Lupus nephritis pathophysiology: Difference between revisions
No edit summary |
|||
| (48 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
__NOTOC__ | __NOTOC__ | ||
{{Lupus nephritis}} | {{Lupus nephritis}} | ||
{{CMG}}; {{AE}} {{CZ}}, {{RT}} | {{CMG}}; {{AE}} {{OK}}, {{CZ}}, {{RT}} , {{AIDA}} | ||
==Overview== | ==Overview== | ||
Systemic lupus erythematosus (SLE, or lupus) is an [[autoimmune]] disease. This means there is a problem with the body's immune system. Normally, the immune system helps protect the body from harmful substances. But in patients with an autoimmune disease, the immune system cannot tell the difference between harmful substances and healthy ones. As a result, the immune system attacks otherwise healthy cells and tissue. | |||
==Pathophysiology== | ==Pathophysiology== | ||
===Pathogenesis=== | |||
[[Immune systems|Immune system]], [[genetic]], and [[Environmental factor|environmenta]]<nowiki/>l factors are considered in the pathogenesis of [[Systemic lupus erythematosus|Lupus Nephritis]] (LN). All tissues of the [[renal]] can be involved in LN. | |||
== | - '''Immune system''' <ref name="pmid25014039">{{cite journal |vauthors=Schwartz N, Goilav B, Putterman C |title=The pathogenesis, diagnosis and treatment of lupus nephritis |journal=Curr Opin Rheumatol |volume=26 |issue=5 |pages=502–9 |date=September 2014 |pmid=25014039 |pmc=4221732 |doi=10.1097/BOR.0000000000000089 |url=}}</ref>: | ||
The | * [[Plasma cell]]<nowiki/>s and [[B lymphocyte]]<nowiki/>s: | ||
[[plasma cells]](PC) and B cells produce [[Autoantibody|autoantibodies]]. | |||
B cells in LN patients have more [[MicroRNA]]<nowiki/>s (miRNAs) which modulate gene expression <ref name="pmid15211354">{{cite journal |vauthors=He L, Hannon GJ |title=MicroRNAs: small RNAs with a big role in gene regulation |journal=Nat. Rev. Genet. |volume=5 |issue=7 |pages=522–31 |date=July 2004 |pmid=15211354 |doi=10.1038/nrg1379 |url=}}</ref>. Over expression of the [[MicroRNA|miR-30a]] could lower the level of Lyn (type of [[Tyrosine kinases|protein tyrosine kinase]]<nowiki/>s), and lower level of Lyn may cause deposition of [[Immune complexes|immune complexe]]<nowiki/>s in the [[kidney]] <ref name="pmid11166177">{{cite journal |vauthors=Yu CC, Yen TS, Lowell CA, DeFranco AL |title=Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn |journal=Curr. Biol. |volume=11 |issue=1 |pages=34–8 |date=January 2001 |pmid=11166177 |doi= |url=}}</ref><ref name="pmid23450709">{{cite journal |vauthors=Liu Y, Dong J, Mu R, Gao Y, Tan X, Li Y, Li Z, Yang G |title=MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn |journal=Arthritis Rheum. |volume=65 |issue=6 |pages=1603–11 |date=June 2013 |pmid=23450709 |doi=10.1002/art.37912 |url=}}</ref>. | |||
High number of PCs in the medulla and activation of [[B cells]] cause [[proteinuria]] and severe damage in LN. | |||
* [[T lymphocytes]]: | |||
[[T cells]] are considered as coordinators of the [[Adaptive immune response|adaptive immune]] response. The [[T-cell receptors|T-cell receptor]] (TCR) complex is a protein receptor composed of [[T cell receptor|TCR]]α, β, and ζ chains. Decreased TCRζ chain expression may cause LN <ref name="pmid26573543">{{cite journal |vauthors=Munroe ME, James JA |title=Genetics of Lupus Nephritis: Clinical Implications |journal=Semin. Nephrol. |volume=35 |issue=5 |pages=396–409 |date=September 2015 |pmid=26573543 |pmc=4653095 |doi=10.1016/j.semnephrol.2015.08.002 |url=}}</ref>. [[Th2]] cells play role in LN by affecting [[B-lymphocytes|B-lymphocyte]] activation. [[Th17]] cells play role in LN by causing [[inflammation]] in [[Nephrons|nephron]]<nowiki/>s. Stat-1 [[Signaling pathway|signaling]] play role on activity of [[IL-17]] which produced by [[Th17]] cells. IL-17–deficient patients are more susceptible to [[SLE]] <ref name="pmid24920843">{{cite journal |vauthors=Amarilyo G, Lourenço EV, Shi FD, La Cava A |title=IL-17 promotes murine lupus |journal=J. Immunol. |volume=193 |issue=2 |pages=540–3 |date=July 2014 |pmid=24920843 |doi=10.4049/jimmunol.1400931 |url=}}</ref>. | |||
* [[Autophagy]] : | |||
Overexpression of [[apolipoprotein]] L1 (APOL1), a protein that induce autophagic cell death, may cause [[fibrosis]] in renal and [[ESRD]] in patients with LN <ref name="pmid18505729">{{cite journal |vauthors=Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA |title=Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death |journal=J. Biol. Chem. |volume=283 |issue=31 |pages=21540–9 |date=August 2008 |pmid=18505729 |pmc=2490785 |doi=10.1074/jbc.M800214200 |url=}}</ref> <ref name="pmid24504811">{{cite journal |vauthors=Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Alarcón GS, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KL, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP |title=End-stage renal disease in African Americans with lupus nephritis is associated with APOL1 |journal=Arthritis Rheumatol |volume=66 |issue=2 |pages=390–6 |date=February 2014 |pmid=24504811 |pmc=4002759 |doi=10.1002/art.38220 |url=}}</ref>. | |||
Low level of myotubularin-related phosphatase 3 (MTMR3), types of the [[phosphatidylinositol 3-phosphate]] that plays a role in initiating [[autophagy]], may cause LN <ref name="pmid20059746">{{cite journal |vauthors=Taguchi-Atarashi N, Hamasaki M, Matsunaga K, Omori H, Ktistakis NT, Yoshimori T, Noda T |title=Modulation of local PtdIns3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy |journal=Traffic |volume=11 |issue=4 |pages=468–78 |date=April 2010 |pmid=20059746 |doi=10.1111/j.1600-0854.2010.01034.x |url=}}</ref>. | |||
* [[Macrophages]]: | |||
Macrophages play role in presenting [[antigens]], removing of dying cells, and producing [[cytokines]]. Increase expression of [[Sialoadhesin]] (Sn), a macrophage-restricted adhesion molecule may play a role in causing sever LN <ref name="pmid18383365">{{cite journal |vauthors=Biesen R, Demir C, Barkhudarova F, Grün JR, Steinbrich-Zöllner M, Backhaus M, Häupl T, Rudwaleit M, Riemekasten G, Radbruch A, Hiepe F, Burmester GR, Grützkau A |title=Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus |journal=Arthritis Rheum. |volume=58 |issue=4 |pages=1136–45 |date=April 2008 |pmid=18383365 |doi=10.1002/art.23404 |url=}}</ref>. | |||
* Inflammatory [[cytokines]]: | |||
[[Tumor necrosis factor]] (TNF) is a [[Cytokines|cytokine]] (cell signaling protein) that play role in inflammation process. One of the sub types of [[TNF]] is TNF-like weak inducer of apoptosis (TWEAK) which has an important role in causing LN <ref name="pmid21303425">{{cite journal |vauthors=Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, Chow KM, Wang G, Li PK, Szeto CC |title=Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis |journal=Nephrology (Carlton) |volume=16 |issue=4 |pages=426–32 |date=May 2011 |pmid=21303425 |doi=10.1111/j.1440-1797.2011.01449.x |url=}}</ref>. Fn14 ( TWEAK receptor) is interacted with TWEAK on renal [[Mesangial cells|mesangial]], [[endothelial]], tubular cells and [[Podocytes|podocyte]]<nowiki/>s <ref name="pmid16424220">{{cite journal |vauthors=Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C |title=Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells |journal=J. Immunol. |volume=176 |issue=3 |pages=1889–98 |date=February 2006 |pmid=16424220 |doi= |url=}}</ref>. This interactions produce multiple inflammatory mediators which lead to LN. | |||
[ | Increased expression of [[interferon alpha]] (IFN-α) inducible [[RNA]] transcripts by [[Mononuclear cells|mononuclear]] cells. | ||
''-'' '''Repair impairment and Tissue Scarring''': | |||
'' ''Impairment in regulation and repair may cause [[Scars|tissue scars]] like <ref name="pmid25401461">{{cite journal |vauthors=Liu Y, Anders HJ |title=Lupus nephritis: from pathogenesis to targets for biologic treatment |journal=Nephron Clin Pract |volume=128 |issue=3-4 |pages=224–31 |date=2014 |pmid=25401461 |doi=10.1159/000368581 |url=}}</ref>'':'' | |||
* [[Necrosis]] and extracellular [[matrix]] production cause global [[glomerulosclerosis]] <ref name="pmid21719782">{{cite journal |vauthors=Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ |title=Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis |journal=J. Am. Soc. Nephrol. |volume=22 |issue=7 |pages=1262–74 |date=July 2011 |pmid=21719782 |pmc=3137574 |doi=10.1681/ASN.2010090970 |url=}}</ref>. | |||
* [[Fibrinogen]] leakage in to [[Bowman's space|Bowman]]’s space cause [[Epithelial cells|epithelial cell]] hyperproliferation. Accumulation of [[epithelial cells]] make [[Glomerular disease|glomerular crescent]]. In severe LN, epithelial cells produce extra amount of matrix in [[Bowman's space|Bowman’s space]], which result in [[glomerulosclerosis]] <ref name="pmid22553158">{{cite journal |vauthors=Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ |title=Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury |journal=J. Pathol. |volume=228 |issue=4 |pages=482–94 |date=December 2012 |pmid=22553158 |doi=10.1002/path.4046 |url=}}</ref>. | |||
* Exaggerated healing response cause hyperproliferation of [[Mesangial cells|mesangial]] and [[endothelial cells]], and [[Podocytes|podocyte]] loss cause damage in [[renal]]. | |||
- '''Environmental factors:''' | |||
* Geographical distribution : | |||
LN is more severe in African, Hispanics and Asian patients with SLE. LN is associated with temperature and season <ref name="pmid23732627">{{cite journal |vauthors=Li Y, Fang X, Li QZ |title=Biomarker profiling for lupus nephritis |journal=Genomics Proteomics Bioinformatics |volume=11 |issue=3 |pages=158–65 |date=June 2013 |pmid=23732627 |pmc=4357827 |doi=10.1016/j.gpb.2013.05.003 |url=}}</ref>. Most flares are happening in spring and hot weather. | |||
* Infections: | |||
** May cause more antibodies production by B cells. | |||
** Include: | |||
*** [[Parvovirus B19]] | |||
*** [[Epstein Barr virus|Epstein-Barr virus (EBV)]] | |||
*** [[Trypanosomiasis]] | |||
*** [[Mycobacterial]] infections | |||
* [[Ultraviolet|Ultraviolet (UV)]] light: | |||
** Can stimulate [[B-cells]] to produce more [[antibodies]] | |||
** May activate [[macrophages]], interfere with [[antigen processing]], and therefore increase the degree of [[autoimmunity]]. | |||
* Diet: | |||
** [[Vitamin D]] and [[vitamin A]] play role in SLE flare by affecting immunological pathways. The role of them in LN is unknown. | |||
==Genetics== | |||
interaction and mutation between below genes from multiple categories may cause severe LN<ref name="pmid25825084">{{cite journal |vauthors=Mohan C, Putterman C |title=Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis |journal=Nat Rev Nephrol |volume=11 |issue=6 |pages=329–41 |date=June 2015 |pmid=25825084 |doi=10.1038/nrneph.2015.33 |url=}}</ref> <ref name="pmid10841565">{{cite journal |vauthors=Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK |title=Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=97 |issue=12 |pages=6670–5 |date=June 2000 |pmid=10841565 |pmc=18697 |doi= |url=}}</ref> <ref name="pmid15003808">{{cite journal |vauthors=Xie S, Mohan C |title=Divide and conquer--the power of congenic strains |journal=Clin. Immunol. |volume=110 |issue=2 |pages=109–11 |date=February 2004 |pmid=15003808 |doi=10.1016/j.clim.2003.09.007 |url=}}</ref> <ref name="pmid15995581">{{cite journal |vauthors=Henry T, Mohan C |title=Systemic lupus erythematosus--recent clues from congenic strains |journal=Arch. Immunol. Ther. Exp. (Warsz.) |volume=53 |issue=3 |pages=207–12 |date=2005 |pmid=15995581 |doi= |url=}}</ref>. | |||
* ''PP2Ac'' <ref name="pmid22422882">{{cite journal |vauthors=Crispín JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, Mayadas TN, Tsokos GC |title=Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism |journal=J. Immunol. |volume=188 |issue=8 |pages=3567–71 |date=April 2012 |pmid=22422882 |pmc=3324672 |doi=10.4049/jimmunol.1200143 |url=}}</ref>: Overexpression of PP2Ac is associated with [[renal]] damage by causing [[neutrophils]] accumulation. | |||
* ''IKZF1'' <ref name="pmid12874250">{{cite journal |vauthors=Jacob CO, Zang S, Li L, Ciobanu V, Quismorio F, Mizutani A, Satoh M, Koss M |title=Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice |journal=J. Immunol. |volume=171 |issue=3 |pages=1564–71 |date=August 2003 |pmid=12874250 |doi= |url=}}</ref> | |||
* ''TNFSF4'' <ref name="pmid23936824">{{cite journal |vauthors=Zhou XJ, Cheng FJ, Qi YY, Zhao MH, Zhang H |title=A replication study from Chinese supports association between lupus-risk allele in TNFSF4 and renal disorder |journal=Biomed Res Int |volume=2013 |issue= |pages=597921 |date=2013 |pmid=23936824 |pmc=3713374 |doi=10.1155/2013/597921 |url=}}</ref> | |||
* ''TLR9'' <ref name="pmid20133703">{{cite journal |vauthors=Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB |title=Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=107 |issue=7 |pages=3012–7 |date=February 2010 |pmid=20133703 |pmc=2840310 |doi=10.1073/pnas.0914902107 |url=}}</ref> | |||
* ''TNFAIP3 (A20)'' <ref name="pmid9461440">{{cite journal |vauthors=Clynes R, Dumitru C, Ravetch JV |title=Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis |journal=Science |volume=279 |issue=5353 |pages=1052–4 |date=February 1998 |pmid=9461440 |doi= |url=}}</ref> | |||
* ''TNIP3 (ABIN3)'' <ref name="pmid24697319">{{cite journal |vauthors=Dang J, Shan S, Li J, Zhao H, Xin Q, Liu Y, Bian X, Liu Q |title=Gene-gene interactions of IRF5, STAT4, IKZF1 and ETS1 in systemic lupus erythematosus |journal=Tissue Antigens |volume=83 |issue=6 |pages=401–8 |date=June 2014 |pmid=24697319 |doi=10.1111/tan.12349 |url=}}</ref> | |||
* ''ACE D allele'' <ref name="pmid22337243">{{cite journal |vauthors=Zhou TB, Liu YG, Lin N, Qin YH, Huang K, Shao MB, Peng DD |title=Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic lupus erythematosus/lupus nephritis: a systematic review and metaanalysis |journal=J. Rheumatol. |volume=39 |issue=4 |pages=686–93 |date=April 2012 |pmid=22337243 |doi=10.3899/jrheum.110863 |url=}}</ref> | |||
* ''KLK'' <ref name="pmid19307730">{{cite journal |vauthors=Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sánchez E, Kelly JA, Li L, Liu Y, Zhou J, Yan M, Ye Q, Liu S, Xie C, Zhou XJ, Chung SA, Pons-Estel B, Witte T, de Ramón E, Bae SC, Barizzone N, Sebastiani GD, Merrill JT, Gregersen PK, Gilkeson GG, Kimberly RP, Vyse TJ, Kim I, D'Alfonso S, Martin J, Harley JB, Criswell LA, Wakeland EK, Alarcón-Riquelme ME, Mohan C |title=Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans |journal=J. Clin. Invest. |volume=119 |issue=4 |pages=911–23 |date=April 2009 |pmid=19307730 |pmc=2662554 |doi=10.1172/JCI36728 |url=}}</ref> | |||
* ''FCGR2A, 3A, 3B'' <ref name="pmid18075791">{{cite journal |vauthors=Brown EE, Edberg JC, Kimberly RP |title=Fc receptor genes and the systemic lupus erythematosus diathesis |journal=Autoimmunity |volume=40 |issue=8 |pages=567–81 |date=December 2007 |pmid=18075791 |doi=10.1080/08916930701763710 |url=}}</ref> | |||
* ''ITGAM'' <ref name="pmid23918926">{{cite journal |vauthors=Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispín JC |title=Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling |journal=J. Biol. Chem. |volume=288 |issue=37 |pages=26775–84 |date=September 2013 |pmid=23918926 |pmc=3772223 |doi=10.1074/jbc.M113.483743 |url=}}</ref> <ref name="pmid17082647">{{cite journal |vauthors=Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R |title=FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis |journal=J. Immunol. |volume=177 |issue=10 |pages=7287–95 |date=November 2006 |pmid=17082647 |doi= |url=}}</ref> <ref name="pmid20497632">{{cite journal |vauthors=Zhou XJ, Lv JC, Cheng WR, Yu L, Zhao MH, Zhang H |title=Association of TLR9 gene polymorphisms with lupus nephritis in a Chinese Han population |journal=Clin. Exp. Rheumatol. |volume=28 |issue=3 |pages=397–400 |date=2010 |pmid=20497632 |doi= |url=}}</ref> | |||
* ''HLA DR and BLK'' <ref name="pmid21948081">{{cite journal |vauthors=Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B |title=Tolerogenic function of Blimp-1 in dendritic cells |journal=J. Exp. Med. |volume=208 |issue=11 |pages=2193–9 |date=October 2011 |pmid=21948081 |pmc=3201204 |doi=10.1084/jem.20110658 |url=}}</ref> | |||
'''Epigenetic modification''' <ref name="pmid237326272">{{cite journal |vauthors=Li Y, Fang X, Li QZ |title=Biomarker profiling for lupus nephritis |journal=Genomics Proteomics Bioinformatics |volume=11 |issue=3 |pages=158–65 |date=June 2013 |pmid=23732627 |pmc=4357827 |doi=10.1016/j.gpb.2013.05.003 |url=}}</ref>''':''' | |||
* [[DNA]] [[methylation]]: | |||
Hypomethylated genes in [[B lymphocyte]]<nowiki/>s activate [[Transcription (genetics)|transcription]], and cause production of many [[Antibodies|anti-DNA antibodi]]<nowiki/>es<ref name="pmid21460598">{{cite journal |vauthors=Renaudineau Y, Youinou P |title=Epigenetics and autoimmunity, with special emphasis on methylation |journal=Keio J Med |volume=60 |issue=1 |pages=10–6 |date=2011 |pmid=21460598 |doi= |url=}}</ref>. | |||
* [[Histone]] modifications: | |||
Histone is a protein in chromatin that play role in [[gene regulation]]. | |||
[[Acetylation]] of histones are concidered targets for [[Autoantibodies|autoantibodi]]<nowiki/>es in LN. | |||
* [[MicroRNA]]<nowiki/>s: | |||
Non-coding RNA sequences that play role in [[gene regulation]] by degradation of [[mRNA]] and [[Translation|protein translation]] blockage. | |||
[ | Some [[MicroRNA|miRNA]]<nowiki/>s are increased in LN like miR-142-3p and miR-181 and some are decreased like miR-106a, miR-17, miR-20a, miR-92a and miR-203 <ref name="pmid23401079">{{cite journal |vauthors=Carlsen AL, Schetter AJ, Nielsen CT, Lood C, Knudsen S, Voss A, Harris CC, Hellmark T, Segelmark M, Jacobsen S, Bengtsson AA, Heegaard NH |title=Circulating microRNA expression profiles associated with systemic lupus erythematosus |journal=Arthritis Rheum. |volume=65 |issue=5 |pages=1324–34 |date=May 2013 |pmid=23401079 |doi=10.1002/art.37890 |url=}}</ref>. | ||
These changes cause dysregulation of genes and LN. | |||
==Associated Conditions== | |||
Morbidity and mortality are increased in patients with LN because of aggressive immunosuppressive therapy. | |||
Anti-DNA, anti-nucleosome and anti-histone Abs are associated with sever poor prognosis LN <ref name="pmid23100145">{{cite journal |vauthors=Sui M, Sui M, Lin Q, Xu Z, Han X, Xie R, Jia X, Guo X, Zhang W, Guan X, Ren H |title=Simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone antibodies is a marker for more severe lupus nephritis |journal=J. Clin. Immunol. |volume=33 |issue=2 |pages=378–87 |date=February 2013 |pmid=23100145 |doi=10.1007/s10875-012-9825-6 |url=}}</ref>. | |||
==Gross Pathology== | |||
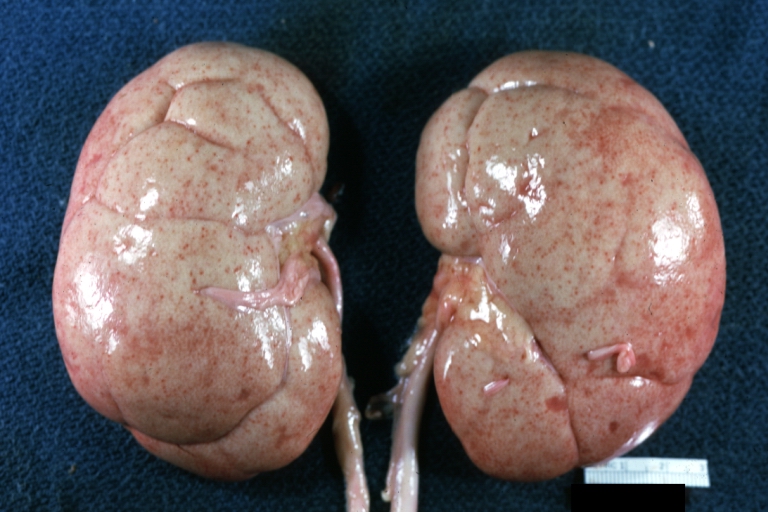
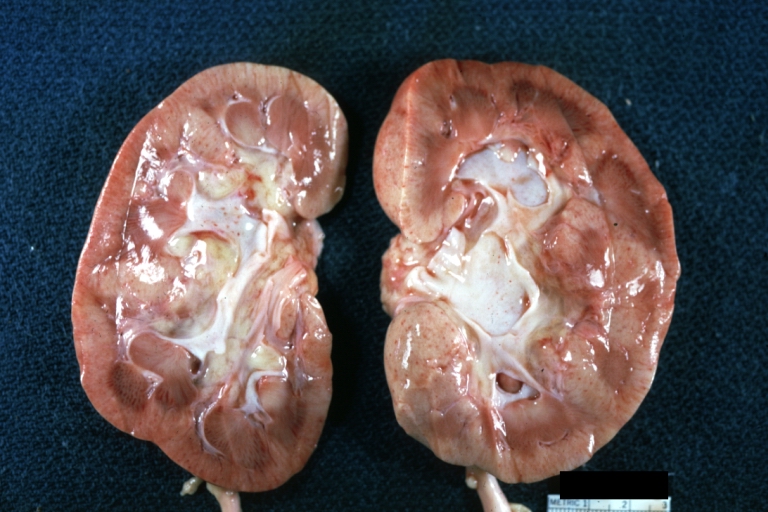
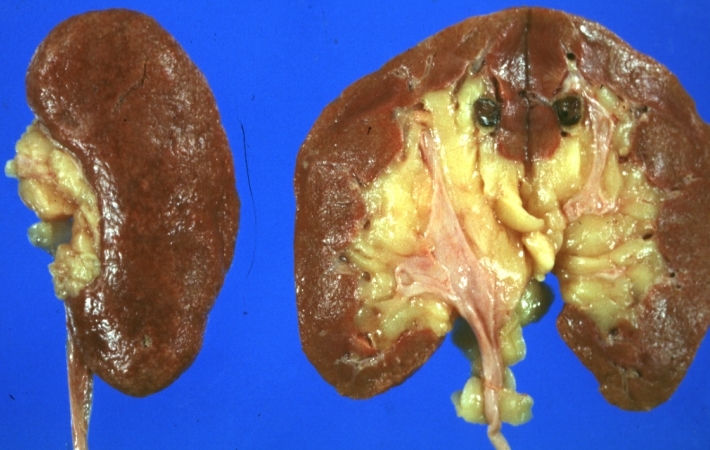
*On gross pathology hypertrophy and pallor of the kidney will be seen. | |||
{| align="row" | {| align="row" | ||
|- | |- | ||
| Line 69: | Line 100: | ||
| [[Image:Systemic lupus erythematosus 002.jpg|thumb|Gross cut surface pale kidneys typical of nephrotic syndrome (subacute glomerulonephritis)]] | | [[Image:Systemic lupus erythematosus 002.jpg|thumb|Gross cut surface pale kidneys typical of nephrotic syndrome (subacute glomerulonephritis)]] | ||
| [[Image:Systemic lupus erythematosus 046.jpg|thumb|Gross natural color nice external and cut surface view of uniformly scarred and moderately shrunken kidneys]] | | [[Image:Systemic lupus erythematosus 046.jpg|thumb|Gross natural color nice external and cut surface view of uniformly scarred and moderately shrunken kidneys]] | ||
|} | |} | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
6 classification for LN on microscopy: | |||
{| class="wikitable" , | |||
|- style="background: blue; color:black" | |||
! Class | |||
! Name | |||
! Light Microscopy | |||
! Light microscopy previews | |||
! Electron microscopy | |||
|- | |||
| I | |||
| Minimal [[Mesangial cells|mesangial]] lupus nephritis | |||
| Normal | |||
| | |||
| Immune deposits in [[Mesangial cells|mesangial]] | |||
|- | |||
| II | |||
| [[Mesangial proliferative glomerulonephritis|Mesangial proliferative]] lupus nephritis | |||
| Mesangial widening and hypercellularity | |||
|[[File:Membranous nephropathy - mpas - very high mag.jpg|thumb|300px|<SMALL><SMALL>''[https://librepathology.org/wiki/Main_Page/ Adapted from Librepathology]''</SMALL></SMALL>]] | |||
| Immune deposits in [[Epithelial|subepithelial]] or subendothelial | |||
|- | |||
| III | |||
| Focal lupus nephritis | |||
| [[Necrotizing]] and [[Sclerosis|sclerosing]] lesions in < 50% [[glomeruli]] | |||
|[[File:1599px-Focal segmental glomerulosclerosis - high mag.jpg|thumb|300px|<SMALL><SMALL>''[https://librepathology.org/wiki/Main_Page/ Adapted from Librepathology]''</SMALL></SMALL>]] | |||
| [[Fibrinoid necrosis|Fibrinoid]] necrosis and crescents in [[Glomeruli|glomeruli,]] Immune deposits in [[Endothelial|subendothelial]] space of the glomerular capillary and mesangium | |||
|- | |||
| IV | |||
| Diffuse lupus nephritis | |||
| mesangial, endocapillary and mesangiocapillary involvement > 50% | |||
| rowspan="2" |[[File:Membranoproliferative glomerulonephritis - very high mag.jpg|thumb|300px|<SMALL><SMALL>''[https://librepathology.org/wiki/Main_Page/ Adapted from Librepathology]''</SMALL></SMALL>]] | |||
| Diffuse wire loop deposits, extensive subendothelial deposits | |||
|- | |||
| V | |||
| Lupus [[membranous nephropathy]] | |||
| thickening of [[capillary]] of the glomeruli | |||
| Global or segmental [[Subepithelial connective tissue graft|subepithelial]] immune deposits | |||
|- | |||
| VI | |||
| Advanced sclerosing lupus nephritis | |||
| [[Sclerosis]] of the glomeruli > 90% | |||
|[[File:Crescentic glomerulonephritis (2).jpg|thumb|300px|<SMALL><SMALL>''[https://librepathology.org/wiki/Main_Page/ Adapted from Librepathology]''</SMALL></SMALL>]] | |||
| Global or segmental subepithelial immune deposits | |||
|} | |||
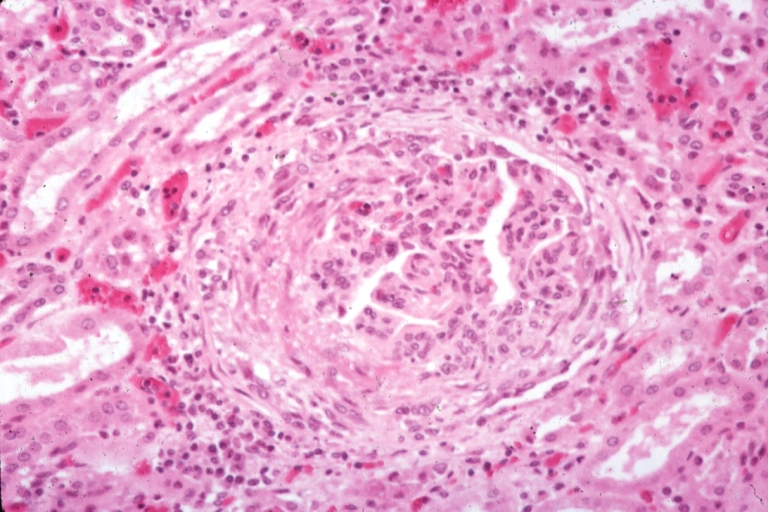
[[Image:Systemic lupus erythematosus 039.jpg|thumb|Kidney: Lupus Erythematosus: Micro high mag H&E. A nice example of a lesion of chronic glomerulonephritis with lobular scarring. A fibrous type crescent.]] | |||
====Videos==== | |||
==References== | |||
[[Category: (name of the system)]] | |||
==References== | |||
[[Category:Disease]] | |||
[[Category:Nephrology]] | |||
[[Category:Autoimmune diseases]] | |||
{| align="row" | {| align="row" | ||
|- | |- | ||
| | | | ||
|- valign="top" | |- valign="top" | ||
[[Image:Systemic lupus erythematosus 039.jpg|thumb|Kidney: Lupus Erythematosus: Micro high mag H&E. A nice example of a lesion of chronic glomerulonephritis with lobular scarring. A fibrous type crescent.]] | |||
====Videos==== | ====Videos==== | ||
| Line 104: | Line 186: | ||
[[Category:Nephrology]] | [[Category:Nephrology]] | ||
[[Category:Autoimmune diseases]] | [[Category:Autoimmune diseases]] | ||
|} | |||
<references /> | |||
Latest revision as of 16:20, 26 October 2018
| https://https://www.youtube.com/watch?v=HwzNQ4Oav00&t=4s |350}} |
|
Lupus nephritis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Lupus nephritis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Lupus nephritis pathophysiology |
|
Risk calculators and risk factors for Lupus nephritis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Omer Kamal, M.D.[2], Cafer Zorkun, M.D., Ph.D. [3], Raviteja Guddeti, M.B.B.S. [4] , Aida Javanbakht, M.D.
Overview
Systemic lupus erythematosus (SLE, or lupus) is an autoimmune disease. This means there is a problem with the body's immune system. Normally, the immune system helps protect the body from harmful substances. But in patients with an autoimmune disease, the immune system cannot tell the difference between harmful substances and healthy ones. As a result, the immune system attacks otherwise healthy cells and tissue.
Pathophysiology
Pathogenesis
Immune system, genetic, and environmental factors are considered in the pathogenesis of Lupus Nephritis (LN). All tissues of the renal can be involved in LN.
- Immune system [1]:
- Plasma cells and B lymphocytes:
plasma cells(PC) and B cells produce autoantibodies.
B cells in LN patients have more MicroRNAs (miRNAs) which modulate gene expression [2]. Over expression of the miR-30a could lower the level of Lyn (type of protein tyrosine kinases), and lower level of Lyn may cause deposition of immune complexes in the kidney [3][4].
High number of PCs in the medulla and activation of B cells cause proteinuria and severe damage in LN.
T cells are considered as coordinators of the adaptive immune response. The T-cell receptor (TCR) complex is a protein receptor composed of TCRα, β, and ζ chains. Decreased TCRζ chain expression may cause LN [5]. Th2 cells play role in LN by affecting B-lymphocyte activation. Th17 cells play role in LN by causing inflammation in nephrons. Stat-1 signaling play role on activity of IL-17 which produced by Th17 cells. IL-17–deficient patients are more susceptible to SLE [6].
Overexpression of apolipoprotein L1 (APOL1), a protein that induce autophagic cell death, may cause fibrosis in renal and ESRD in patients with LN [7] [8].
Low level of myotubularin-related phosphatase 3 (MTMR3), types of the phosphatidylinositol 3-phosphate that plays a role in initiating autophagy, may cause LN [9].
Macrophages play role in presenting antigens, removing of dying cells, and producing cytokines. Increase expression of Sialoadhesin (Sn), a macrophage-restricted adhesion molecule may play a role in causing sever LN [10].
- Inflammatory cytokines:
Tumor necrosis factor (TNF) is a cytokine (cell signaling protein) that play role in inflammation process. One of the sub types of TNF is TNF-like weak inducer of apoptosis (TWEAK) which has an important role in causing LN [11]. Fn14 ( TWEAK receptor) is interacted with TWEAK on renal mesangial, endothelial, tubular cells and podocytes [12]. This interactions produce multiple inflammatory mediators which lead to LN.
Increased expression of interferon alpha (IFN-α) inducible RNA transcripts by mononuclear cells.
- Repair impairment and Tissue Scarring:
Impairment in regulation and repair may cause tissue scars like [13]:
- Necrosis and extracellular matrix production cause global glomerulosclerosis [14].
- Fibrinogen leakage in to Bowman’s space cause epithelial cell hyperproliferation. Accumulation of epithelial cells make glomerular crescent. In severe LN, epithelial cells produce extra amount of matrix in Bowman’s space, which result in glomerulosclerosis [15].
- Exaggerated healing response cause hyperproliferation of mesangial and endothelial cells, and podocyte loss cause damage in renal.
- Environmental factors:
- Geographical distribution :
LN is more severe in African, Hispanics and Asian patients with SLE. LN is associated with temperature and season [16]. Most flares are happening in spring and hot weather.
- Infections:
- May cause more antibodies production by B cells.
- Include:
- Ultraviolet (UV) light:
- Can stimulate B-cells to produce more antibodies
- May activate macrophages, interfere with antigen processing, and therefore increase the degree of autoimmunity.
- Diet:
Genetics
interaction and mutation between below genes from multiple categories may cause severe LN[17] [18] [19] [20].
- PP2Ac [21]: Overexpression of PP2Ac is associated with renal damage by causing neutrophils accumulation.
- IKZF1 [22]
- TNFSF4 [23]
- TLR9 [24]
- TNFAIP3 (A20) [25]
- TNIP3 (ABIN3) [26]
- ACE D allele [27]
- KLK [28]
- FCGR2A, 3A, 3B [29]
- ITGAM [30] [31] [32]
- HLA DR and BLK [33]
Epigenetic modification [34]:
Hypomethylated genes in B lymphocytes activate transcription, and cause production of many anti-DNA antibodies[35].
- Histone modifications:
Histone is a protein in chromatin that play role in gene regulation.
Acetylation of histones are concidered targets for autoantibodies in LN.
- MicroRNAs:
Non-coding RNA sequences that play role in gene regulation by degradation of mRNA and protein translation blockage.
Some miRNAs are increased in LN like miR-142-3p and miR-181 and some are decreased like miR-106a, miR-17, miR-20a, miR-92a and miR-203 [36].
These changes cause dysregulation of genes and LN.
Associated Conditions
Morbidity and mortality are increased in patients with LN because of aggressive immunosuppressive therapy.
Anti-DNA, anti-nucleosome and anti-histone Abs are associated with sever poor prognosis LN [37].
Gross Pathology
- On gross pathology hypertrophy and pallor of the kidney will be seen.
Microscopic Pathology
6 classification for LN on microscopy:
| Class | Name | Light Microscopy | Light microscopy previews | Electron microscopy |
|---|---|---|---|---|
| I | Minimal mesangial lupus nephritis | Normal | Immune deposits in mesangial | |
| II | Mesangial proliferative lupus nephritis | Mesangial widening and hypercellularity |  |
Immune deposits in subepithelial or subendothelial |
| III | Focal lupus nephritis | Necrotizing and sclerosing lesions in < 50% glomeruli |  |
Fibrinoid necrosis and crescents in glomeruli, Immune deposits in subendothelial space of the glomerular capillary and mesangium |
| IV | Diffuse lupus nephritis | mesangial, endocapillary and mesangiocapillary involvement > 50% |  |
Diffuse wire loop deposits, extensive subendothelial deposits |
| V | Lupus membranous nephropathy | thickening of capillary of the glomeruli | Global or segmental subepithelial immune deposits | |
| VI | Advanced sclerosing lupus nephritis | Sclerosis of the glomeruli > 90% |  |
Global or segmental subepithelial immune deposits |
Videos
References
References

Videos
{{#ev:youtube|Tw07BFaDEo0}}
References
- ↑ Schwartz N, Goilav B, Putterman C (September 2014). "The pathogenesis, diagnosis and treatment of lupus nephritis". Curr Opin Rheumatol. 26 (5): 502–9. doi:10.1097/BOR.0000000000000089. PMC 4221732. PMID 25014039.
- ↑ He L, Hannon GJ (July 2004). "MicroRNAs: small RNAs with a big role in gene regulation". Nat. Rev. Genet. 5 (7): 522–31. doi:10.1038/nrg1379. PMID 15211354.
- ↑ Yu CC, Yen TS, Lowell CA, DeFranco AL (January 2001). "Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn". Curr. Biol. 11 (1): 34–8. PMID 11166177.
- ↑ Liu Y, Dong J, Mu R, Gao Y, Tan X, Li Y, Li Z, Yang G (June 2013). "MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn". Arthritis Rheum. 65 (6): 1603–11. doi:10.1002/art.37912. PMID 23450709.
- ↑ Munroe ME, James JA (September 2015). "Genetics of Lupus Nephritis: Clinical Implications". Semin. Nephrol. 35 (5): 396–409. doi:10.1016/j.semnephrol.2015.08.002. PMC 4653095. PMID 26573543.
- ↑ Amarilyo G, Lourenço EV, Shi FD, La Cava A (July 2014). "IL-17 promotes murine lupus". J. Immunol. 193 (2): 540–3. doi:10.4049/jimmunol.1400931. PMID 24920843.
- ↑ Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA (August 2008). "Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death". J. Biol. Chem. 283 (31): 21540–9. doi:10.1074/jbc.M800214200. PMC 2490785. PMID 18505729.
- ↑ Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Alarcón GS, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KL, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP (February 2014). "End-stage renal disease in African Americans with lupus nephritis is associated with APOL1". Arthritis Rheumatol. 66 (2): 390–6. doi:10.1002/art.38220. PMC 4002759. PMID 24504811.
- ↑ Taguchi-Atarashi N, Hamasaki M, Matsunaga K, Omori H, Ktistakis NT, Yoshimori T, Noda T (April 2010). "Modulation of local PtdIns3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy". Traffic. 11 (4): 468–78. doi:10.1111/j.1600-0854.2010.01034.x. PMID 20059746.
- ↑ Biesen R, Demir C, Barkhudarova F, Grün JR, Steinbrich-Zöllner M, Backhaus M, Häupl T, Rudwaleit M, Riemekasten G, Radbruch A, Hiepe F, Burmester GR, Grützkau A (April 2008). "Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus". Arthritis Rheum. 58 (4): 1136–45. doi:10.1002/art.23404. PMID 18383365.
- ↑ Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, Chow KM, Wang G, Li PK, Szeto CC (May 2011). "Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis". Nephrology (Carlton). 16 (4): 426–32. doi:10.1111/j.1440-1797.2011.01449.x. PMID 21303425.
- ↑ Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C (February 2006). "Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells". J. Immunol. 176 (3): 1889–98. PMID 16424220.
- ↑ Liu Y, Anders HJ (2014). "Lupus nephritis: from pathogenesis to targets for biologic treatment". Nephron Clin Pract. 128 (3–4): 224–31. doi:10.1159/000368581. PMID 25401461.
- ↑ Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ (July 2011). "Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis". J. Am. Soc. Nephrol. 22 (7): 1262–74. doi:10.1681/ASN.2010090970. PMC 3137574. PMID 21719782.
- ↑ Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ (December 2012). "Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury". J. Pathol. 228 (4): 482–94. doi:10.1002/path.4046. PMID 22553158.
- ↑ Li Y, Fang X, Li QZ (June 2013). "Biomarker profiling for lupus nephritis". Genomics Proteomics Bioinformatics. 11 (3): 158–65. doi:10.1016/j.gpb.2013.05.003. PMC 4357827. PMID 23732627.
- ↑ Mohan C, Putterman C (June 2015). "Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis". Nat Rev Nephrol. 11 (6): 329–41. doi:10.1038/nrneph.2015.33. PMID 25825084.
- ↑ Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK (June 2000). "Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains". Proc. Natl. Acad. Sci. U.S.A. 97 (12): 6670–5. PMC 18697. PMID 10841565.
- ↑ Xie S, Mohan C (February 2004). "Divide and conquer--the power of congenic strains". Clin. Immunol. 110 (2): 109–11. doi:10.1016/j.clim.2003.09.007. PMID 15003808.
- ↑ Henry T, Mohan C (2005). "Systemic lupus erythematosus--recent clues from congenic strains". Arch. Immunol. Ther. Exp. (Warsz.). 53 (3): 207–12. PMID 15995581.
- ↑ Crispín JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, Mayadas TN, Tsokos GC (April 2012). "Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism". J. Immunol. 188 (8): 3567–71. doi:10.4049/jimmunol.1200143. PMC 3324672. PMID 22422882.
- ↑ Jacob CO, Zang S, Li L, Ciobanu V, Quismorio F, Mizutani A, Satoh M, Koss M (August 2003). "Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice". J. Immunol. 171 (3): 1564–71. PMID 12874250.
- ↑ Zhou XJ, Cheng FJ, Qi YY, Zhao MH, Zhang H (2013). "A replication study from Chinese supports association between lupus-risk allele in TNFSF4 and renal disorder". Biomed Res Int. 2013: 597921. doi:10.1155/2013/597921. PMC 3713374. PMID 23936824.
- ↑ Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB (February 2010). "Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages". Proc. Natl. Acad. Sci. U.S.A. 107 (7): 3012–7. doi:10.1073/pnas.0914902107. PMC 2840310. PMID 20133703.
- ↑ Clynes R, Dumitru C, Ravetch JV (February 1998). "Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis". Science. 279 (5353): 1052–4. PMID 9461440.
- ↑ Dang J, Shan S, Li J, Zhao H, Xin Q, Liu Y, Bian X, Liu Q (June 2014). "Gene-gene interactions of IRF5, STAT4, IKZF1 and ETS1 in systemic lupus erythematosus". Tissue Antigens. 83 (6): 401–8. doi:10.1111/tan.12349. PMID 24697319.
- ↑ Zhou TB, Liu YG, Lin N, Qin YH, Huang K, Shao MB, Peng DD (April 2012). "Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic lupus erythematosus/lupus nephritis: a systematic review and metaanalysis". J. Rheumatol. 39 (4): 686–93. doi:10.3899/jrheum.110863. PMID 22337243.
- ↑ Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sánchez E, Kelly JA, Li L, Liu Y, Zhou J, Yan M, Ye Q, Liu S, Xie C, Zhou XJ, Chung SA, Pons-Estel B, Witte T, de Ramón E, Bae SC, Barizzone N, Sebastiani GD, Merrill JT, Gregersen PK, Gilkeson GG, Kimberly RP, Vyse TJ, Kim I, D'Alfonso S, Martin J, Harley JB, Criswell LA, Wakeland EK, Alarcón-Riquelme ME, Mohan C (April 2009). "Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans". J. Clin. Invest. 119 (4): 911–23. doi:10.1172/JCI36728. PMC 2662554. PMID 19307730.
- ↑ Brown EE, Edberg JC, Kimberly RP (December 2007). "Fc receptor genes and the systemic lupus erythematosus diathesis". Autoimmunity. 40 (8): 567–81. doi:10.1080/08916930701763710. PMID 18075791.
- ↑ Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispín JC (September 2013). "Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling". J. Biol. Chem. 288 (37): 26775–84. doi:10.1074/jbc.M113.483743. PMC 3772223. PMID 23918926.
- ↑ Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R (November 2006). "FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis". J. Immunol. 177 (10): 7287–95. PMID 17082647.
- ↑ Zhou XJ, Lv JC, Cheng WR, Yu L, Zhao MH, Zhang H (2010). "Association of TLR9 gene polymorphisms with lupus nephritis in a Chinese Han population". Clin. Exp. Rheumatol. 28 (3): 397–400. PMID 20497632.
- ↑ Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B (October 2011). "Tolerogenic function of Blimp-1 in dendritic cells". J. Exp. Med. 208 (11): 2193–9. doi:10.1084/jem.20110658. PMC 3201204. PMID 21948081.
- ↑ Li Y, Fang X, Li QZ (June 2013). "Biomarker profiling for lupus nephritis". Genomics Proteomics Bioinformatics. 11 (3): 158–65. doi:10.1016/j.gpb.2013.05.003. PMC 4357827. PMID 23732627.
- ↑ Renaudineau Y, Youinou P (2011). "Epigenetics and autoimmunity, with special emphasis on methylation". Keio J Med. 60 (1): 10–6. PMID 21460598.
- ↑ Carlsen AL, Schetter AJ, Nielsen CT, Lood C, Knudsen S, Voss A, Harris CC, Hellmark T, Segelmark M, Jacobsen S, Bengtsson AA, Heegaard NH (May 2013). "Circulating microRNA expression profiles associated with systemic lupus erythematosus". Arthritis Rheum. 65 (5): 1324–34. doi:10.1002/art.37890. PMID 23401079.
- ↑ Sui M, Sui M, Lin Q, Xu Z, Han X, Xie R, Jia X, Guo X, Zhang W, Guan X, Ren H (February 2013). "Simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone antibodies is a marker for more severe lupus nephritis". J. Clin. Immunol. 33 (2): 378–87. doi:10.1007/s10875-012-9825-6. PMID 23100145.