayed ossification was seen at all doses. Maternal body weight gain and fetal body weights were reduced in rabbits given 3,600 mg/m2/day (approximately 2-fold the adult dose), but could not account for the increased incidence of malformations seen at this or lower administered doses.
- There are no adequate and well-controlled studies of ARRANON in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of child-bearing potential should be advised to avoid becoming pregnant while receiving treatment with ARRANON.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nelarabine in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Nelarabine during labor and delivery.
Nursing Mothers
- It is not known whether nelarabine or ara-G are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ARRANON, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of ARRANON has been established in pediatric patients.
Geriatic Use
- Clinical studies of ARRANON did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In an exploratory analysis, increasing age, especially age 65 years and older, appeared to be associated with increased rates of neurologic adverse reactions. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
- There is no FDA guidance on the use of Nelarabine with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Nelarabine with respect to specific racial populations.
Renal Impairment
- Ara-G clearance decreased as renal function decreased. Because the risk of adverse reactions to this drug may be greater in patients with moderate (CLcr 30 to 50 mL/min) or severe (CLcr <30 mL/min) renal impairment, these patients should be closely monitored for toxicities when treated with ARRANON.
Hepatic Impairment
- The influence of hepatic impairment on the pharmacokinetics of nelarabine has not been evaluated. Because the risk of adverse reactions to this drug may be greater in patients with severe hepatic impairment (total bilirubin >3 times upper limit of normal), these patients should be closely monitored for toxicities when treated with ARRANON.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Nelarabine in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Nelarabine in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosage
- This product is for intravenous use only.
- The recommended duration of treatment for adult and pediatric patients has not been clearly established. In clinical trials, treatment was generally continued until there was evidence of disease progression, the patient experienced unacceptable toxicity, the patient became a candidate for bone marrow transplant, or the patient no longer continued to benefit from treatment.
- Adult Dosage: The recommended adult dose of ARRANON is 1,500 mg/m² administered intravenously over 2 hours on Days 1, 3, and 5 repeated every 21 days. ARRANON is administered undiluted.
- Pediatric Dosage: The recommended pediatric dose of ARRANON is 650 mg/m² administered intravenously over 1 hour daily for 5 consecutive days repeated every 21 days. ARRANON is administered undiluted.
Dosage Modification
- Administration of ARRANON should be discontinued for neurologic adverse reactions of NCI Common Toxicity Criteria Grade 2 or greater. Dosage may be delayed for other toxicity including hematologic toxicity.
Adjustment of Dose in Special Populations
- ARRANON has not been studied in patients with renal or hepatic dysfunction. No dose adjustment is recommended for patients with a creatinine clearance (CLcr) ≥50 mL/min. There are insufficient data to support a dose recommendation for patients with a CLcr <50 mL/min.
Prevention of Hyperuricemia
- Appropriate measures (e.g., hydration, urine alkalinization, and prophylaxis with allopurinol) must be taken to prevent hyperuricemia.
Instructions for Handling, Preparation, and Administration
- Handling: ARRANON is a cytotoxic agent. Caution should be used during handling and preparation. Use of gloves and other protective clothing to prevent skin contact is recommended. Proper aseptic technique should be used. Guidelines for proper handling and disposal of anticancer drugs have been published.
- Preparation and Administration: Do not dilute ARRANON prior to administration. The appropriate dose of ARRANON is transferred into polyvinylchloride (PVC) infusion bags or glass containers and administered as a 2-hour infusion in adult patients and as a 1-hour infusion in pediatric patients.
- Prior to administration, inspect the drug product visually for particulate matter and discoloration.
- Stability: ARRANON Injection is stable in polyvinylchloride (PVC) infusion bags and glass containers for up to 8 hours at up to 30° C.
DOSAGE FORMS AND STRENGTHS
- 250 mg/50 mL (5 mg/mL) vial
Monitoring
- There is limited information regarding Monitoring of Nelarabine in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Nelarabine in the drug label.
Overdosage
- There is no known antidote for overdoses of ARRANON. It is anticipated that overdosage would result in severe neurotoxicity (possibly including paralysis, coma), myelosuppression, and potentially death. In the event of overdose, supportive care consistent with good clinical practice should be provided.
- Nelarabine has been administered in clinical trials up to a dose of 2,900 mg/m2 on Days 1, 3, and 5 to 2 adult patients. At a dose of 2,200 mg/m2 given on Days 1, 3, and 5 every 21 days, 2 patients developed a significant Grade 3 ascending sensory neuropathy. MRI evaluations of the 2 patients demonstrated findings consistent with a demyelinating process in the cervical spine.
Pharmacology
Mechanism of Action
Nelarabine is a prodrug of the deoxyguanosine analogue 9-β-D-arabinofuranosylguanine (ara-G), a nucleoside metabolic inhibitor. Nelarabine is demethylated by adenosine deaminase (ADA) to ara-G, mono-phosphorylated by deoxyguanosine kinase and deoxycytidine kinase, and subsequently converted to the active 5’-triphosphate, ara-GTP. Accumulation of ara-GTP in leukemic blasts allows for incorporation into deoxyribonucleic acid (DNA), leading to inhibition of DNA synthesis and cell death. Other mechanisms may contribute to the cytotoxic and systemic toxicity of nelarabine.
Structure
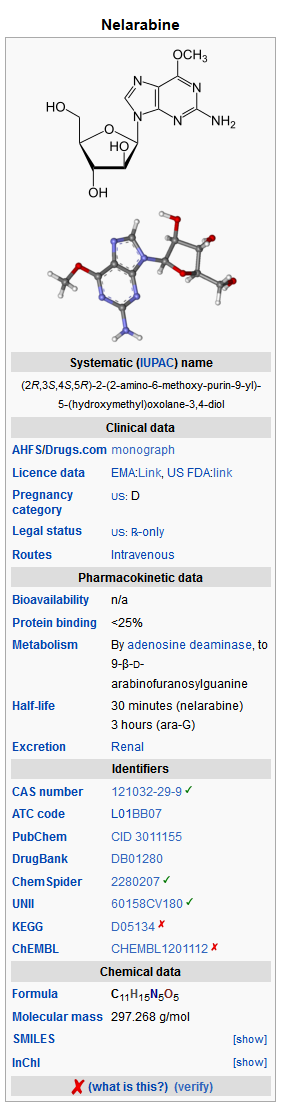
- ARRANON (nelarabine) is a prodrug of the cytotoxic deoxyguanosine analogue, 9-β-D-arabinofuranosylguanine (ara-G).
- The chemical name for nelarabine is 2-amino-9-β-D-arabinofuranosyl-6-methoxy-9H-purine. It has the molecular formula C11H15N5O5 and a molecular weight of 297.27. Nelarabine has the following structural formula:

- Nelarabine is slightly soluble to soluble in water and melts with decomposition between 209° and 217° C.
- ARRANON Injection is supplied as a clear, colorless, sterile solution in glass vials. Each vial contains 250 mg of nelarabine (5 mg nelarabine per mL) and the inactive ingredient sodium chloride (4.5 mg per mL) in 50 mL Water for Injection, USP. ARRANON is intended for intravenous infusion.
- Hydrochloric acid and sodium hydroxide may have been used to adjust the pH. The solution pH ranges from 5.0 to 7.0.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Nelarabine in the drug label.
Pharmacokinetics
- Absorption: Following intravenous administration of nelarabine to adult patients with refractory leukemia or lymphoma, plasma ara-G Cmax values generally occurred at the end of the nelarabine infusion and were generally higher than nelarabine Cmax values, suggesting rapid and extensive conversion of nelarabine to ara-G. Mean plasma nelarabine and ara-G Cmax values were 5.0 ± 3.0 mcg/mL and 31.4 ± 5.6 mcg/mL, respectively, after a 1,500 mg/m2 nelarabine dose infused over 2 hours in adult patients. The area under the concentration-time curve (AUC) of ara-G is 37 times higher than that for nelarabine on Day 1 after nelarabine IV infusion of 1,500 mg/m2 dose (162 ± 49 mcg.h/mL versus 4.4 ± 2.2 mcg.h/mL, respectively). Comparable Cmax and AUC values were obtained for nelarabine between Days 1 and 5 at the nelarabine adult dosage of 1,500 mg/m2, indicating that nelarabine does not accumulate after multiple-dosing. There are not enough ara-G data to make a comparison between Day 1 and Day 5. After a nelarabine adult dose of 1,500 mg/m2, intracellular Cmax for ara-GTP appeared within 3 to 25 hours on Day 1. Exposure (AUC) to intracellular ara-GTP was 532 times higher than that for nelarabine and 14 times higher than that for ara-G (2,339 ± 2,628 mcg.h/mL versus 4.4 ± 2.2 mcg.h/mL and 162 ± 49 mcg.h/mL, respectively). Because the intracellular levels of ara-GTP were so prolonged, its elimination half-life could not be accurately estimated.
- Distribution: Nelarabine and ara-G are extensively distributed throughout the body. For nelarabine, VSS values were 197 ± 216 L/m2 in adult patients. For ara-G, VSS/F values were 50 ± 24 L/m2 in adult patients.
- Nelarabine and ara-G are not substantially bound to human plasma proteins (<25%) in vitro, and binding is independent of nelarabine or ara-G concentrations up to 600 μM.
- Metabolism: The principal route of metabolism for nelarabine is O-demethylation by adenosine deaminase to form ara-G, which undergoes hydrolysis to form guanine. In addition, some nelarabine is hydrolyzed to form methylguanine, which is O-demethylated to form guanine. Guanine is N-deaminated to form xanthine, which is further oxidized to yield uric acid.
- Excretion: Nelarabine and ara-G are partially eliminated by the kidneys. Mean urinary excretion of nelarabine and ara-G was 6.6 ± 4.7% and 27 ± 15% of the administered dose, respectively, in 28 adult patients over the 24 hours after nelarabine infusion on Day 1. Renal clearance averaged 24 ± 23 L/h for nelarabine and 6.2 ± 5.0 L/h for ara-G in 21 adult patients. Combined Phase I pharmacokinetic data at nelarabine doses of 199 to 2,900 mg/m2 (n = 66 adult patients) indicate that the mean clearance (CL) of nelarabine is 197 ± 189 L/h/m2 on Day 1. The apparent clearance of ara-G (CL/F) is 10.5 ± 4.5 L/h/m2 on Day 1. Nelarabine and ara-G are rapidly eliminated from plasma with a mean half-life of 18 minutes and 3.2 hours, respectively, in adult patients.
- Pediatrics: No pharmacokinetic data are available in pediatric patients at the once-daily 650 mg/m2 nelarabine dosage. Combined Phase I pharmacokinetic data at nelarabine doses of 104 to 2,900 mg/m2 indicate that the mean clearance (CL) of nelarabine is about 30% higher in pediatric patients than in adult patients (259 ± 409 L/h/m2 versus 197 ± 189 L/h/m2, respectively) (n = 66 adults, n = 22 pediatric patients) on Day 1. The apparent clearance of ara-G (CL/F) is comparable between the two groups (10.5 ± 4.5 L/h/m2 in adult patients and 11.3 ± 4.2 L/h/m2 in pediatric patients) on Day 1. Nelarabine and ara-G are extensively distributed throughout the body. For nelarabine, VSS values were 213 ± 358 L/m2 in pediatric patients. For ara-G, VSS/F values were 33 ± 9.3 L/m2 in pediatric patients. Nelarabine and ara-G are rapidly eliminated from plasma in pediatric patients, with a half-life of 13 minutes and 2 hours, respectively.
- Effect of Age: Age has no effect on the pharmacokinetics of nelarabine or ara-G in adults. Decreased renal function, which is more common in the elderly, may reduce ara-G clearance.
- Effect of Gender: Gender has no effect on nelarabine or ara-G pharmacokinetics.
- Effect of Race: In general, nelarabine mean clearance and volume of distribution values tend to be higher in whites (n = 63) than in blacks (by about 10%) (n = 15). The opposite is true for ara-G; mean apparent clearance and volume of distribution values tend to be lower in whites than in blacks (by about 15% to 20%). No differences in safety or effectiveness were observed between these groups.
- Effect of Renal Impairment: The pharmacokinetics of nelarabine and ara-G have not been specifically studied in renally impaired or hemodialyzed patients. Nelarabine is excreted by the kidney to a small extent (5% to 10% of the administered dose). Ara-G is excreted by the kidney to a greater extent (20% to 30% of the administered nelarabine dose). In the combined Phase I trials, patients were categorized into 3 groups: normal with CLcr >80 mL/min (n = 67), mild with CLcr = 50 to 80 mL/min (n = 15), and moderate with CLcr <50 mL/min (n = 3). The mean apparent clearance (CL/F) of ara-G was about 15% and 40% lower in patients with mild and moderate renal impairment, respectively, than in patients with normal renal function. No differences in safety or effectiveness were observed.
- Effect of Hepatic Impairment: The influence of hepatic impairment on the pharmacokinetics of nelarabine has not been evaluated.
- Drug Interactions: Cytochrome P450: Nelarabine and ara-G did not significantly inhibit the activities of the human hepatic cytochrome P450 isoenzymes 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4 in vitro at concentrations of nelarabine and ara-G up to 100 μM.
- Fludarabine: Administration of fludarabine 30 mg/m2 as a 30-minute infusion 4 hours before a 1,200-mg/m2 infusion of nelarabine did not affect the pharmacokinetics of nelarabine, ara-G, or ara-GTP in 12 patients with refractory leukemia.
Pentostatin: There is in vitro evidence that pentostatin is a strong inhibitor of adenosine deaminase. Inhibition of adenosine deaminase may result in a reduction in the conversion of the prodrug nelarabine to its active moiety and consequently in a reduction in efficacy of nelarabine and/or change in adverse reaction profile of either drug.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity testing of nelarabine has not been done. However, nelarabine was mutagenic when tested in vitro in L5178Y/TK mouse lymphoma cells with and without metabolic activation. No studies have been conducted in animals to assess genotoxic potential or effects on fertility. The effect on human fertility is unknown.
Clinical Studies
- The safety and efficacy of ARRANON were evaluated in two open-label, single-arm, multicenter trials.
Adult Clinical Trial
- The safety and efficacy of ARRANON in adult patients were studied in a clinical trial which included 39 treated patients, 28 who had T-cell acute lymphoblastic leukemia (T-ALL) or T-cell lymphoblastic lymphoma (T-LBL) that had relapsed following or was refractory to at least two prior induction regimens. A 1,500-mg/m2 dose of ARRANON was administered intravenously over 2 hours on Days 1, 3, and 5 repeated every 21 days. Patients who experienced signs or symptoms of Grade 2 or greater neurologic toxicity on therapy were to be discontinued from further therapy with ARRANON. Seventeen patients had a diagnosis of T-ALL and 11 had a diagnosis of T-LBL. For patients with ≥2 prior inductions, the age range was 16 to 65 years (mean 34 years) and most patients were male (82%) and Caucasian (61%). Patients with central nervous system (CNS) disease were not eligible.
- Complete response (CR) in this trial was defined as bone marrow blast counts ≤5%, no other evidence of disease, and full recovery of peripheral blood counts. Complete response without complete hematologic recovery (CR*) was also assessed. The results of the trial for patients who had received ≥2 prior inductions are shown in Table 5.
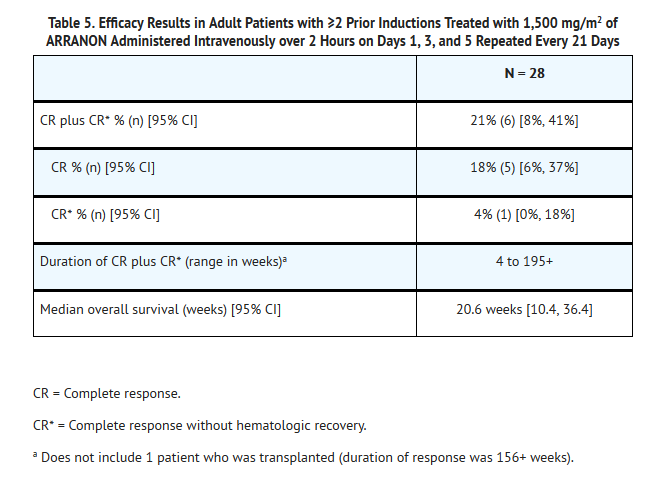
- The mean number of days on therapy was 56 days (range of 10 to 136 days). Time to CR plus CR* ranged from 2.9 to 11.7 weeks.
Pediatric Clinical Trial
- The safety and efficacy of ARRANON in pediatric patients were studied in a clinical trial which included patients aged 21 years and younger, who had relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) or T-cell lymphoblastic lymphoma (T-LBL). Eighty-four (84) patients, 39 of whom had received two or more prior induction regimens, were treated with 650 mg/m2/day of ARRANON administered intravenously over 1 hour daily for 5 consecutive days repeated every 21 days (see Table 6). Patients who experienced signs or symptoms of Grade 2 or greater neurologic toxicity on therapy were to be discontinued from further therapy with ARRANON.

- The 84 patients ranged in age from 2.5 to 21.7 years (overall mean: 11.9 years), 52% were 3 to 12 years of age and most were male (74%) and Caucasian (62%). The majority (77%) of patients had a diagnosis of T-ALL.
- Complete response (CR) in this trial was defined as bone marrow blast counts ≤5%, no other evidence of disease, and full recovery of peripheral blood counts. Complete response without full hematologic recovery (CR*) was also assessed as a meaningful outcome in this heavily pretreated population. Duration of response is reported from date of response to date of relapse, and may include subsequent stem cell transplant. Efficacy results are presented in Table 7.
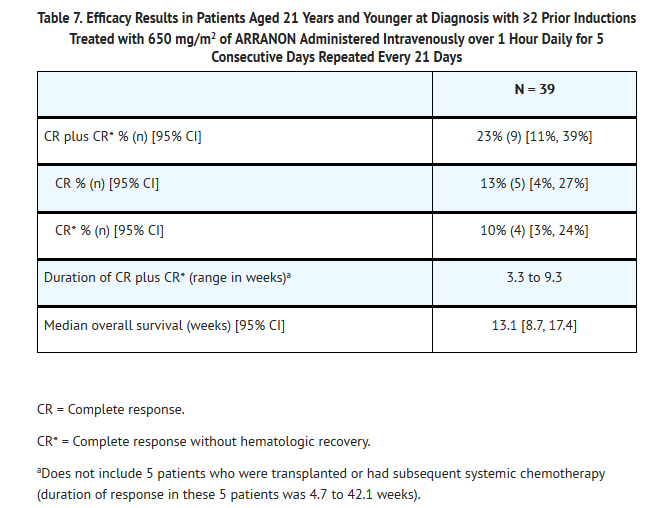
- The mean number of days on therapy was 46 days (range: 7 to 129 days). Median time to CR plus CR* was 3.4 weeks (95% CI: 3.0, 3.7).
How Supplied
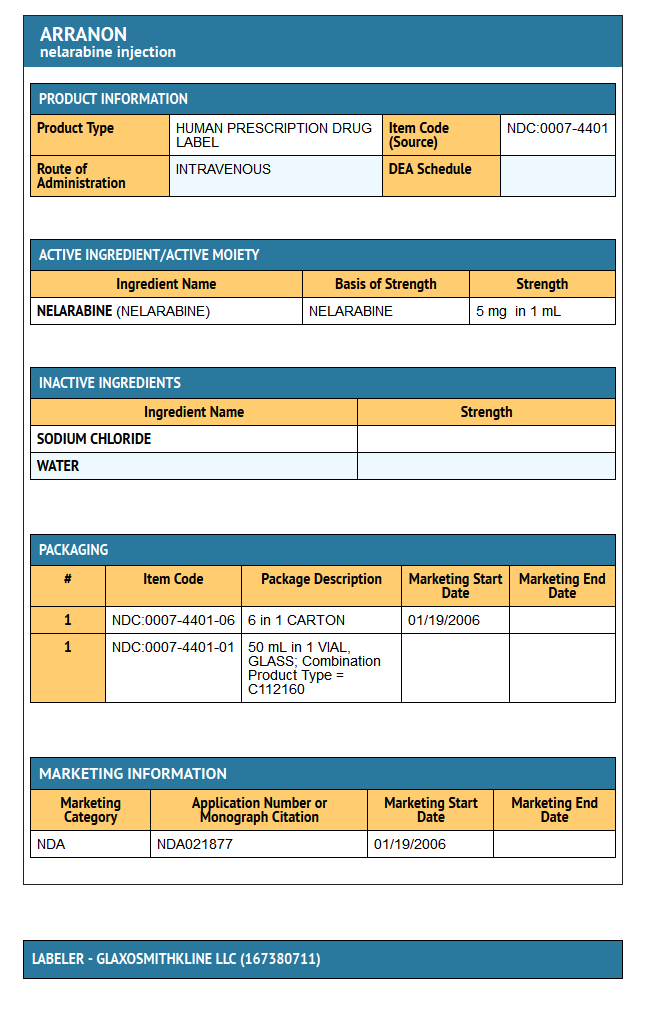
- ARRANON Injection is supplied as a clear, colorless, sterile solution in Type I, clear glass vials with a gray bromobutyl rubber stopper (not made with natural rubber latex) and a red snap-off aluminum seal. Each vial contains 250 mg of nelarabine (5 mg nelarabine per mL) and the inactive ingredient sodium chloride (4.5 mg per mL) in 50 mL Water for Injection, USP. Vials (NDC 0007-4401-01) are available in the following carton size:
- NDC 0007-4401-06 (package of 6)
Storage
- Store at 25° C (77° F); excursions permitted to 15° to 30° C (59° to 86° F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Nelarabine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Nelarabine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patient labeling is provided as a tear-off leaflet at the end of this full prescribing information. However, inform the patients of the following:
- Since patients receiving nelarabine therapy may experience somnolence, they should be cautioned about operating hazardous machinery, including automobiles.
- Patients should be instructed to contact their physician if they experience new or worsening symptoms of peripheral neuropathy. These signs and symptoms include: tingling or numbness in fingers, hands, toes, or feet; difficulty with the fine motor coordination tasks such as buttoning clothing; unsteadiness while walking; weakness arising from a low chair; weakness in climbing stairs; increased tripping while walking over uneven surfaces.
- Patients should be instructed that seizures have been known to occur in patients who receive nelarabine. If a seizure occurs, the physician administering ARRANON should be promptly informed.
- Patients who develop fever or signs of infection while on therapy should notify their physician promptly.
- Patients should be advised to use effective contraceptive measures to prevent pregnancy and to avoid breastfeeding during treatment with ARRANON.

Precautions with Alcohol
- Alcohol-Nelarabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Arranon
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Nelarabine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Nelarabine |Label Name=Nelarabine11.png
}}
{{#subobject:
|Label Page=Nelarabine |Label Name=Nelarabine11.png
}}