Nadolol (tablet): Difference between revisions
No edit summary |
No edit summary |
||
| Line 98: | Line 98: | ||
[[Beta-blockers]] may mask certain clinical signs (e.g., [[tachycardia]]) of [[hyperthyroidism]]. Patients suspected of developing [[thyrotoxicosis]] should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blockade which might precipitate a [[thyroid storm]]. | [[Beta-blockers]] may mask certain clinical signs (e.g., [[tachycardia]]) of [[hyperthyroidism]]. Patients suspected of developing [[thyrotoxicosis]] should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blockade which might precipitate a [[thyroid storm]]. | ||
|clinicalTrials====== | |clinicalTrials======Cardiovascular===== | ||
* [[Bradycardia]] with [[heart rate]]s of less than 60 beats per minute occurs commonly, and [[heart rate]]s below 40 beats per minute and/or symptomatic [[bradycardia]] were seen in about 2 of 100 patients. Symptoms of peripheral vascular insufficiency, usually of the [[Raynaud]] type, have occurred in approximately 2 of 100 patients. | |||
* [[Cardiac failure]], [[hypotension]], and [[rhythm]]/[[conduction]] disturbances have each occurred in about 1 of 100 patients. | |||
* Single instances of [[first degree heart block]] and [[third degree heart block]] have been reported; intensification of [[AV block]] is a known effect of [[beta-blockers]]. | |||
===== | =====Central Nervous System==== | ||
* [[Dizziness]] or [[fatigue]] has been reported in approximately 2 of 100 patients. | |||
* [[Paresthesias, [[sedation]], change in behavior have each been reported in approximately 6 of 1000 patients. | |||
=====Respiratory===== | |||
* [[Bronchospasm]] has been reported in approximately 1 of 1000 patients. | |||
=====Gastrointestinal===== | |||
* [[Nausea]], [[diarrhea]], [[abdominal discomfort]], [[constipation]], [[vomiting]], [[indigestion]], [[anorexia]], [[bloating]], and [[flatulence]] have been reported in 1 to 5 of 1000 patients. | |||
===== | =====Miscellaneous===== | ||
: | Each of the following has been reported in 1 to 5 of 1000 patients: | ||
* [[Rash]] | |||
* [[Pruritus]] | |||
* [[Headache]] | |||
* Dry mouth, eyes, or skin | |||
* [[Impotence]] or decreased [[libido]] | |||
* Facial [[swelling]] | |||
* Weight gain | |||
* Slurred speech | |||
* [[Cough]] | |||
* Nasal stuffiness | |||
* [[Sweating]] | |||
* [[Tinnitus]] | |||
* [[Blurred vision]] | |||
* Reversible [[alopecia]] has been reported infrequently. | |||
The following adverse reactions have been reported in patients taking nadolol and/or other beta-adrenergic blocking agents, but no causal relationship to nadolol has been established. | |||
=====Central Nervous System===== | |||
* Reversible [[mental depression]] progressing to [[catatonia]] | |||
* Visual disturbances | |||
* [[Hallucinations]] | |||
* An acute reversible syndrome characterized by [[disorientation]] for time and place, [[short-term memory loss]], [[emotional lability]] with slightly clouded [[sensorium]], and decreased performance on neuropsychometrics. | |||
====== | ======Gastrointestinal===== | ||
* [[Mesenteric arterial thrombosis]] | |||
* [[Ischemic colitis]] | |||
* [[Elevated liver enzymes]] | |||
===== | =====Hematologic===== | ||
* [[Agranulocytosis]] | |||
* [[Thrombocytopenic purpura]] or [[nonthrombocytopenic purpura]] | |||
===== | =====Allergic===== | ||
* Fever combined with aching and [[sore throat]] | |||
* [[Laryngospasm]] | |||
* [[Respiratory distress]] | |||
===== | =====Miscellaneous===== | ||
* [[Pemphigoid rash]] | |||
* Hypertensive reaction in patients with [[pheochromocytoma]] | |||
* [[Sleep disorders]] | |||
* [[Peyronie's disease]] | |||
* The [[oculomucocutaneous syndrome]] associated with the [[beta-blocker]] practolol has not been reported with nadolol. | |||
|drugInteractions=* Drug 1 | |drugInteractions=* Drug 1 | ||
* Drug 2 | * Drug 2 | ||
Revision as of 19:23, 7 July 2014
{{DrugProjectFormSinglePage |authorTag=Alonso Alvarado, M.D. [1] |genericName=nadolol |aOrAn=a |drugClass=beta-adrenergic blocker |indication=agina pectoris, hypertension |hasBlackBoxWarning=Yes |adverseReactions=bradyarrhythmia, dizziness, fatigue |blackBoxWarningTitle=WARNING |blackBoxWarningBody=Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal: Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered nadolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of one to two weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, nadolol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue nadolol therapy abruptly even in patients treated only for hypertension. |fdaLIADAdult======Angina Pectoris=====
- Dosing infromation
- The usual initial dose is 40 mg nadolol once daily. Dosage may be gradually increased in 40 to 80 mg increments at 3 to 7 day intervals until optimum clinical response is obtained or there is pronounced slowing of the heart rate. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 160 or 240 mg administered once daily may be needed.
- The usefulness and safety in angina pectoris of dosage exceeding 240 mg per day have not been established. If treatment is to be discontinued, reduce the dosage gradually over a period of one to two weeks
Hypertension
- Dosing infromation
- The usual initial dose is 40 mg nadolol once daily, whether it is used alone or in addition to diuretic therapy. Dosage may be gradually increased in 40 to 80 mg increments until optimum blood pressure reduction is achieved. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 240 or 320 mg administered once daily may be needed.
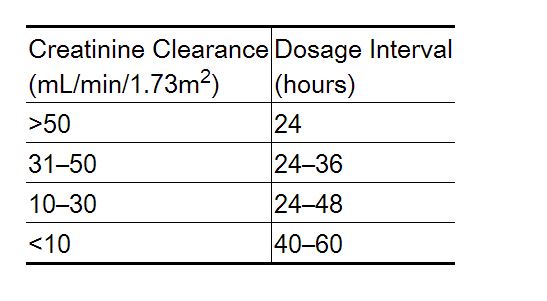
Dosage Adjustment in Renal Failure
Absorbed nadolol is excreted principally by the kidneys and, although nonrenal elimination does occur, dosage adjustments are necessary in patients with renal impairment. The following dose intervals are recommended:
|offLabelAdultGuideSupport======Atrial Fibrillation, Heart Rate Control=====
- Developed by: AHA/ACC
- Class of Recommendation: Class I
- Strength of Evidence: Level B
- Dosing Information/Recommendation
- 10 to 240 mg PO daily[1]
|offLabelAdultNoGuideSupport======Supraventricular Tachycardia=====
- Dosing information
- Initial dose of 0.01 followed by 0.02 and then 0.04 mg/kg administered every 10 minutes until achieving a positive response or the maximum dose of 0.07 mg/kg is reached. Do not exceed 10 mg.[2]
Gastrointestinal Hemorrhage
- Dosing Information
- 40 to 160 mg/day[3]
Hyperthyroidism
- Dosing Information
- 80 to 160 mg/day[4]
Migraine Prophylaxis
- Dosing Information
Tremor
- Dosing information
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Nadolol in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Nadolol in pediatric patients. |contraindications=* Bronchial asthma
- Sinus bradycardia
- Second degree conduction block
- Third degree conduction block
- Cardiogenic shock
- Cardiac failure
|warnings======Cardiac Failure=====
Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, they can be used with caution in patients with a history of failure who are well-compensated, usually with digitalis and diuretics. Beta-blockers do not abolish the inotropic action of digitalis on heart muscle.
In patients without a history of heart failure, continued use of beta-blockers can, in some cases, lead to cardiac failure. Therefore, at the first sign or symptom of heart failure, the patient should be digitalized and/or treated with diuretics, and the response observed closely, or nadolol should be discontinued (gradually, if possible).
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema)
Patients with bronchoespastic disease should in general not receive beta-blockers. Nadolol should be administered with caution since it may block bronchodilation produced by endogenous or exogenous catecholamine stimulation of beta2 receptors.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
Beta-blockers may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia and blood pressure changes) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; therefore, it may be necessary to adjust the dose of antidiabetic drugs.
Thyrotoxicosis
Beta-blockers may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blockade which might precipitate a thyroid storm. |clinicalTrials======Cardiovascular=====
- Bradycardia with heart rates of less than 60 beats per minute occurs commonly, and heart rates below 40 beats per minute and/or symptomatic bradycardia were seen in about 2 of 100 patients. Symptoms of peripheral vascular insufficiency, usually of the Raynaud type, have occurred in approximately 2 of 100 patients.
- Cardiac failure, hypotension, and rhythm/conduction disturbances have each occurred in about 1 of 100 patients.
- Single instances of first degree heart block and third degree heart block have been reported; intensification of AV block is a known effect of beta-blockers.
=Central Nervous System
- Dizziness or fatigue has been reported in approximately 2 of 100 patients.
- [[Paresthesias, sedation, change in behavior have each been reported in approximately 6 of 1000 patients.
Respiratory
- Bronchospasm has been reported in approximately 1 of 1000 patients.
Gastrointestinal
- Nausea, diarrhea, abdominal discomfort, constipation, vomiting, indigestion, anorexia, bloating, and flatulence have been reported in 1 to 5 of 1000 patients.
Miscellaneous
Each of the following has been reported in 1 to 5 of 1000 patients:
- Rash
- Pruritus
- Headache
- Dry mouth, eyes, or skin
- Impotence or decreased libido
- Facial swelling
- Weight gain
- Slurred speech
- Cough
- Nasal stuffiness
- Sweating
- Tinnitus
- Blurred vision
- Reversible alopecia has been reported infrequently.
The following adverse reactions have been reported in patients taking nadolol and/or other beta-adrenergic blocking agents, but no causal relationship to nadolol has been established.
Central Nervous System
- Reversible mental depression progressing to catatonia
- Visual disturbances
- Hallucinations
- An acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability with slightly clouded sensorium, and decreased performance on neuropsychometrics.
=Gastrointestinal
Hematologic
Allergic
- Fever combined with aching and sore throat
- Laryngospasm
- Respiratory distress
Miscellaneous
- Pemphigoid rash
- Hypertensive reaction in patients with pheochromocytoma
- Sleep disorders
- Peyronie's disease
- The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with nadolol.
|drugInteractions=* Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description) |useInPregnancyFDA=(Description) |useInPregnancyAUS=(Description) |useInLaborDelivery=(Description) |useInNursing=(Description) |useInPed=(Description) |useInGeri=(Description) |useInGender=(Description) |useInRace=(Description) |useInRenalImpair=(Description) |useInHepaticImpair=(Description) |useInReproPotential=(Description) |useInImmunocomp=(Description) |othersTitle=Others |useInOthers=(Description) |administration=(Oral/Intravenous/etc) |monitoring======Condition 1=====
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section) |IVCompat====Solution===
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
|overdose====Acute Overdose===
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description) |drugBox=
Nadolol (tablet)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
|mechAction=(Description) |structure=(Description with picture) |PD=(Description) |PK=(Description) |nonClinToxic=(Description) |clinicalStudies======Condition 1=====
(Description)
Condition 2
(Description)
Condition 3
(Description) |howSupplied=(Description) |fdaPatientInfo=(Patient Counseling Information) |alcohol=Alcohol-Nadolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |lookAlike=* (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
|nlmPatientInfo=(Link to patient information page) |drugShortage=Drug Shortage }}
- ↑ January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE; et al. (2014). "2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". J Am Coll Cardiol. doi:10.1016/j.jacc.2014.03.022. PMID 24685669.
- ↑ Olukotun AY, Klein GJ (1987). "Efficacy and safety of intravenous nadolol for supraventricular tachycardia". Am J Cardiol. 60 (6): 59D–62D. PMID 3630923.
- ↑ Gatta A, Merkel C, Sacerdoti D, Bolognesi M, Caregaro L, Zuin R; et al. (1987). "Nadolol for prevention of variceal rebleeding in cirrhosis: a controlled clinical trial". Digestion. 37 (1): 22–8. PMID 3301478.
- ↑ Lazarus JH, Kingswood JC, John R (1987). "The effect of nadolol on heart rate in hyperthyroidism. A controlled trial". Acta Endocrinol (Copenh). 114 (1): 102–6. PMID 3544631.
- ↑ Pascual J, Rivas MT, Leira R (2007). "Testing the combination beta-blocker plus topiramate in refractory migraine". Acta Neurol Scand. 115 (2): 81–3. doi:10.1111/j.1600-0404.2006.00772.x. PMID 17212609.
- ↑ Ryan RE, Ryan RE, Sudilovsky A (1983). "Nadolol: its use in the prophylactic treatment of migraine". Headache. 23 (1): 26–31. PMID 6131052.
- ↑ Edwards RV (1982). "Nadolol use for cerebellar tremor". Am J Psychiatry. 139 (11): 1522. PMID 6127958.
- ↑ Koller WC (1983). "Nadolol in essential tremor". Neurology. 33 (8): 1076–7. PMID 6348587.