Lisinopril
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2], Amr Marawan, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
USE IN PREGNANCY
See full prescribing information for complete Boxed Warning.
* When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus.
|
Overview
Lisinopril is an Angiotensin converting enzyme inhibitor, Thiazide diuretic that is FDA approved for the {{{indicationType}}} of hypertension, heart failure. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypotension, syncope, hyperkalemia, dizziness, headache, renal function tests abnormal and cough.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- Initial dose (not receiving a diuretic): Lisinopril 10 mg PO qd should be used.
- Initial dose (with receiving a diuretic): Lisinopril 5 mg PO qd should be used.
- Maintenance dose: Lisinopril 20-40 mg PO qd on two divided doses, adjust dose based on response (MAX 80 mg/day)
Heart failure
- Dosing Information: Adjunct
- Initial dose : Lisinopril 5 mg PO qd
- Maintenance dose: Lisinopril 5-20 mg PO qd, titrate no more than 10 mg increments in at least 2 weeks intervals (MAX 40 mg/day)
Acute myocardial infarction
- Dosing Information
- Initial dose : Lisinopril 2.5 - 5 mg PO qd
- Maintenance dose: Lisinopril titrate up to 10 mg PO or higher as tolerated
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
Diabetic nephropathy
- Dosing information
- Recommended dosage: 40 mg PO qd[1]
Diabetic retinopathy
- Dosing information
- Recommended dosage: 10 mg/day[2]
Erythrocytosis
- Dosing information
Hypertension - Transplantation
- Dosing information
- Initial dosage: 10 mg/day
- Maximum dosage: 40 mg/day[5]
Kidney disease, Nondiabetic
- Dosing information
- Monotherapy: 10 mg/day[6]
- Combination Therapy: Adding candesartan to ACE inhibitor therapy produced significant reductions in blood pressure and urinary protein excretion among normotensive patients with chronic renal disease and proteinuria, based on an open-label, controlled, crossover trial (n=60)[7]
Prophylaxis treatment of Migraine
- Dosing information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hypertension
- Dosing Information for children 6 years or older
- Initial dose : Lisinopril 0.07 mg/kg po qd (up to 5 mg total) should be used.
- Maintenance dose: Lisinopril adjust based on response; doses above 0.61 mg/kg/day or 40 mg/day have not been studied.
Off-Label Use and Dosage (Pediatric)
Contraindications
- History of hypersensitivity or angioedema related to previous treatment with an angiotensin converting enzyme inhibitor.
- Patients with hereditary or idiopathic angioedema.
Warnings
|
USE IN PREGNANCY
See full prescribing information for complete Boxed Warning.
* When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus.
|
There is limited information regarding Lisinopril Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- Lisinopril have been found to be generally well tolerated in controlled clinical trials involving 1969 patients with hypertension or heart failure. For the most part, adverse experiences were mild and transient.
Hypertension
- In clinical trials in patients with hypertension treated with lisinopril, discontinuation of therapy due to clinical adverse experiences occurred in 5.7% of patients. The overall frequency of adverse experiences could not be related to total daily dosage within the recommended therapeutic dosage range.
- For adverse experiences occurring in greater than 1% of patients with hypertension treated with lisinopril or lisinopril plus hydrochlorothiazide in controlled clinical trials, and more frequently with lisinopril and/or lisinopril plus hydrochlorothiazide than placebo, comparative incidence data are listed in the table below.
Heart Failure
- In patients with heart failure treated with lisinopril for up to four years, discontinuation of therapy due to clinical adverse experiences occurred in 11.0% of patients. In controlled studies in patients with heart failure, therapy was discontinued in 8.1% of patients treated with lisinopril for 12 weeks, compared to 7.7% of patients treated with placebo for 12 weeks.
- The following table lists those adverse experiences which occurred in greater than 1% of patients with heart failure treated with lisinopril or placebo for up to 12 weeks in controlled clinical trials, and more frequently on lisinopril than placebo.
- Also observed at >1% with lisinopril but more frequent or as frequent on placebo than lisinopril in controlled trials were asthenia, angina pectoris, nausea, dyspnea, cough, and pruritus.
- Worsening of heart failure, anorexia, increased salivation, muscle cramps, back pain, myalgia, depression, chest sound abnormalities, and pulmonary edema were also seen in controlled clinical trials, but were more common on placebo than lisinopril.
Acute Myocardial Infarction
- In the GISSI-3 trial, in patients treated with lisinopril for six weeks following acute myocardial infarction, discontinuation of therapy occurred in 17.6% of patients.
- Patients treated with lisinopril had a significantly higher incidence of hypotension and renal dysfunction compared with patients not taking lisinopril.
- In the GISSI-3 trial, hypotension (9.7%), renal dysfunction (2.0%), cough (0.5%), post infarction angina (0.3%), skin rash and generalized edema (0.01%), and angioedema (0.01%) resulted in withdrawal of treatment. In elderly patients treated with lisinopril, discontinuation due to renal dysfunction was 4.2%.
- Other clinical adverse experiences occurring in 0.3% to 1.0% of patients with hypertension or heart failure treated with lisinopril in controlled clinical trials and rarer, serious, possibly drug-related events reported in uncontrolled studies or marketing experience are listed below, and within each category are in order of decreasing severity.
Body as a Whole
- Anaphylactoid reactions , syncope, orthostatic effects, chest discomfort, pain, pelvic pain, flank pain, edema, facial edema, virus infection, fever, chills, malaise.
Cardiovascular
- Cardiac arrest; myocardial infarction or cerebrovascular accident possibly secondary to excessive hypotension in high risk patients; pulmonary embolism and infarction, arrhythmias (including ventricular tachycardia, atrial tachycardia, atrial fibrillation, bradycardia and premature ventricular contractions), palpitations, transient ischemic attacks, paroxysmal nocturnal dyspnea, orthostatic hypotension, decreased blood pressure, peripheral edema, vasculitis.
Digestive
- Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) , vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth.
Hematologic
- Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia, and thrombocytopenia.
Endocrine
Metabolic
- Weight loss, dehydration, fluid overload, gout, weight gain.
Musculoskeletal
- Arthritis, arthralgia, neck pain, hip pain, low back pain, joint pain, leg pain, knee pain, shoulder pain, arm pain, lumbago.
Nervous System/Psychiatric
- Stroke, ataxia, memory impairment, tremor, peripheral neuropathy (e.g., dysesthesia), spasm, paresthesia, confusion, insomnia, somnolence, hypersomnia, irritability and nervousness.
Respiratory System
- Malignant lung neoplasms, hemoptysis, pulmonary infiltrates, bronchospasm, asthma, pleural effusion, pneumonia, eosinophilic pneumonitis, bronchitis, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngeal pain, pharyngitis, rhinitis, rhinorrhea.
Skin
- Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson syndrome, pemphigus, herpes zoster, erythema multiforme, urticaria, pruritus, alopecia, flushing, diaphoresis, photosensitivity.
Special Senses
- Blurred vision, taste alteration, anosmia, tinnitus, conjunctivitis, dry eyes, tearing.
Urogenital
- Renal failure, oliguria, renal dysfunction (see [[Lisinopril#[[Lisinopril#Warnings|Warnings and Warnings and Precautions]]|[[Lisinopril#Warnings|Warnings and Warnings and Precautions]] and Warnings and Precautions]] and Dosage and Administration, flank pain, gynecomastia, impotence.
Miscellaneous
- A symptom complex has been reported which may include some or all of the following: a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia/myositis, fever, serositis, vasculitis, leukocytosis, eosinophilia, photosensitivity, rash and other dermatologic manifestations.
Angioedema
- Angioedema has been reported in patients receiving enalaprilat, with an incidence higher in black than in non-black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with enalaprilat should be discontinued and appropriate therapy instituted immediately (see [[Lisinopril#[[Lisinopril#Warnings|Warnings and Warnings and Precautions]]|[[Lisinopril#Warnings|Warnings and Warnings and Precautions]] and Warnings and Precautions]]).
Hypotension
- In the hypertensive patients, hypotension occurred in 0.9 percent and syncope occurred in 0.5 percent of patients following the initial dose or during extended therapy. Hypotension or syncope was a cause for discontinuation of therapy in 0.1 percent of hypertensive patients. In heart failure patients, hypotension occurred in 6.7 percent and syncope occurred in 2.2 percent of patients. Hypotension or syncope was a cause for discontinuation of therapy in 1.9 percent of patients with heart failure (see [[Lisinopril#Warnings|Warnings and Warnings and Precautions]]).
Fetal/Neonatal Morbidity and Mortality
- See [[Lisinopril#Warnings|Warnings and Warnings and Precautions]], Fetal/Neonatal Morbidity and Mortality.
Cough
Pediatric Patients
- The adverse experience profile for pediatric patients appears to be similar to that seen in adult patients.
Clinical Laboratory Test Findings
Serum Electrolytes
Creatinine, Blood Urea Nitrogen
- In controlled clinical trials minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 0.2 percent of patients with essential hypertension treated with enalaprilat alone. Increases are more likely to occur in patients receiving concomitant diuretics or in patients with renal artery stenosis (see Warnings and Precautions). In patients with heart failure who were also receiving diuretics with or without digitalis, increases in blood urea nitrogen or serum creatinine, usually reversible upon discontinuation of enalaprilat and/or other concomitant diuretic therapy, were observed in about 11 percent of patients. Increases in blood urea nitrogen or creatinine were a cause for discontinuation in 1.2 percent of patients.
Hematology
- Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.3 g percent and 1.0 vol percent, respectively) occur frequently in either hypertension or congestive heart failure patients treated with enalaprilat but are rarely of clinical importance unless another cause of anemia coexists. In clinical trials, less than 0.1 percent of patients discontinued therapy due to anemia. Hemolytic anemia, including cases of hemolysis in patients with G-6-PD deficiency, has been reported; a causal relationship to enalapril cannot be excluded.
Liver Function Tests
- Elevations of liver enzymes and/or serum bilirubin have occurred (see [[Lisinopril#Warnings|Warnings and Warnings and Precautions]], Hepatic Failure).
Postmarketing Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Drug Interactions
Hypotension - Patient on Diuretic Therapy
- Patients on diuretics and especially those in whom diuretic therapywas recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with lisinopril. The possibility of hypotensive effects with lisinopril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If it is necessary to continue the diuretic, initiate therapy with lisinopril at a dose of 5 mg daily, and provide close medical supervision after the initial dose until blood pressure has stabilized. When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed. Studies with ACE inhibitors in combination with diuretics indicate that the dose of the ACE inhibitor can be reduced when it is given with a diuretic.
Indomethacin
- In a study in 36 patients with mild to moderate hypertension where the antihypertensive effects of lisinopril alone were compared to lisinopril given concomitantly with indomethacin, the use of indomethacin was associated with a reduced effect, although the difference between the two regimens was not significant.
Other Agents
- Lisinopril have been used concomitantly with nitrates and/or digoxin without evidence of clinically significant adverse interactions. This included post myocardial infarction patients who were receiving intravenous or transdermal nitroglycerin. No clinically important pharmacokinetic interactions occurred when lisinopril were used concomitantly with propranolol or hydrochlorothiazide. The presence of food in the stomach does not alter the bioavailability of lisinopril.
Agents Increasing Serum Potassium
- Lisinopril attenuate potassium loss caused by thiazide-type diuretics. Use of lisinopril with potassium-sparing diuretics (e.g., spironolactone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium. Potassium sparing agents should generally not be used in patients with heart failure who are receiving lisinopril.
Lithium
- Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. It is recommended that serum lithium levels be monitored frequently if lisinopril are administered concomitantly with lithium.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Pregnancy Categories C (first trimester) and D (second and third trimesters).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lisinopril in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lisinopril during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Lisinopril in women who are nursing.
Pediatric Use
- The usual recommended starting dose is 0.07 mg/kg once daily (up to 5 mg total). Dosage should be adjusted according to blood pressure response. Doses above 0.61 mg/kg (or in excess of 40 mg) have not been studied in pediatric patients.
- Lisinopril is not recommended in pediatric patients < 6 years or in pediatric patients with glomerular filtration rate < 30 mL/ min/1.73m2.
Geriatic Use
- In general, blood pressure response and adverse experiences were similar in younger and older patients given similar doses of lisinopril tablets. Pharmacokinetic studies, however, indicate that maximum blood levels and area under the plasma concentration time curve (AUC) are doubled in older patients, so that dosage adjustments should be made with particular caution.
Gender
There is no FDA guidance on the use of Lisinopril with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lisinopril with respect to specific racial populations.
Renal Impairment
Dosage Adjustment in Renal Impairment
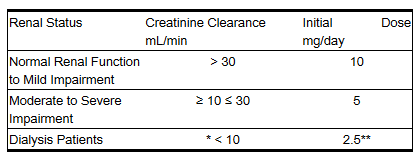
- The usual dose of lisinopril tablets (10 mg) is recommended for patients with creatinine clearance >30 mL/min (serum creatinine of up to approximately 3 mg/dL). For patients with creatinine clearance ≥10 mL/min ≤30 mL/min (serum creatinine ≥3 mg/dL), the first dose is 5 mg once daily. For patients with creatinine clearance <10 mL/min (usually on hemodialysis) the recommended initial dose is 2.5 mg. The dosage may be titrated upward until blood pressure is controlled or to a maximum of 40 mg daily.
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia
- In patients with heart failure who have hyponatremia (serum sodium <130 mEq/L) or moderate to severe renal impairment (creatinine clearance ≤30 mL/min or serum creatinine >3 mg/dL), therapy with lisinopril tablets should be initiated at a dose of 2.5 mg once a day under close medical supervision.
Dosage Adjustment in Patients With Myocardial Infarction with Renal Impairment
- In acute myocardial infarction, treatment with lisinopril tablets should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. No evaluation of dosing adjustments in myocardial infarction patients with severe renal impairment has been performed.
Hepatic Impairment
There is no FDA guidance on the use of Lisinopril in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lisinopril in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lisinopril in patients who are immunocompromised.
Usage in other medical conditions
Heart Failure
- Lisinopril tablets are indicated as adjunctive therapy with diuretics and (usually) digitalis. The recommended starting dose is 5 mg once a day. When initiating treatment with lisinopril in patients with heart failure, the initial dose should be administered under medical observation, especially in those patients with low blood pressure (systolic blood pressure below 100 mmHg). The mean peak blood pressure lowering occurs six to eight hours after dosing. Observation should continue until blood pressure is stable. The concomitant diuretic dose should be reduced, if possible, to help minimize hypovolemia which may contribute to hypotension. The appearance of hypotension after the initial dose of lisinopril tablets does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension. The usual effective dosage range is 5 to 20 mg per day administered as a single daily dose.
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia
- In patients with heart failure who have hyponatremia (serum sodium <130 mEq/L) or moderate to severe renal impairment (creatinine clearance ≤30 mL/min or serum creatinine >3 mg/dL), therapy with lisinopril tablets should be initiated at a dose of 2.5 mg once a day under close medical supervision.
Acute Myocardial Infarction
- In hemodynamically stable patients within 24 hours of the onset of symptoms of acute myocardial infarction, the first dose of lisinopril tablets is 5 mg given orally, followed by 5 mg after 24 hours, 10 mg after 48 hours and then 10 mg of lisinopril tablets once daily. Dosing should continue for six weeks. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin, and beta-blockers. Patients with a low systolic blood pressure (≤120 mmHg) when treatment is started or during the first 3 days after the infarct should be given a lower 2.5 mg oral dose of lisinopril tablets. If hypotension occurs (systolic blood pressure ≤100 mmHg) a daily maintenance dose of 5 mg may be given with temporary reductions to 2.5 mg if needed. If prolonged hypotension occurs (systolic blood pressure ≤90 mmHg for more than 1 hour) lisinopril tablets should be withdrawn. For patients who develop symptoms of heart failure.
Dosage Adjustment in Patients With Myocardial Infarction with Renal Impairment
- In acute myocardial infarction, treatment with lisinopril tablets should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. No evaluation of dosing adjustments in myocardial infarction patients with severe renal impairment has been performed.
Administration and Monitoring
Administration
There is limited information regarding Lisinopril Administration in the drug label.
Monitoring
There is limited information regarding Lisinopril Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Lisinopril and IV administrations.
Overdosage
There is limited information regarding Lisinopril overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Lisinopril Pharmacology in the drug label.
Mechanism of Action
- Lisinopril inhibits angiotensin converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex.
- The beneficial effects of isinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with lisinopril alone for up to 24 weeks, the mean increase in serum potassium was approximately 0.1 mEq/L; however, approximately 15% of patients had increases greater than 0.5 mEq/L and approximately 6% had a decrease greater than 0.5 mEq/L. In the same study, patients treated with lisinopril and hydrochlorothiazide for up to 24 weeks had a mean decrease in serum potassium of 0.1 mEq/L; approximately 4% of patients had increases greater than 0.5 mEq/L and approximately 12% had a decrease greater than 0.5 mEq/L. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity. ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of lisinopril remains to be elucidated. While the mechanism through which lisinopril lower blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, lisinopril are antihypertensive even in patients with low-renin hypertension.
- Although lisinopril were antihypertensive in all races studied, black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to monotherapy than nonblack patients.
- Concomitant administration of lisinopril and hydrochlorothiazide further reduced blood pressure in black and non-black patients and any racial differences in blood pressure response were no longer evident.
Structure
There is limited information regarding Lisinopril Structure in the drug label.
Pharmacodynamics
- Administration of lisinopril to patients with hypertension results in a reduction of both supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is usually not observed although it can occur and should be anticipated in volume and/or salt-depleted patients. When given together with thiazide-type diuretics, the blood pressure lowering effects of the two drugs are approximately additive.
- In most patients studied, onset of antihypertensive activity was seen at one hour after oral administration of an individual dose of lisinopril, with peak reduction of blood pressure achieved by 6 hours. Although an antihypertensive effect was observed 24 hours after dosing with recommended single daily doses, the effect was more consistent and the mean effect was considerably larger in some studies with doses of 20 mg or more than with lower doses. However, at all doses studied, the mean antihypertensive effect was substantially smaller 24 hours after dosing than it was 6 hours after dosing.
- In some patients achievement of optimal blood pressure reduction may require two to four weeks of therapy. The antihypertensive effects of lisinopril are maintained during long term therapy. Abrupt withdrawal of lisinopril has not been associated with a rapid increase in blood pressure, or a significant increase in blood pressure compared to pretreatment levels.
- Two dose-response studies utilizing a once daily regimen were conducted in 438 mild to moderate hypertensive patients not on a diuretic. Blood pressure was measured 24 hours after dosing. An antihypertensive effect of lisinopril was seen with 5 mg in some patients. However, in both studies blood pressure reduction occurred sooner and was greater in patients treated with 10, 20 or 80 mg of lisinopril. In controlled clinical studies, lisinopril 20-80 mg have been compared in patients with mild to moderate hypertension to hydrochlorothiazide 12.5-50 mg and with atenolol 50-200 mg; and in patients with moderate to severe hypertension to metoprolol 100-200 mg. It was superior to hydrochlorothiazide in effects on systolic and diastolic pressure in a population that was 3/4 caucasian. Lisinopril were approximately equivalent to atenolol and metoprolol in effects on diastolic blood pressure, and had somewhat greater effects on systolic blood pressure.
- Lisinopril had similar effectiveness and adverse effects in younger and older (>65 years) patients. They were less effective in blacks than in caucasians.
- In hemodynamic studies in patients with essential hypertension, blood pressure reduction was accompanied by a reduction in peripheral arterial resistance with little or no change in cardiac output and in heart rate. In a study in nine hypertensive patients, following administration of lisinopril, there was an increase in mean renal blood flow that was not significant. Data from several small studies are inconsistent with respect to the effect of lisinopril on glomerular filtration rate in hypertensive patients with normal renal function, but suggest that changes, if any, are not large.
- In patients with renovascular hypertension lisinopril have been shown to be well tolerated and effective in controlling blood pressure.
Pharmacokinetics
- Following oral administration of lisinopril, peak serum concentrations of lisinopril occur within about 7 hours, although there was a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients. Declining serum concentrations exhibit a prolonged terminal phase which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose. Lisinopril does not appear to be bound to other serum proteins. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25%, with large intersubject variability (6%-60%) at all doses tested (5-80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract. \
- The absolute bioavailability of lisinopril is reduced to 16% in patients with stable NYHA Class II-IV congestive heart failure, and the volume of distribution appears to be slightly smaller than that in normal subjects. The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers. Upon multiple dosing, lisinopril exhibits an effective half-life of accumulation of 12 hours.
- Impaired renal function decreases elimination of lisinopril, which is excreted principally through the kidneys, but this decrease becomes clinically important only when the glomerular filtration rate is below 30 mL/min. Above this glomerular filtration rate, the elimination half-life is little changed. With greater impairment, however, peak and trough lisinopril levels increase, time to peak concentration increases and time to attain steady state is prolonged. Older patients, on average, have (approximately doubled) higher blood levels and the area under the plasma concentration time curve (AUC) than younger patients. Lisinopril can be removed by hemodialysis.
- Studies in rats indicate that lisinopril crosses the blood-brain barrier poorly. Multiple doses of lisinopril in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C lisinopril. By whole body autoradiography, radioactivity was found in the placenta following administration of labeled drug to pregnant rats, but none was found in the fetuses.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 mg/kg/day (about 56 or 9 times* the maximum recommended daily human dose,
based on body weight and body surface area, respectively).
- There was no evidence of carcinogenicity when lisinopril was administered for 92 weeks to (male and female) mice at doses up to 135 mg/kg/day (about 84 times* the maximum recommended daily human dose). This dose was 6.8 times the maximum human dose based on body surface area in mice. *Calculations assume a human weight of 50 kg and human body surface area of 1.62 m2. Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
Clinical Studies
Acute Myocardial Infarction
- GISSI-3 trial
- In the GISSI-3 trial, in patients treated with lisinopril for six weeks following acute myocardial infarction, discontinuation of therapy occurred in 17.6% of patients.Patients treated with lisinopril had a significantly higher incidence of hypotension and renal dysfunction compared with patients not taking lisinopril.
- In the GISSI-3 trial, hypotension (9.7%), renal dysfunction (2.0%), cough (0.5%), post infarction angina (0.3%), skin rash and generalized edema (0.01%), and angioedema (0.01%) resulted in withdrawal of treatment. In elderly patients treated with lisinopril, discontinuation due to renal dysfunction was 4.2%. Other clinical adverse experiences occurring in 0.3% to 1.0% of patients with hypertension or heart failure treated with lisinopril in controlled clinical trials and rarer, serious, possibly drug-related events reported in uncontrolled studies or marketing experience are listed below, and within each category are in order of decreasing severity
How Supplied
2.5 mg Tablets
2.5 mg Tablets (NDC 0310-0135) white, round, biconvex, uncoated tablets identified as “ZESTRIL 2 1/2” on one side and “135” on the other side are supplied in bottles of 100 tablets.
5 mg Tablets
5 mg Tablets (NDC 0310-0130) pink, capsule-shaped, biconvex, bisected, uncoated tablets, identified “ZESTRIL” on one side and “130” on the other side are supplied in bottles of 100 tablets and unit dose packages of 100 tablets.
10 mg Tablets
10 mg Tablets (NDC 0310-0131) pink, round, biconvex, uncoated tablets identified “ZESTRIL 10” debossed on one side, and “131” debossed on the other side are supplied in bottles of 100 tablets and unit dose packages of 100 tablets.
20 mg Tablets
20 mg Tablets (NDC 0310-0132) red, round, biconvex, uncoated tablets identified “ZESTRIL 20” debossed on one side, and “132” debossed on the other side are supplied in bottles of 100 tablets and unit dose packages of 100 tablets.
30 mg Tablets
30 mg Tablets (NDC 0310-0133) red, round, biconvex, uncoated tablets identified “ZESTRIL 30” debossed on one side, and “133” debossed on the other side are supplied in bottles of 100 tablets.
40 mg Tablets
40 mg Tablets (NDC 0310-0134) yellow, round, biconvex, uncoated tablets identified “ZESTRIL 40” debossed on one side, and “134” debossed on the other side are supplied in bottles of 100 tablets.
Storage
There is limited information regarding Lisinopril Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Lisinopril |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lisinopril |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Lisinopril Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Lisinopril tablet interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Lisinopril Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Lisinopril Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Schjoedt KJ, Astrup AS, Persson F, Frandsen E, Boomsma F, Rossing K et al. (2009) Optimal dose of lisinopril for renoprotection in type 1 diabetic patients with diabetic nephropathy: a randomised crossover trial. Diabetologia 52 (1):46-9. DOI:10.1007/s00125-008-1184-8 PMID: 18974967
- ↑ Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A et al. (1998) Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 351 (9095):28-31. PMID: 9433426
- ↑ Midtvedt K, Stokke ES, Hartmann A (1996) Successful long-term treatment of post-transplant erythrocytosis with losartan. Nephrol Dial Transplant 11 (12):2495-7. PMID: 9017632
- ↑ Pisani F, Tisone G, Alciati E, Vennarecci G, Pieragostini E, Casciani CU (1994) Role of ACE inhibitors in the treatment of erythrocytosis in patients with renal allograft. Transplant Proc 26 (5):2602-3. PMID: 7940809
- ↑ Brozena SC, Johnson MR, Ventura H, Hobbs R, Miller L, Olivari MT et al. (1996) Effectiveness and safety of diltiazem or lisinopril in treatment of hypertension after heart transplantation. Results of a prospective, randomized multicenter trail. J Am Coll Cardiol 27 (7):1707-12. PMID: 8636558
- ↑ Shiigai T, Shichiri M (2001) Late escape from the antiproteinuric effect of ace inhibitors in nondiabetic renal disease. Am J Kidney Dis 37 (3):477-83. PMID: 11228170
- ↑ Kincaid-Smith P, Fairley K, Packham D (2002) Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant 17 (4):597-601. PMID: 11917051
- ↑ Schrader H, Stovner LJ, Helde G, Sand T, Bovim G (2001) Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ 322 (7277):19-22. PMID: 11141144
- ↑ Bender WI (1995) ACE inhibitors for prophylaxis of migraine headaches. Headache 35 (8):470-1. PMID: 7591740
{{#subobject:
|Page Name=Lisinopril |Pill Name=Lisinopril 2.5 MG Oral Tablet |Drug Name=Lisinopril |Pill Ingred=starch, corn / calcium phosphate, dibasic, dihydrate / magnesium stearate / mannitol / talc|+sep=; |Pill Imprint=4209;V |Pill Dosage=2.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=7.00 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=0603-4209-21
}}
{{#subobject:
|Page Name=Lisinopril
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_03.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_04.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_02.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_01.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_label_03.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_label_02.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_label_01.jpg
}}