Multiple endocrine neoplasia type 2 surgery
|
Multiple endocrine neoplasia type 2 Microchapters |
|
Differentiating Multiple endocrine neoplasia type 2 from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Multiple endocrine neoplasia type 2 surgery On the Web |
|
American Roentgen Ray Society Images of Multiple endocrine neoplasia type 2 surgery |
|
Directions to Hospitals Treating Multiple endocrine neoplasia type 2 |
|
Risk calculators and risk factors for Multiple endocrine neoplasia type 2 surgery |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Overview
Surgery is the mainstay of treatment for multiple endocrine neoplasia type 2.
Surgery
Management of multiple endocrine neoplasia type 2 patients includes thyroidectomy including cervical central and bilateral lymph nodes dissection for medullary thyroid carcinoma, unilateral adrenalectomy for unilateral pheochromocytoma or bilateral adrenalectomy when both glands are involved and selective resection of pathologic parathyroid glands for primary hyperparathyroidism.
Medullary Thyroid Cancer
Conventional Therapy
- The treatment of choice for primary medullary thyroid carcinoma, both sporadic or hereditary, is total thyroidectomy with systematic dissection of all lymph nodes of the central compartment. Total thyroidectomy is necessary as medullary thyroid carcinoma is multicentric in 65–90% of patients in multiple endocrine neoplasia type 2 and extensive central lymph node dissection has been reported to improve survival and recurrence rates compared to less aggressive procedures.[1][2] Lymph node dissection of laterocervical compartments is not performed on principle but only when the neck ultrasound suggests the presence of metastatic nodes.
- Endoscopic adrenal-sparing surgery has become the method of choice for the surgical therapy of pheochromocytoma.[3] In cases with an asynchronous development of pheochromocytoma, the adrenal gland without pheochromocytoma can be preserved, but the patient must be aware that the probability to repeat the surgical treatment in the near future is very high. The advantage of a monolateral adrenal surgery is the possibility to avoid substitutive therapy until the second surgery will be performed.
- The parathyroid glands are frequently found to be enlarged at the time of the thyroidectomy for medullary thyroid carcinoma and should, therefore, be carefully evaluated. The goal in multiple endocrine neoplasia type 2 patients with primary hyperparathyroidism (PHPT) is to excise the enlarged glands and to leave at least one apparently normal parathyroid gland intact. If all glands are enlarged, a subtotal parathyroidectomy or total parathyroidectomy with autotransplantation should be performed. In patients with persistent or recurrent primary hyperparathyroidism (PHPT), the long-term oral administration of calcimimetic drugs as cinacalcet to achieve long-term reductions in serum calcium and PTH concentration should be considered.
Prophylactic or Precocious Thyroidectomy in RET Gene Carrier
- Prophylactic thyroidectomy is advised in gene carriers to guarantee a definitive cure in these subjects. Four different risk levels (from A, the lowest, to D the highest) for RET mutations have been suggested by the American Thyroid Association task force, which developed the most recent guidelines for the management of medullary thyroid carcinoma patients.[4] According to these guidelines, these levels of risk, which are related to the clinical aggressiveness of the corresponding medullary thyroid carcinoma, should be taken into consideration when planning surgical treatment. In particular patients with a level D, RET mutation (i.e., Met918Thr) should be treated as soon as possible in the first year of life; patients with level B and C mutations (located in exons 10, 11, 13, 14, and 15) should be operated on before 5 years of age; only for patients with a level A mutation (exon 8 and 5 mutations), total thyroidectomy can be delayed after five years of age or until the CT positivity.
- During the Seventh International Multiple Endocrine Neoplasia Meeting in Gubbio in 1999, the risk of MTC has been stratified in three categories according to the mutations of c-RET as following.
| Gene | Risk | Treatment |
|---|---|---|
| Children with MEN2B and/or c-RET codon 883, 918,
922 |
Highest risk of aggressive medullary thyroid carcinoma | Total thyroidectomy with
central node dissection, within the first six months. |
| Children with any c-RET codon 611, 618, 620 or 634
mutations |
High risk of medullary thyroid carcinoma | Total thyroidectomy should be performed before age of
five years, with or without central node dissection. |
| Children with c-RET codon 609, 768, 790, 791, 804
and 891 mutations |
Less aggressive and slowly growing medullary thyroid carcinoma | Operated at a later stage |
- Recently, some evidences in big series of RET gene carriers demonstrated that gene carriers with undetectable levels of basal calcitonin have an almost null risk to have already developed the medullary thyroid carcinoma.[5][6] Moreover, a serum Ct <30–40 pg/mL is always associated to an intrathyroidal micro-medullary thyroid carcinoma without any evidence of lymph node metastases. Taking into account these observation, Elisei et al. [7] designed a study in which they operated on only RET gene carriers on the basis of basal and stimulated CT. According to their results, the time of surgical treatment could be personalized and safely planned when the stimulated serum CT becomes positive at the annual control, independently from the type of RET mutation and its associated level of risk. Of course, both cysteine RET mutations and older age are risk factors for having an earlier positive result for either basal or Pg-stimulated serum calcitonin. For these reasons, the follow-up controls should be more or less frequent in cysteine or noncysteine RET-mutated gene carriers, respectively. This strategy obviously implies a high compliance of the RET gene carriers to the scheduled followup with the advantage that young children can be treated later, sometime even after the puberty, close to the adulthood.

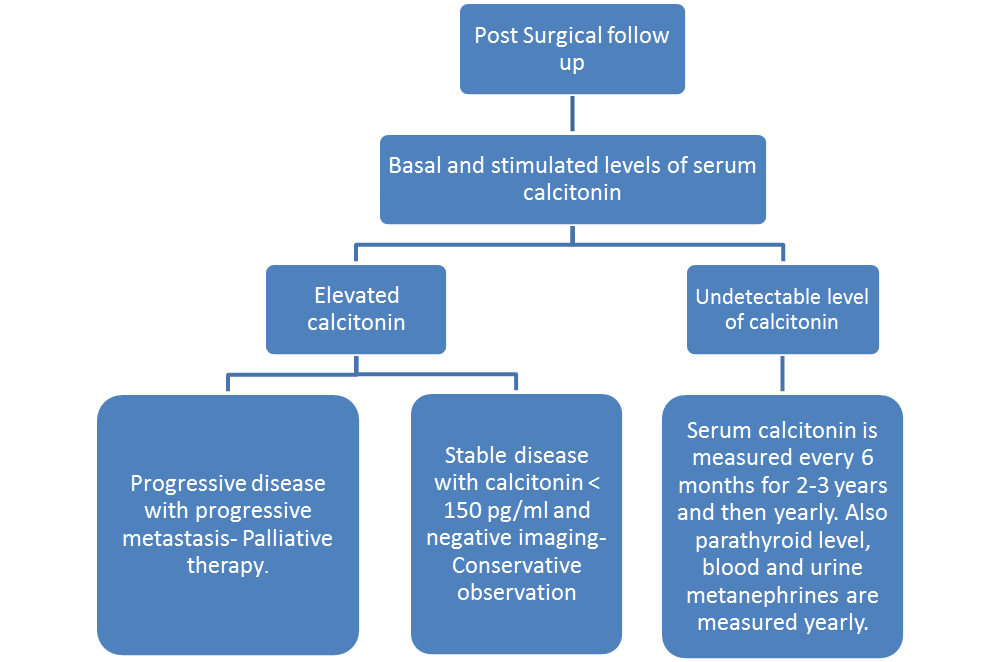
Post Surgery
- Thyroid should supplemented be supplemented for patients undergoing total thyroidectomy.[8]
- Serum calcitonin and carcinoembryonic antigen doubling time (CEA DT) are measured during post surgical follow-up.
- Provacative pentagastrin or calcium test is administered and serum calcitonin level is measured.
- If there is no significant elevation in serum calcitonin level, serum calcitonin is measured every 6 months for 2-3 years and then yearly.
- If the calcitonin is below 150 pg/ml, USG neck is recommended.
- If the basal serum calcitonin is above 150 pg/ml, screening for distant metastasis is recommended.
References
- ↑ Machens A, Hauptmann S, Dralle H (2007). "Increased risk of lymph node metastasis in multifocal hereditary and sporadic medullary thyroid cancer". World J Surg. 31 (10): 1960–5. doi:10.1007/s00268-007-9185-1. PMID 17665245.
- ↑ Russell CF, Van Heerden JA, Sizemore GW, Edis AJ, Taylor WF, ReMine WH; et al. (1983). "The surgical management of medullary thyroid carcinoma". Ann Surg. 197 (1): 42–8. PMC 1352852. PMID 6128962.
- ↑ Walz MK, Alesina PF (2009). "Single access retroperitoneoscopic adrenalectomy (SARA)--one step beyond in endocrine surgery". Langenbecks Arch Surg. 394 (3): 447–50. doi:10.1007/s00423-008-0418-z. PMID 18784938.
- ↑ American Thyroid Association Guidelines Task Force. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF; et al. (2009). "Medullary thyroid cancer: management guidelines of the American Thyroid Association". Thyroid. 19 (6): 565–612. doi:10.1089/thy.2008.0403. PMID 19469690.
- ↑ Lau GS, Lang BH, Lo CY, Tso A, Garcia-Barcelo MM, Tam PK; et al. (2009). "Prophylactic thyroidectomy in ethnic Chinese patients with multiple endocrine neoplasia type 2A syndrome after the introduction of genetic testing". Hong Kong Med J. 15 (5): 326–31. PMID 19801688.
- ↑ Schmoldt A, Benthe HF, Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. PMID http://dx.doi.org/10.1210/jc.2010-1234 Check
|pmid=value (help). - ↑ Schmoldt A, Benthe HF, Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. PMID http://dx.doi.org/10.1210/jc.2011-2046 Check
|pmid=value (help). - ↑ Pacini F, Castagna MG, Brilli L, Pentheroudakis G, ESMO Guidelines Working Group (2012). "Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Ann Oncol. 23 Suppl 7: vii110–9. doi:10.1093/annonc/mds230. PMID 22997443.