Hydromorphone (oral): Difference between revisions
No edit summary |
Kiran Singh (talk | contribs) m (Kiran Singh moved page Hydromorphone to Hydromorphone (oral) without leaving a redirect) |
||
| (6 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{chetan}} | |authorTag={{chetan}} | ||
|genericName=Hydromorphone | |genericName=Hydromorphone | ||
|aOrAn=an | |aOrAn=an | ||
|drugClass=analgesic opioid | |drugClass=analgesic opioid | ||
| Line 7: | Line 7: | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[dermatologic]]: [[flushing]] (extended-release, less than 2% ), [[pruritus]] (extended-release, 1% to 8% ), [[sweating]],[[gastrointestinal]]: [[constipation]] (extended-release, 7% to 31% ), [[nausea]] (extended-release, 9% to 28% .), [[vomiting]] (extended-release, 6% to 14% ), [[neurologic]]: [[asthenia]] (1% to 11% ), [[dizziness]] (1% to 11% ), [[headache]] (1% to 12% ), [[somnolence]] (less than 2% ) | |adverseReactions=[[dermatologic]]: [[flushing]] (extended-release, less than 2% ), [[pruritus]] (extended-release, 1% to 8% ), [[sweating]],[[gastrointestinal]]: [[constipation]] (extended-release, 7% to 31% ), [[nausea]] (extended-release, 9% to 28% .), [[vomiting]] (extended-release, 6% to 14% ), [[neurologic]]: [[asthenia]] (1% to 11% ), [[dizziness]] (1% to 11% ), [[headache]] (1% to 12% ), [[somnolence]] (less than 2% ) | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;"> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">Warning: addiction, abuse, and misuse; life-threatening respiratory depression; accidental ingestion; neonatal opioid withdrawal syndrome; and interaction with alcohol</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> | ||
* hydromorphone exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk before prescribing, and monitor regularly for development of these behaviors or conditions. | |||
* Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. Instruct patients to swallow hydromorphone capsules whole to avoid exposure to a potentially fatal dose of hydromorphone. | |||
* Accidental ingestion of hydromorphone, especially in children, can result in fatal overdose of hydromorphone. | |||
* Prolonged use of hydromorphone during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. | |||
* Instruct patients not to consume alcohol or any products containing alcohol while taking hydromorphone because co-ingestion can result in fatal plasma hydromorphone levels. | |||
|fdaLIADAdult=* Rectal suppository formulation has not been found by the US Food and Drug Administration (FDA) to be safe and effective, and the drug product labeling has not been approved. | |||
* High-potency (HP) and extended-release formulations (Dilaudid-HP(R) Injection, Exalgo(R) ER Tablets, Palladone(R) ER Capsules) are used only in opioid-tolerant patients, defined as those using at least 60 mg of oral morphine daily, 25 mcg transdermal fentanyl/hr, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day or an equianalgesic dose of another opioid, for a week or longer. | |||
* Confusion between the different concentrations of hydromorphone and hydromorphone HP may result in an accidental overdose and death; when prescribing, provide both the total dose in mg and the total volume in mL. | |||
* Avoid medication errors, be aware that hydromorphone 8 mg tablets are available as both immediate-release and extended-release. | |||
* Pain, When opioid analgesics are appropriate: 1 to 2 mg IM/subQ every 2 to 3 hours as needed, may reduce in opioid-naive. | |||
* Pain, When opioid analgesics are appropriate: 0.2 to 1 mg IV over at least 2 to 3 minutes every 2 to 3 hours as needed. | |||
* Pain, When opioid analgesics are appropriate: patient-controlled analgesia (opioid-naive patients), concentration 0.2 mg/mL; usual initial dose is 0.2 mg (range, 0.05 to 0.4 mg) with lockout period of 6 minutes (range, 5 to 10 min). | |||
* Pain, When opioid analgesics are appropriate: (immediate-release oral liquid) 2.5 to 10 mg (2.5 to 10 mL) orally every 3 to 6 hr as needed; higher doses may be required in some cases. | |||
* Pain, When opioid analgesics are appropriate: (immediate-release oral tablet) 2 to 4 mg orally every 4 to 6 hours as needed; gradually increase until adequate analgesia. | |||
* Pain, When opioid analgesics are appropriate: (rectal suppository) 3 mg rectallyevery 6 to 8 hours [9] OR 3 to 6 mg rectallyevery 3 to 4 hours. | |||
* Pain (Moderate to Severe), Opioid-tolerant: do not use hydromorphone HP if the amount of hydromorphone required cannot be delivered accurately with this formulation; never administer hydromorphone HP injection to opioid-naïve patients; use hydromorphone HP only if higher concentration and lower total volume are required. | |||
* Pain (Moderate to Severe), Opioid-tolerant: conversion from hydromorphone to hydromorphone HP: initiate with an equipotent dose based on previous hydromorphone requirement and administer IV/IM/subQ in divided doses; titrate gradually based on response and tolerability [6] | |||
* Pain (Moderate to Severe), Opioid-tolerant: conversion from other opioids: initiate with one-half of the estimated daily hydromorphone dose based on previous 24-hour analgesic requirement; administer IV/IM/subQ in divided doses; may titrate dose gradually based on response and tolerability. | |||
* Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: individualize dose; after all other extended-release or around-the-clock opioids have been discontinued, initial dose selection must take into account patient's prior analgesic treatment experience and risk factors for addiction, abuse, and misuse; due to substantial inter-patient variability in relative potency of different opioid products, when converting it is recommended to underestimate a patient's 24-hour oral hydromorphone requirements and provide rescue mediation as needed. | |||
* Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Exalgo(R)) conversion from oral immediate-release (IR) hydromorphone: initiate dose equal to total daily hydromorphone requirement orally once daily; may titrate by increments of 4 to 8 mg every 3 to 4 days as needed. | |||
* Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Exalgo) conversion from other oral opioids: initiate with one-half of the estimated daily hydromorphone dose orally once daily using the following conversion factors to Exalgo(R): hydromorphone (1), codeine (0.06), hydrocodone (0.4), methadone (0.6), morphine (0.2), oxycodone (0.4), oxymorphone (0.6)); provide rescue medication as needed and titrate by increments of 4 to 8 mg every 3 to 4 days as needed. | |||
* Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Palladone(R)) conversion from other oral opioids: initiate with one-half of the estimated daily hydromorphone dose orally once daily using the following conversion factors to Palladone(R): hydromorphone (1), codeine (0.04), hydrocodone (0.22), methadone (0.38), morphine (0.12), oxycodone (0.25); provide rescue medication as needed and titrate by increments of 4 to 8 mg every 3 to 4 days as needed. | |||
* Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Exalgo(R) or Palladone(R)) conversion from transdermal fentanyl: starting 18 hours after the removal of the fentanyl patch, initiate with one-half of the 24-hour hydromorphone dose orally once daily, using a conversion factor of 25 mcg/hr fentanyl transdermal patch to 12 mg of hydromorphone; provide rescue medication as needed and titrate by increments of 4 to 8 mg every 3 to 4 days as needed | |||
|offLabelAdultGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of hydromorphone in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of hydromorphone in adult patients. | |||
|fdaLIADPed=* Safety and efficacy not established in pediatric patients. | |||
* Pain, When opioid analgesics are appropriate: adolescents, 1 to 4 mg orally every 4 to 6 hr as needed has been used. | |||
* Pain, When opioid analgesics are appropriate: children, 0.05 to 0.1 mg/kg/dose orally every 6 hr; MAX 5 mg/dose. | |||
* Pain, When opioid analgesics are appropriate: adolescents, initial (opiate-naive patients) 0.2 to 0.6 mg IV (slow) every 2 to 3 hours as needed; usual, 1 to 2 mg/dose IM/IV/subQ every 4 to 6 hours. | |||
* Pain, When opioid analgesics are appropriate: children, 0.015 mg/kg/dose IV every 4 to 6 hours. | |||
|offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of hydromorphone in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of hydromorphone in pediatric patients. | |||
|contraindications=* hydromorphone is contraindicated in: | |||
:* Opioid non-tolerant patients. Fatal respiratory depression could occur in patients who are not opioid tolerant. | |||
:* Patients with significant respiratory depression | |||
:* Patients with acute or severe [[bronchial asthma]] in an unmonitored setting or in the absence of resuscitative equipment. | |||
:* Patients with known or suspected [[paralytic ileus]]. | |||
:* Patients who have had surgical procedures and/or underlying disease resulting in narrowing of the gastrointestinal tract, or have “blind loops” of the gastrointestinal tract or gastrointestinal obstruction. | |||
:* Patients with hypersensitivity (e.g., [[anaphylaxis]]) to [[hydromorphone]] or sulfite-containing medications [see Warnings and Precautions]. | |||
|warnings=* Addiction, Abuse, and Misuse | |||
:* hydromorphone contains hydromorphone, a Schedule II controlled substance. As an opioid, hydromorphone exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence]. As modified-release products such as hydromorphone deliver the opioid over an extended period of time, there is a greater risk for overdose and death due to the larger amount of hydromorphone present. | |||
:* Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed hydromorphone and in those who obtain the drug illicitly. Addiction can occur at recommended doses and if the drug is misused or abused. | |||
:* Assess each patient’s risk for opioid addiction, abuse, or misuse prior to prescribing hydromorphone, and monitor all patients receiving hydromorphone for the development of these behaviors or conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of hydromorphone for the proper management of pain in any given patient. Patients at increased risk may be prescribed modified-release opioid formulations such as hydromorphone, but use in such patients necessitates intensive counseling about the risks and proper use of hydromorphone along with intensive monitoring for signs of addiction, abuse, and misuse. | |||
:* Abuse or misuse of hydromorphone by crushing, chewing, snorting, or injecting the dissolved product will result in the uncontrolled delivery of hydromorphone and can result in overdose and death [see Overdosage]. | |||
:* Opioid agonists such as hydromorphone are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. | |||
:* Consider these risks when prescribing or dispensing hydromorphone. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on the proper disposal of unused drug [see Patient Counseling Information]. | |||
:* Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product. | |||
* Life-threatening Respiratory Depression | |||
:* Serious, life-threatening, or fatal respiratory depression has been reported with the use of modified-release opioids, even when used as recommended. Respiratory depression from opioid use, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see Overdosage]. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids. | |||
:* While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of hydromorphone, the risk is greatest during the initiation of therapy or following a dose increase. Closely monitor patients for respiratory depression when initiating therapy with hydromorphone and following dose increases. | |||
:* To reduce the risk of respiratory depression, proper dosing and titration of hydromorphone are essential [see Dosage and Administration]. | |||
:* Overestimating the hydromorphone dose when converting patients from another opioid product can result in fatal overdose with the first dose. | |||
:* Accidental ingestion of even one dose of hydromorphone, especially by children, can result in respiratory depression and death due to an overdose of hydromorphone. | |||
* Neonatal Opioid Withdrawal Syndrome | |||
:* Prolonged use of hydromorphone during pregnancy can result in withdrawal signs in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. | |||
:* Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn. | |||
* Interactions with Central Nervous System Depressants | |||
:* [[Hypotension]], profound sedation, [[coma]], [[respiratory depression]], and death may result if hydromorphone is used concomitantly with alcohol or other central nervous system (CNS) depressants (e.g., [[sedatives]], [[anxiolytics]], [[hypnotics]], [[neuroleptics]], other opioids). | |||
:* When considering the use of hydromorphone in a patient taking a CNS depressant, assess the duration use of the CNS depressant and the patient’s response, including the degree of tolerance that has developed to CNS depression. Additionally, evaluate the patient’s use of alcohol or illicit drugs that cause CNS depression. If the decision to begin hydromorphone is made, start with 1/3 to 1/2 the calculated starting dose of hydromorphone, monitor patients for signs of sedation and respiratory depression, and consider using a lower dose of the concomitant CNS depressant [see Drug Interactions]. | |||
* Use in Ederly, Cachectic, and Debilitated Patients | |||
:* Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients. Monitor such patients closely, particularly when initiating and titrating hydromorphone and when hydromorphone is given concomitantly with other drugs that depress respiration [see Warnings and Precautions]. | |||
* Use in Patients with Chronic Pulmonary Disease | |||
:* Monitor patients with significant [[chronic obstructive pulmonary disease]] or [[cor pulmonale]], and patients having a substantially decreased respiratory reserve, [[hypoxia]], [[hypercapnia]], or pre-existing respiratory depression for respiratory depression, particularly when initiating therapy and titrating with hydromorphone, as in these patients, even usual therapeutic doses of hydromorphone may decrease respiratory drive to the point of apnea [see Warnings and Precautions]. Consider the use of alternative non-opioid analgesics in these patients if possible. | |||
* Hypotensive Effect | |||
:* hydromorphone may cause severe hypotension including [[orthostatic hypotension]] and [[syncope]] in ambulatory patients. There is an increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g. [[phenothiazines]] or [[general anesthetics]]) [see Drug Interactions]. Monitor these patients for signs of hypotension after initiating or titrating the dose of hydromorphone. | |||
* Use in Patients with Head Injury or Increased Intracranial Pressure | |||
: Monitor patients taking hydromorphone who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors) for signs of sedation and respiratory depression, particularly when initiating therapy with hydromorphone. hydromorphone may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of hydromorphone in patients with impaired consciousness or coma. | |||
* Use in Patients with Gastrointestinal Conditions | |||
:* hydromorphone is contraindicated in patients with paralytic ileus. Avoid the use of hydromorphone in patients with other GI obstruction. | |||
:* Because the hydromorphone capsule is nondeformable and does not appreciably change in shape in the GI tract, hydromorphone is contraindicated in patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic, for example: [[esophageal motility]] disorders, small bowel inflammatory disease, “[[short gut]]” syndrome due to adhesions or decreased transit time, past history of [[peritonitis]], [[cystic fibrosis]], chronic [[intestinal pseudoobstruction]], or [[Meckel’s diverticulum]]). There have been reports of obstructive symptoms in patients with known strictures or risk of strictures, such as previous GI surgery, in association with the ingestion of drugs in nondeformable extended-release formulations. | |||
:* It is possible that hydromorphone capsules may be visible on abdominal x-rays under certain circumstances, especially when digital enhancing techniques are utilized. | |||
:* The hydromorphone in hydromorphone may cause spasm of the sphincter of Oddi. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms. | |||
* Sulfites | |||
:* hydromorphone contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people. | |||
* Use in Patients with Convulsive or Seizure Disorders | |||
:* The hydromorphone in hydromorphone may aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings. Monitor patients with a history of seizure disorders for worsened seizure control during hydromorphone therapy. | |||
* Avoidance of Withdrawal | |||
:* Avoid the use of mixed agonist/antagonist (i.e., [[pentazocine]], [[nalbuphine]], and [[butorphanol]]) or partial agonists (buprenorphine) analgesics in patients who have received or are receiving a course of therapy with a full opioid agonist analgesic, including hydromorphone . In these patients, mixed agonists/antagonist and partial agonist analgesics may reduce the analgesic effect and/or may precipitate withdrawal symptoms [see Drug Interactions]. | |||
:* When discontinuing hydromorphone , gradually taper the dose [see Dosage and Administration (2.3)]. Do not abruptly discontinue hydromorphone . | |||
* Driving and Operating Machinery | |||
:* hydromorphone may impair the mental and/or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of hydromorphone and know how they will react to the medication. | |||
|clinicalTrials=* The following serious adverse reactions are discussed elsewhere in the labeling: | |||
:* Addiction, Abuse, and Misuse [see Warnings and Precautions] | |||
:* Life Threatening Respiratory Depression [see Warnings and Precautions] | |||
:* Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions] | |||
:* Interactions with Other CNS Depressants [see Warnings and Precautions] | |||
:* Hypotensive Effect [see Warnings and Precautions] | |||
:* Gastrointestinal Effects [see Warnings and Precautions] | |||
:* Seizures [see Warnings and Precautions] | |||
* Clinical Trial Experience | |||
:* The safety of hydromorphone was evaluated in double-blind clinical trials involving 612 patients with moderate to severe pain. An open-label extension study involving 143 patients with cancer pain was conducted to evaluate the safety of hydromorphone when used for longer periods of time in higher doses than in the controlled trials. Patients were treated with doses averaging 40 to 50 mg of hydromorphone per day (ranging between 12 and 500 mg/day) for several months (range 1 to 52 weeks). | |||
:* Serious adverse reactions which may be associated with hydromorphone therapy in clinical use are similar to those of other opioid analgesics, including respiratory depression, apnea, respiratory arrest, and to a lesser degree, circulatory depression, hypotension, shock or cardiac arrest [see OVERDOSAGE]. | |||
* Adverse Events Reported in Controlled Trials | |||
:* Table 2 lists treatment emergent signs and symptoms that were reported in at least 2% of patients in the placebo-controlled trials for which the rate of occurrence was greater for those treated with hydromorphone 12 mg capsules than those treated with placebo. | |||
Adverse Events Reported in Controlled Trials | |||
Table 2 lists treatment emergent signs and symptoms that were reported in at least 2% of patients in the placebo-controlled trials for which the rate of occurrence was greater for those treated with | |||
[[File:Hydromoprhone adverse 1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:Hydromoprhone adverse 1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
:* Average exposure was 21 days for hydromorphone and 15 days for placebo. | |||
Musculoskeletal: | * Adverse Events Observed in Clinical Trials | ||
:* hydromorphone has been administered to 785 individuals during completed clinical trials. The conditions and duration of exposure to hydromorphone varied greatly, and included open-label and double-blind studies, uncontrolled and controlled studies, inpatient and outpatient studies, fixed dose and titration studies. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. | |||
:* These categories are used in the listing below. The frequencies represent the proportion of 785 patients from these trials who experienced that event while receiving hydromorphone . All adverse events included in this tabulation occurred in at least one patient. Events are classified by body system and listed using the following definitions: frequent adverse events - those occurring in at least 1/100 patients; adverse events occurring with an incidence less than 1% are considered infrequent. These adverse events are not necessarily related to hydromorphone treatment and in most cases were observed at a similar frequency in placebo-treated patients in the controlled studies. | |||
* Frequent Adverse Events | |||
:* Body as a Whole: [[headache]], [[]asthenia]], pain, [[abdominal pain]], fever, chest pain, infection, chills, malaise, neck pain, carcinoma, accidental injury | |||
:* [[Cardiovascular System]]: [[vasodilatation]], [[tachycardia]], [[migraine]] | |||
:* [[Digestive System]]: nausea, constipation, vomiting, diarrhea, dyspepsia, anorexia, dry mouth, nausea and vomiting, dysphagia, flatulence | |||
:* Hemic and Lymphatic System: [[anemia]], [[leukopenia]] | |||
:* Metabolic and Nutritional Disorders: peripheral edema, dehydration, edema, generalized edema, hypokalemia, weight loss | |||
:* Musculoskeletal: arthralgia, bone pain, leg cramps, [[myalgia]] | |||
:* Nervous System: somnolence, dizziness, nervousness, confusion, insomnia, anxiety, depression, hypertonia, hypesthesia, paresthesia, tremor, thinking abnormal, hallucinations, speech disorder, agitation, amnesia, tinnitus, abnormal gait | |||
:* Respiratory System: dyspnea, cough increased, [[rhinitis]], [[pharyngitis]], [[pneumonia]], [[epistaxis]], [[hiccup]], hypoxia, pleural effusion | |||
:* Skin and Appendages: [[pruritus]], sweating, rash | |||
:* Special Senses: [[amblyopia]], taste perversion | |||
:* [[Urogenital System]]: [[dysuria]], [[urinary incontinence]] | |||
Nervous System: abnormal dreams, emotional lability, paranoid reaction, sleep disorder euphoria, incoordination, stupor, ataxia, convulsion, hallucination, hostility, myoclonus, psychosis, vertigo, withdrawal syndrome, apathy, delirium, dementia, drug dependence, nystagmus, twitching, depersonalization, aphasia, cerebrovascular accident, circumoral parasthesia, seizure, hyperkinesia, hypotonia, increased salivation, neuralgia | * Infrequent Adverse Events | ||
:* Body as a Whole: face edema, [[ascites]], allergic reaction, [[cellulitis]], overdose, [[hypothermia]], [[neoplasm]], [[photosensitivity]] reaction, sepsis, flank pain | |||
:* [[Cardiovascular System]]: [[hypertension]], [[hypotension]], [[syncope]], [[deep thrombophlebitis]], [[arrhythmia]], postural hypotension, [[atrial fibrillation]], [[pallor]], [[bradycardia]], electrocardiogram abnormal, [[myocardial infarction]], [[palpitation]], [[angina pectoris]], [[congestive heart failure]], [[QT interval prolonged]], [[supraventricular tachycardia]], [[thrombosis]], [[cardiomegaly]], [[hemorrhage]] | |||
:* Digestive System: fecal impaction, [[intestinal obstruction]], abnormal stools, [[fecal incontinence]], [[hepatic failure]], increased appetite, [[cholangitis]], [[cholecystitis]], [[colitis]], [[enterocolitis]], [[hepatomegaly]], jaundice, liver function tests abnormal, [[biliary spasm]], [[ileus]], [[eructation]], [[rectal hemorrhage]], [[esophagitis]], [[glossitis]], [[melena]], [[mouth ulceration]], [[gastrointestinal hemorrhage]], [[tongue edema]] | |||
:* [[Endocrine]]: [[adrenal cortex insufficiency]] | |||
:* Hemic and Lymphatic System: [[ecchymosis]], [[thrombocytopenia]], [[leukocytosis]], [[lymphadenopathy]], [[agranulocytosis]], lymphoma like reaction, [[pancytopenia]], [[petechia]] | |||
:* Metabolic and Nutritional Disorders: [[hyperglycemia]], [[hyponatremia]], cachexia, [[hypercalcemia]], [[hypomagnesemia]], [[cyanosis]], [[diabetes mellitus]], [[gout]], [[respiratory acidosis]], elevated liver enzymes, thirst | |||
:* [[Musculoskeletal]]: [[myasthenia]] | |||
:* [[Nervous System]]: abnormal dreams, [[emotional lability]], [[paranoid reaction]], sleep disorder [[euphoria]], [[incoordination]], [[stupor]], [[ataxia]], [[convulsion]], [[hallucination]], [[hostility]], [[myoclonus]], [[psychosis]], [[vertigo]], withdrawal syndrome, apathy, [[delirium]], [[dementia]], [[drug dependence]], [[nystagmus]], [[twitching]], depersonalization, [[aphasia]], cerebrovascular accident, circumoral parasthesia, [[seizure]], [[hyperkinesia]], [[hypotonia]], increased salivation, [[neuralgia]] | |||
:* Respiratory System: [[hypoventilation]], apnea, [[atelectasis]], [[hemoptysis]], asthma, hyperventilation, [[pulmonary embolus]], laryngismus | |||
:* Skin and Appendages: urticaria, maculopapular rash, alopecia | |||
:* Special Senses: abnormal vision, diplopia, dry eyes, lacrimation disorder, hyperacusis | |||
:* Urogenital: urinary retention, hematuria, impotence, urinary frequency, urination impaired, dysmenorrhea, creatinine increased, urinary urgency | |||
* Additional Adverse Events From Non-U.S. Experience | |||
:* Addiction, blurred vision, drowsiness, dysphoria, sedation, seizure, physical dependence, biliary spasm, and ileus | |||
|drugInteractions=* Alcohol | |||
:* Concomitant use of alcohol with hydromorphone can result in an increase of hydromorphone plasma levels and potentially fatal overdose of hydromorphone. Instruct patients not to consume alcoholic beverages or use prescription or non-prescription products containing alcohol while on hydromorphone therapy [see Clinical Pharmacology]. | |||
* CNS Depressants | |||
:* The concomitant use of hydromorphone with other CNS depressants including sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and alcohol can increase the risk of respiratory depression, profound sedation, coma and death. Monitor patients receiving CNS depressants and hydromorphone for signs of respiratory depression, sedation and hypotension. | |||
:* When combined therapy with any of the above medications is considered, the dose of one or both agents should be reduced [see Dosage and Administration and Warnings and Precautions]. | |||
* Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics | |||
:* Mixed agonist/antagonist (pentazocine, nalbuphine, and butorphanol) and partial agonist (buprenorphine) analgesics may reduce the analgesic effect of hydromorphone and/or may precipitate withdrawal symptoms in these patients. Avoid the use of mixed agonist/antagonist analgesics in patients receiving hydromorphone . | |||
* Monoamine Oxidase Inhibitors (MAOIs) | |||
:* The effects of opioid analgesics may be potentiated by MAOIs. hydromorphone is not recommended for use in patients who have received MAOIs within 14 days. If concurrent therapy with an MAOI and hydromorphone is unavoidable, monitor patients for increased respiratory and central nervous system depression. | |||
The effects of opioid analgesics may be potentiated by MAOIs. | |||
* Anticholinergics | |||
:* Anticholinergics or other medications with anticholinergic activity when used concurrently with hydromorphone may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. Monitor patients for signs of urinary retention or reduced gastric motility when hydromorphone is used concurrently with anticholinergic drugs. | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA= | |useInPregnancyFDA=* Fetal/neonatal adverse reactions | ||
:* Prolonged use of opioid analgesics during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth. Observe newborns for symptoms of neonatal opioid withdrawal syndrome, such as poor feeding, diarrhea, irritability, tremor, rigidity, and seizures, and manage accordingly [see Warnings and Precautions (5.3)]. | |||
* Teratogenic Effects - Pregnancy Category C | |||
:* There are no adequate and well-controlled studies in pregnant women. hydromorphone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
:* hydromorphone was not teratogenic in female rats given oral doses up to 10 mg/kg or female rabbits given oral doses up to 50 mg/kg during the major period of organ development. Estimated exposures in the female rat and rabbit were approximately 3-fold and 6-fold higher than a 32 mg human daily oral dose based on exposure (AUC0-24h). | |||
:* hydromorphone administration to pregnant Syrian hamsters and CF-1 mice during major organ development revealed teratogenicity likely the result of maternal toxicity associated with sedation and hypoxia. In Syrian hamsters given single subcutaneous doses from 14 to 278 mg/kg during organogenesis (gestation days 8 to10), doses ≥ 19 mg/kg hydromorphone produced skull malformations (exencephaly and cranioschisis). | |||
:* Continuous infusion of hydromorphone (5 mg/kg, s.c.) via implanted osmotic mini pumps during organogenesis (gestation days 7 to10) produced soft tissue malformations (cryptorchidism, cleft palate, malformed ventricals and retina), and skeletal variations (supraoccipital, checkerboard and split sternebrae, delayed ossification of the paws and ectopic ossification sites). The malformations and variations observed in the hamsters and mice were at doses approximately 3-fold higher and <1-fold lower, respectively, than a 32 mg human daily oral dose on a body surface area basis. | |||
* Nonteratogenic Effect | |||
:* In a rat pre- and post-natal study, an increase in pup mortality and a decrease in pup body weight which was associated with maternal toxicity was observed at doses of 2 and 5 mg/kg/day. The maternal no effect level for hydromorphone was 0.5 mg/kg/day which is <1-fold lower than a 32 mg human daily oral dose on a body surface area. hydromorphone had no effect on pup development or reproduction when given to female rats during the pre-natal and postnatal periods up to a dose of 5 mg/kg which is equivalent to a 32 mg human daily oral dose on a body surface area basis. | |||
:* Neonates born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Approaches to the treatment of the syndrome have included supportive care and, if indicated, drugs such as paregoric or phenobarbital. | |||
|useInLaborDelivery=* hydromorphone is not for use in women during and immediately prior to labor, where shorter acting analgesics or other analgesic techniques are more appropriate [see Indications and Usage (1)]. Occasionally, opioid analgesics may prolong labor by temporarily reducing the strength, duration, and frequency of uterine contractions. However, these effects are not consistent and may be offset by an increased rate of cervical dilatation which tends to shorten labor. | |||
* Opioids cross the placenta and may produce respiratory depression and psychophysiologic effects in neonates. Closely observe neonates whose mothers received opioid analgesics during labor for signs of respiratory depression. An opioid antagonist, such as naloxone, should be available for reversal of opioid-induced respiratory depression in the neonate in such situations. | |||
|useInNursing=* Low concentrations of hydromorphone have been detected in human milk in clinical trials. Withdrawal symptoms can occur in breastfeeding infants when maternal administration of an opioid analgesic is stopped. Nursing should not be undertaken while a patient is receiving hydromorphone since hydromorphone is excreted in the milk. | |||
|useInPed=* The safety and effectiveness of hydromorphone in pediatric patients below the age of 18 have not been established. | |||
|useInGeri=* Elderly patients have been shown to be more sensitive to the adverse effects of opioids compared to the younger population. Of the total number of subjects in clinical studies of hydromorphone , 22% were 65 and over, and 6% were 75 and over. Dosages should be adjusted according to the clinical situation. As with all opioids, the starting dose should be reduced to 1/3 to 1/2 of the usual dosage in debilitated patients. Respiratory depression is the chief hazard in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration. Therefore, closely monitor elderly patients for respiratory and central nervous system depression when prescribing hydromorphone , particularly during initiation and titration. | |||
|useInRenalImpair=* In patients with mild to moderate renal impairment, based on calculated creatinine clearance, the concentrations of hydromorphone in plasma were slightly higher than in subjects with normal renal function. | |||
|useInHepaticImpair=* hydromorphone was not studied in patients with severe hepatic impairment and are not recommended for use in such patients. Care in initial dose selection and careful observation are recommended in patients with evidence of mild to moderate hepatic impairment. | |||
|overdose=* Clinical Presentation | |||
:* Acute overdosage with opioids can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes pulmonary edema, bradycardia, hypotension and death. Marked mydriasis rather than miosis may be seen due to severe hypoxia in overdose situations. | |||
* Treatment of Overdose | |||
:* In case of overdose, priorities are the re-establishment of a patent and protected airway and institution of assisted or controlled ventilation if needed. Employ other supportive measures (including oxygen, vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life support techniques. | |||
:* The opioid antagonists, such as naloxone and naltrexone, are specific antidotes to respiratory depression resulting from opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to hydromorphone overdose. Such agents should be administered cautiously to patients who are known, or suspected to be, physically dependent on hydromorphone . In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute withdrawal syndrome. | |||
:* Because the duration of reversal would be expected to be less than the duration of action of hydromorphone in hydromorphone , carefully monitor the patient until spontaneous respiration is reliably reestablished. hydromorphone will continue to release hydromorphone adding to the hydromorphone load for up to 24 hours after administration, necessitating prolonged monitoring for at least 24 to 48 hours beyond the overdose. If the response to opioid antagonists is suboptimal or not sustained, additional antagonist should be given as directed in the product’s prescribing information. | |||
:* In an individual physically dependent on opioids, administration of an opioid receptor antagonist may precipitate an acute withdrawal. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist. | |||
Treatment of Overdose | |||
In case of overdose, priorities are the re-establishment of a patent and protected airway and institution of assisted or controlled ventilation if needed. Employ other supportive measures (including oxygen, vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life support techniques. | |||
The opioid antagonists, such as naloxone and naltrexone, are specific antidotes to respiratory depression resulting from opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to hydromorphone overdose. Such agents should be administered cautiously to patients who are known, or suspected to be, physically dependent on | |||
Because the duration of reversal would be expected to be less than the duration of action of hydromorphone in | |||
In an individual physically dependent on opioids, administration of an opioid receptor antagonist may precipitate an acute withdrawal. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist. | |||
|drugBox={{drugbox2 | |drugBox={{drugbox2 | ||
| verifiedrevid = 464191262 | | verifiedrevid = 464191262 | ||
| Line 290: | Line 208: | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| brand name = Dilaudid | | brand name = Dilaudid | ||
| Drugs.com = {{drugs.com|monograph| | | Drugs.com = {{drugs.com|monograph|Hydromorphone-hydrochloride}} | ||
| MedlinePlus = a682013 | | MedlinePlus = a682013 | ||
| pregnancy_AU = C | | pregnancy_AU = C | ||
| Line 300: | Line 218: | ||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = Oral: 30–35%, Intranasal: 52–58%<ref name="pmid12818953">{{cite journal | author = Coda BA, Rudy AC, Archer SM, Wermeling DP | title = Pharmacokinetics and bioavailability of single-dose intranasal | | bioavailability = Oral: 30–35%, Intranasal: 52–58%<ref name="pmid12818953">{{cite journal | author = Coda BA, Rudy AC, Archer SM, Wermeling DP | title = Pharmacokinetics and bioavailability of single-dose intranasal Hydromorphone hydrochloride in healthy volunteers | journal = Anesth. Analg. | volume = 97 | issue = 1 | pages = 117–23, table of contents |date=July 2003 | pmid = 12818953 | doi = 10.1213/01.ANE.0000066311.40978.4F| url = }}</ref> | ||
| protein_bound = 20% | | protein_bound = 20% | ||
| metabolism = Hepatic | | metabolism = Hepatic | ||
| elimination_half-life = 2–3 hours<ref name="pmid6165742">{{cite journal | author = Vallner JJ, Stewart JT, Kotzan JA, Kirsten EB, Honigberg IL | title = Pharmacokinetics and bioavailability of | | elimination_half-life = 2–3 hours<ref name="pmid6165742">{{cite journal | author = Vallner JJ, Stewart JT, Kotzan JA, Kirsten EB, Honigberg IL | title = Pharmacokinetics and bioavailability of Hydromorphone following intravenous and oral administration to human subjects | journal = J Clin Pharmacol | volume = 21 | issue = 4 | pages = 152–6 |date=April 1981 | pmid = 6165742 | doi = 10.1002/j.1552-4604.1981.tb05693.x| url = }}</ref> | ||
| excretion = Renal | | excretion = Renal | ||
| Line 312: | Line 230: | ||
| ATC_prefix = N02 | | ATC_prefix = N02 | ||
| ATC_suffix = AA03 | | ATC_suffix = AA03 | ||
| ATC_supplemental = | | ATC_supplemental = | ||
| PubChem = 5284570 | | PubChem = 5284570 | ||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| Line 328: | Line 246: | ||
<!--Chemical data--> | <!--Chemical data--> | ||
| C=17 | H=19 | N=1 | O=3 | | C=17 | H=19 | N=1 | O=3 | ||
| molecular_weight = 285.3g/mol | | molecular_weight = 285.3g/mol | ||
| smiles = O=C4[C@@H]5Oc1c2c(ccc1O)C[C@H]3N(CC[C@]25[C@H]3CC4)C | | smiles = O=C4[C@@H]5Oc1c2c(ccc1O)C[C@H]3N(CC[C@]25[C@H]3CC4)C | ||
| Line 339: | Line 257: | ||
| solubility = HCl: 333 | | solubility = HCl: 333 | ||
}} | }} | ||
|mechAction=Hydromorphone, a semi-synthetic morphine derivative, is a hydrogenated ketone of morphine. Hydromorphone is principally an agonist of mu-receptors, showing a weak affinity for к-receptors. Comparing relative binding affinity for mu- and к-opioid receptors, | |mechAction=* Hydromorphone, a semi-synthetic morphine derivative, is a hydrogenated ketone of morphine. Hydromorphone is principally an agonist of mu-receptors, showing a weak affinity for к-receptors. Comparing relative binding affinity for mu- and к-opioid receptors, Hydromorphone binds more specifically to mu- receptors than structurally related morphine. As an opioid agonist, the principle therapeutic action of Hydromorphone is analgesia. The precise mechanism of action of opioid analgesics is not known but the effects are thought to be mediated through opioid-specific receptors located predominantly in the central nervous system (CNS). Interaction with the mu-opioid receptor subtype is believed to be responsible for most of Hydromorphone’s clinical effects. There is no intrinsic limit to the analgesic effect of Hydromorphone. Clinically, however, dosage limitations are imposed by the adverse effects, primarily respiratory depression, sedation, nausea, and vomiting, which can result from high doses. | ||
|structure= | |structure=* Hydromorphone extended-release capsules are for oral use and contain Hydromorphone hydrochloride, a mu-opioid. | ||
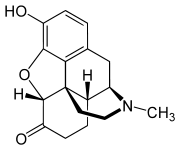
* Hydromorphone hydrochloride USP is 4,5α-epoxy-3-hydroxy-17-methlymorphinan-6-one hydrochloride. Hydromorphone hydrochloride is a white or almost white crystalline powder that is freely soluble in water, very slightly soluble in ethanol (96%), and practically insoluble in methylene chloride. Its empirical formula is C17H19NO3•HCl. The compound has the following structural formula: | |||
Hydromorphone hydrochloride USP is 4,5α-epoxy-3-hydroxy-17-methlymorphinan-6-one hydrochloride. Hydromorphone hydrochloride is a white or almost white crystalline powder that is freely soluble in water, very slightly soluble in ethanol (96%), and practically insoluble in methylene chloride. Its empirical formula is C17H19NO3•HCl. The compound has the following structural formula: | |||
[[File:Ydromorphone structure.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:Ydromorphone structure.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
| Line 348: | Line 265: | ||
(C17H19NO3•HCl) MW 321.80 | (C17H19NO3•HCl) MW 321.80 | ||
* Hydromorphone also contains the following inactive ingredients: ammonio methacrylate copolymer type B, ethylcellulose, and stearyl alcohol, synthetic black iron oxide, gelatin, red iron oxide (12 mg & 16 mg only), FD&C Blue No. 2 (24 mg only), and titanium dioxide. | |||
|PD=* CNS Depressant/Alcohol Interaction | |||
:* Additive pharmacodynamic effects may be expected when Hydromorphone is used in conjunction with alcohol, other opioids, legal or illicit drugs that cause central nervous system depression. | |||
* Effects on the Central Nervous System | |||
:* Hydromorphone produces dose-related respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation. | |||
:* Hydromorphone depresses the cough reflex by direct effect on the cough center in the medulla. | |||
:* Hydromorphone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomic. Marked mydriasis, rather than miosis, may be seen due to severe hypoxia in overdose situations. | |||
* Effects on the Gastrointestinal Tract and Other Smooth Muscle | |||
:* Gastric, biliary and pancreatic secretions are decreased by Hydromorphone. Hydromorphone causes a reduction in motility associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm. The end result is constipation. Hydromorphone also can cause an increase in biliary tract pressure as a result of spasm of the sphincter of Oddi. | |||
* Effects on the Cardiovascular System | |||
:* Hydromorphone produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Release of histamine may be induced by Hydromorphone and can contribute to opioid-induced hypotension. Manifestations of histamine release or peripheral vasodilation may include pruritus, flushing, red eyes, and sweating. | |||
* Effects on the Endocrine System | |||
:* Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon. | |||
Effects on the | * Effects on the Immune System | ||
:* Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive. | |||
|PK=* Absorption | |||
Hydromorphone | :* Hydromorphone is an extended-release formulation of Hydromorphone. Administration of a single Hydromorphone dose is characterized by biphasic absorption, a relatively rapid rise to an initial peak concentration, followed by a second broader peak with therapeutic plasma concentrations maintained over the 24-hour dosing interval. The absolute bioavailability of Hydromorphone from Hydromorphone has not been determined. Under conditions of multiple dosing, the bioavailability of a once-daily dose of Hydromorphone is equivalent to the same total daily dose of immediate-release Hydromorphone given in divided doses every 6 hours. Dose proportionality has been established in terms of Cmax and AUC for the 12 mg and 24 mg dosage strengths. Dosage form proportionality on a dose-adjusted basis has been demonstrated for three 12 mg capsules to one 32 mg capsule. | ||
:* In a study comparing 12 mg Hydromorphone dosed every 24 hours to 3 mg of immediate-release Hydromorphone dosed every 6 hours in healthy human subjects, the two treatments were found to be equivalent in terms of extent of absorption (AUC) (see Figure 1). The extended-release characteristics of Hydromorphone resulted in lower steady-state peak levels (Cmax), higher trough levels (Cmin), and an approximately twofold to threefold reduction in the fluctuation seen with the immediate-release Hydromorphone tablets. | |||
In a study comparing 12 mg | |||
[[File:Hydromorphone pharmacokinetics 1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:Hydromorphone pharmacokinetics 1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
Steady-state plasma concentrations with | :* Steady-state plasma concentrations with Hydromorphone were achieved within 2 to 3 days after initiation of dosing. This is consistent with the mean apparent terminal elimination half-life for Hydromorphone of approximately 18.6 hours. Hydromorphone did not accumulate significantly after multiple dosing with once-daily administration. | ||
:* Food had no significant effect on the peak (Cmax), AUC or the elimination of Hydromorphone from Hydromorphone (see Figure 2). | |||
Food had no significant effect on the peak (Cmax), AUC or the elimination of | |||
[[File:Hydromorphone pharmacokinetics 2.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:Hydromorphone pharmacokinetics 2.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
* Food Effect | |||
:* The pharmacokinetics of Hydromorphone is not affected by food as indicated by bioequivalence when administered under fed and fasting conditions. Therefore, Hydromorphone may be administered without regard to meals. | |||
The pharmacokinetics of | |||
Following intravenous administration of | * Distribution | ||
:* Following intravenous administration of Hydromorphone, the reported volume of distribution is 295 L (4 L/kg). Hydromorphone is approximately 20% bound to human plasma proteins. | |||
Metabolism | * Metabolism | ||
:* Hydromorphone is metabolized by direct conjugation, or by 6-keto reduction followed by conjugation. Following absorption, Hydromorphone is metabolized to the major metabolites Hydromorphone-3-glucuronide, Hydromorphone-3-glucoside and dihydroisomorphine-6-glucuronide. Also observed were the less prevalent metabolites, dihydroisomorphine-6-glucoside, dihydromorphine and dihydroisomorphine. | |||
:* Hydromorphone metabolites have been found in plasma, urine and in human hepatocyte test systems. However, it is not known whether Hydromorphone is metabolized by the cytochrome P450 enzyme system. Hydromorphone is a poor inhibitor of human recombinant CYP isoforms including CYP1A2, 2A6, 2C8, 2D6, and 3A4 with an IC50 > 50 µM. Therefore, Hydromorphone is not expected to inhibit the metabolism of other drugs metabolized by these CYP isoforms. | |||
* Specific Populations | |||
:* Geriatric Patients | |||
::* Age-related increases in exposure in clinical studies were observed between geriatric and younger adult subjects. Greater sensitivity of some older individuals cannot be excluded. Dosages should be adjusted according to the clinical situation. | |||
Hydromorphone | :* Pediatric Patients | ||
::* The safety and effectiveness of Hydromorphone have not been established in patients below the age of 18. | |||
* Gender | |||
:* Pharmacokinetics of Hydromorphone from Hydromorphone is comparable in men and women. | |||
* Race | |||
:* The pharmacokinetics of Hydromorphone in African Americans and Caucasians in the clinical population were comparable. | |||
* Hepatic Impairment | |||
:* Hydromorphone was not studied in patients with severe hepatic insufficiency and is not recommended for use in such patients. Start patients with moderate hepatic impairment on 25% of the usual dose of Hydromorphone and closely monitor for respiratory and central nervous system depression during dose titration. Consider alternate analgesic therapy for patients with severe hepatic impairment [see Dosage and Administration and Specific Populations]. | |||
* Renal Impairment | |||
:* In patients with mild renal impairment, based on calculated creatinine clearance, the concentrations of Hydromorphone in plasma were slightly higher than in subjects with normal renal function. Start patients with moderate renal impairment on 50% of the usual Hydromorphone dose for patients with normal renal function and closely monitor for respiratory and central nervous system depression during dose titration. As Hydromorphone is only intended for once-daily administration, consider use of an alternate analgesic that may permit more flexibility with the dosing interval in patients with severe renal impairment [see Dosage and Administration and Use in Specific Populations]. | |||
* Drug Interaction/Alcohol Interaction | |||
:* A pharmacokinetic study in healthy subjects showed that co-ingestion of a 12 mg Hydromorphone capsule with 240 mL (8 ounces) of 40% (80 proof) alcohol resulted in an average peak Hydromorphone concentration approximately six times greater than when taken with water. One subject in this study experienced a 16-fold increase when the drug was ingested with 40% alcohol compared with water. In certain subjects, 8 ounces of 4% alcohol (equivalent to 2/3 of a typical serving of beer) resulted in almost twice the peak plasma Hydromorphone concentration than when the drug was ingested with water. | |||
:* This pharmacokinetic study was an open-label, four-arm, crossover design study and included twenty-four healthy adult subjects who were tested under fasted conditions and 24 healthy adult subjects who were tested under standardized fed conditions. Subjects were pretreated with naltrexone to block the opiate effects, and then administered one of the following four treatments: | |||
* Group A Hydromorphone , 12 mg + 240 mL of 40% ethanol | |||
* Group B Hydromorphone , 12 mg + 240 mL of 20% ethanol | |||
* Group C Hydromorphone , 12 mg + 240 mL of 4% ethanol | |||
* Group D Hydromorphone , 12 mg + 240 mL of water | |||
:* Plasma was sampled and analyzed for Hydromorphone concentration at appropriate intervals. Each subject received each of the four treatments, thereby acting as his or her own control (Group D). | |||
:* The effects of alcohol co-ingestion were more marked in the fasted state and are summarized below. | |||
The effects of alcohol co-ingestion were more marked in the fasted state and are summarized below. | |||
[[File:Hydromorphone pharmacokinetics 3.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:Hydromorphone pharmacokinetics 3.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
* Ratio of values when co-ingested with 240 mL of 40% ethanol compared to co-ingestion with 240 mL of water, i.e. if the peak plasma concentration was 6 ng/mL when administered with alcohol and 1 ng/mL when administered with water, this ratio would be 6 | |||
* Ratio of values (as above) when co-ingested with 240 mL of 20% ethanol compared to co-ingestion with 240 mL of water | |||
†Ratio of values (as above) when co-ingested with 240 mL of 4% ethanol compared to co-ingestion with 240 mL of water | * †Ratio of values (as above) when co-ingested with 240 mL of 4% ethanol compared to co-ingestion with 240 mL of water | ||
‡Peak plasma concentration | * ‡Peak plasma concentration | ||
* Measure of total drug exposure | |||
In the fed state, the mean peak plasma concentration ratio (40% alcohol:water) was 3.5 with a maximum of 6. | * In the fed state, the mean peak plasma concentration ratio (40% alcohol:water) was 3.5 with a maximum of 6. | ||
* In summary, the study showed that ingesting Hydromorphone with alcohol in clinically relevant amounts results in significantly higher peak plasma concentrations of Hydromorphone. The effect is more pronounced with increasing concentrations of alcohol and in a fasted state. | |||
* The effects of co-ingestion of smaller volumes and with other concentrations of alcohol have not been studied. | |||
|nonClinToxic=* Carcinogenesis, Mutagenesis, Impairment of Fertility | |||
:* Carcinogenesis: No carcinogenicity studies have been conducted in animals. | |||
:* Hydromorphone was negative in the in vitro bacterial reverse mutation assay and in the in vivo mouse micronucleus assay. Hydromorphone was negative in the mouse lymphoma assay in the absence of metabolic activation, but was positive in the mouse lymphoma assay in the presence of metabolic activation. Morphinone, an impurity, tested as a besylate salt was negative in the in vitro bacterial reverse mutation assay and negative in the in vivo mouse micronucleus assay. Morphinone was positive in the Chinese Hamster Ovary Cell Chromosomal Aberration test in the absence and presence of metabolic activation. | |||
:* Hydromorphone did not affect fertility in rats at oral doses up to 5 mg/kg which is equivalent to a 32 mg human daily oral dose on a body surface area basis. | |||
* Mutagenesis | |||
:* Hydromorphone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay). Hydromorphone was not clastogenic in either the in vitro human lymphocyte chromosome aberration assay or the in vivo mouse micronucleus assay. | |||
:* Impairment of Fertility: Hydromorphone given orally to rats during the mating period caused a slight but statistically significant reduction in implantations at 6.25 mg/kg/day (~1.2 times the human exposure following to 32 mg/day). | |||
|clinicalStudies=* The efficacy of Hydromorphone was established in a double-blind, randomized, parallel group, multicenter, placebo-controlled, four-week trial of patients with pain that was present for at least one month. The majority of these patients experienced moderate to severe pain due to musculoskeletal disorders while maintained on one or more opioid analgesics, often in addition to non-opioid analgesics. * Two hundred twenty-one patients with chronic moderate to severe pain were randomized to receive once daily 12 mg Hydromorphone capsule or placebo after they had demonstrated that they needed approximately 12 mg of immediate-release Hydromorphone (in addition to non-opioid medication) around-the-clock to improve their pain control. Patients randomized to Hydromorphone maintained adequate analgesia for a significantly longer period of time (P<0.0001) than patients randomized to placebo. | |||
|howSupplied=* 2 mg: cinnamon-colored capsules imprinted with “P-XL” on the cap and “12 mg” on the body and available as follows: | |||
* 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-008-06 | |||
* 20 capsules, Unit Dose (2 X 10) ......…………………………. NDC 42858-008-11 | |||
* 16 mg: pink-colored capsules imprinted with “P-XL” on the cap and “16 mg” on the body and available as follows: | |||
* 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-027-06 | |||
* 20 capsules, Unit Dose (2 X 10) ......…………………………. NDC 42858-027-11 | |||
* 24 mg: blue-colored capsules imprinted with “P-XL” on the cap and “24 mg” on the body and available as follows: | |||
* 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-039-06 | |||
* 20 capsules, Unit Dose (2 X 10) ......…………………………. NDC 42858-039-11 | |||
* 32 mg: white capsules imprinted with “P-XL” on the cap and “32 mg” on the body and available as follows: | |||
* 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-204-06 | |||
* 20 capsules, Unit Dose (2 X 10) ......……………………….… NDC 42858-204-11 | |||
|storage=* Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. | |||
* Avoid temperatures above 40°C (104°F) [See USP Excessive Heat] | |||
* Dispense in a tight, light-resistant container. | |||
|fdaPatientInfo=* Advise the patient to read the FDA-approved patient labeling (Medication Guide). | |||
* Addiction, Abuse, and Misuse | |||
:* Inform patients that the use of Hydromorphone , even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose or death [see Warnings and Precautions]. Instruct patients not to share Hydromorphone with others and to take steps to protect Hydromorphone from theft or misuse. | |||
* Life-threatening Respiratory Depression | |||
:* Inform patients of the risk of life-threatening of respiratory depression, including information that the risk is greatest when starting Hydromorphone or when the dose is increased, and that it can occur even at recommended doses [see Warnings and Precautions (5.2)]. Advise patients how to recognize respiratory depression and to seek medical attention if breathing difficulties develop. | |||
* Accidental Ingestion | |||
:* Inform patients that accidental ingestion, especially in children, may result in respiratory depression or death [see Warnings and Precautions]. Instruct patients to take steps to store Hydromorphone securely and to dispose of unused Hydromorphone by flushing the capsules down the toilet. | |||
Hydromorphone | * Neonatal Opioid Withdrawal Syndrome | ||
:* Inform female patients of reproductive potential that prolonged use of Hydromorphone during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated [see Warnings and Precautions]. | |||
Hydromorphone | * Interactions with Alcohol and other CNS Depressants | ||
:* Inform patients that potentially serious additive effects may occur if Hydromorphone is used with alcohol or other CNS depressants, and not to use such drugs unless supervised by a health care provider. | |||
* Important Administration Instructions | |||
:* Instruct patients how to properly take Hydromorphone , including the following: | |||
::* Swallowing Hydromorphone whole | |||
::* Not crushing, chewing, splitting or dissolving the capsule | |||
::* Using Hydromorphone exactly as prescribed to reduce the risk of life-threatening adverse reactions (e.g., respiratory depression) | |||
::* Not discontinuing Hydromorphone without first discussing the need for a tapering regimen with the prescriber | |||
* Gastrointestinal Blockage | |||
:* Advise patients that people with certain stomach or intestinal problems such as narrowing of the intestines or previous surgery may be at higher risk of developing a blockage. Symptoms include abdominal distension, abdominal pain, severe constipation, or vomiting. Instruct patients to contact their healthcare provider immediately if they develop these symptoms. | |||
* Hypotension | |||
:* Inform patients that Hydromorphone may cause orthostatic hypotension and syncope. Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position). | |||
* Driving or Operating Heavy Machinery | |||
:* Inform patients that Hydromorphone may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how they will react to the medication. | |||
* Constipation | |||
:* Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention. | |||
* Anaphylaxis | |||
:* Inform patients that anaphylaxis has been reported with ingredients contained in Hydromorphone . Advise patients how to recognize such a reaction and when to seek medical attention. | |||
* Pregnancy | |||
:* Advise female patients that Hydromorphone can cause fetal harm and to inform the prescriber if they are pregnant or plan to become pregnant. | |||
* Disposal | |||
:* Advise patients to flush the unused capsules down the toilet when Hydromorphone is no longer needed. | |||
Advise patients to flush the unused capsules down the toilet when | |||
Marketed by: | Marketed by: | ||
Rhodes Pharmaceuticals L.P. | Rhodes Pharmaceuticals L.P. | ||
Coventry, RI 02816 | Coventry, RI 02816 | ||
Manufactured by: | Manufactured by: | ||
The PF Laboratories Inc. | The PF Laboratories Inc. | ||
Totowa, New Jersey 07512 | Totowa, New Jersey 07512 | ||
U.S. Patent Numbers 5,958,452; 5,965,161; 5,968,551, 6,294,195, 6,335,033; 6,706,281 | U.S. Patent Numbers 5,958,452; 5,965,161; 5,968,551, 6,294,195, 6,335,033; 6,706,281 | ||
303260-0A | 303260-0A | ||
Revision: April 2014 | Revision: April 2014 | ||
Medication Guide | Medication Guide | ||
PALLADONE (pal-a-d-own) | PALLADONE (pal-a-d-own) | ||
(Hydromorphone hydrochloride) extended-release capsules, CII | |||
( | ====PALLADONE is:==== | ||
* A strong prescription pain medicine that contains an opioid (narcotic) that is used to manage pain severe enough to require daily around-the-clock, long-term treatment with an opioid, in people who are already regularly using opioid pain medicine, when other pain treatments such as non-opioid pain medicines or immediate-release opioid medicines do not treat your pain well enough or you cannot tolerate them. | |||
* A long-acting (extended-release) opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death. | |||
* Not for use to treat pain that is not around-the-clock. | |||
PALLADONE is | ====Important information about PALLADONE:==== | ||
* Get emergency help right away if you take too much PALLADONE (overdose). When you first start taking PALLADONE, when your dose is changed, or if you take too much (overdose), serious or life threatening breathing problems that can lead to death may occur. | |||
* Never give anyone your PALLADONE. They could die from taking it. Store PALLADONE away from children and in a safe place to prevent stealing or abuse. Selling or giving away PALLADONE is against the law. | |||
====Do not take PALLADONE if you have:==== | |||
* Severe asthma, trouble breathing, or other lung problems. | |||
* A bowel blockage or have narrowing of the stomach or intestines. | |||
====Before taking PALLADONE, tell your healthcare provider if you have a history of:==== | |||
* Head injury, seizures | |||
* Liver, kidney, thyroid problems | |||
* Allergy to sulfites | |||
* Problems urinating | |||
* Pancreas or gallbladder problems | |||
* Abuse of street or prescription drugs, alcohol addiction, or mental health problems | |||
* Tell your healthcare provider if you are: | |||
:* Pregnant or planning to become pregnant. Prolonged use of PALLADONE during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated. | |||
:* Breastfeeding. PALLADONE passes into breast milk and may harm your baby. | |||
:* Taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking PALLADONE with certain other medicines can cause serious side effects. | |||
Do not take PALLADONE if you | * When taking PALLADONE: | ||
:* Do not change your dose. Take PALLADONE exactly as prescribed by your healthcare provider. | |||
:* Take your prescribed dose every 24 hours, at the same time every day. Do not take more than your prescribed dose in 24 hours. If you miss a dose, take your next dose at your usual time the next day. | |||
:* Swallow PALLADONE whole. Do not cut, break, chew, crush, dissolve, snort, or inject PALLADONE because this may cause you to overdose and die. | |||
::* Call your healthcare provider if the dose you are taking does not control your pain. | |||
::* Do not stop taking PALLADONE without talking to your healthcare provider. | |||
::* After you stop taking PALLADONE, flush any unused tablets down the toilet. | |||
* While taking PALLADONE DO NOT: | |||
:* Drive or operate heavy machinery until you know how PALLADONE affects you. PALLADONE can make you sleepy, dizzy, or lightheaded. | |||
:* Drink alcohol or use prescription or over-the-counter medicines containing alcohol. Using products containing alcohol during treatment with PALLADONE may cause you to overdose and die. | |||
* The possible side effects of PALLADONE are: | |||
:* Constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain. Call your healthcare provider if you have any of these symptoms and they are severe. | |||
* Get emergency medical help if you have: | |||
:* Trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue or throat, extreme drowsiness, light-headedness when changing positions, or you are feeling faint. | |||
* These are not all the possible side effects of PALLADONE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov | |||
These are not all the possible side effects of PALLADONE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov | |||
* Manufactured for: Rhodes Pharmaceuticals L.P., or call 1-888-827-0616 | |||
This Medication Guide has been approved by the U.S. Food and Drug Administration. | This Medication Guide has been approved by the U.S. Food and Drug Administration. | ||
Revised: April 2014 | Revised: April 2014 | ||
|alcohol=Alcohol-Hydromorphone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Hydromorphone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
{{PillImage | {{PillImage | ||
|fileName= | |fileName=No_image.jpg | ||
|drugName= Hydromorphone | |drugName=Hydromorphone | ||
|NDC=42858-039-06 | |NDC=42858-039-06 | ||
|drugAuthor= Purdue Pharma L.P. | |drugAuthor=Purdue Pharma L.P. | ||
|ingredients=Ammonio methacrylate copolymer type b, ethylcellulose, steryl alcohol, synthetic black iron oxide, titanium dioxide | |ingredients=Ammonio methacrylate copolymer type b, ethylcellulose, steryl alcohol, synthetic black iron oxide, titanium dioxide | ||
|pillImprint=P-XL;32;mg | |pillImprint=P-XL;32;mg | ||
|dosageValue=32 | |dosageValue=32 | ||
|dosageUnit=mg | |dosageUnit=mg | ||
|pillColor=White | |pillColor=White | ||
Latest revision as of 19:14, 19 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: addiction, abuse, and misuse; life-threatening respiratory depression; accidental ingestion; neonatal opioid withdrawal syndrome; and interaction with alcohol
See full prescribing information for complete Boxed Warning.
|
Overview
Hydromorphone (oral) is an analgesic opioid that is FDA approved for the {{{indicationType}}} of pain, when opioid analgesics are appropriate, pain (moderate to severe), opioid-tolerant, pain (severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include dermatologic: flushing (extended-release, less than 2% ), pruritus (extended-release, 1% to 8% ), sweating,gastrointestinal: constipation (extended-release, 7% to 31% ), nausea (extended-release, 9% to 28% .), vomiting (extended-release, 6% to 14% ), neurologic: asthenia (1% to 11% ), dizziness (1% to 11% ), headache (1% to 12% ), somnolence (less than 2% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Rectal suppository formulation has not been found by the US Food and Drug Administration (FDA) to be safe and effective, and the drug product labeling has not been approved.
- High-potency (HP) and extended-release formulations (Dilaudid-HP(R) Injection, Exalgo(R) ER Tablets, Palladone(R) ER Capsules) are used only in opioid-tolerant patients, defined as those using at least 60 mg of oral morphine daily, 25 mcg transdermal fentanyl/hr, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day or an equianalgesic dose of another opioid, for a week or longer.
- Confusion between the different concentrations of hydromorphone and hydromorphone HP may result in an accidental overdose and death; when prescribing, provide both the total dose in mg and the total volume in mL.
- Avoid medication errors, be aware that hydromorphone 8 mg tablets are available as both immediate-release and extended-release.
- Pain, When opioid analgesics are appropriate: 1 to 2 mg IM/subQ every 2 to 3 hours as needed, may reduce in opioid-naive.
- Pain, When opioid analgesics are appropriate: 0.2 to 1 mg IV over at least 2 to 3 minutes every 2 to 3 hours as needed.
- Pain, When opioid analgesics are appropriate: patient-controlled analgesia (opioid-naive patients), concentration 0.2 mg/mL; usual initial dose is 0.2 mg (range, 0.05 to 0.4 mg) with lockout period of 6 minutes (range, 5 to 10 min).
- Pain, When opioid analgesics are appropriate: (immediate-release oral liquid) 2.5 to 10 mg (2.5 to 10 mL) orally every 3 to 6 hr as needed; higher doses may be required in some cases.
- Pain, When opioid analgesics are appropriate: (immediate-release oral tablet) 2 to 4 mg orally every 4 to 6 hours as needed; gradually increase until adequate analgesia.
- Pain, When opioid analgesics are appropriate: (rectal suppository) 3 mg rectallyevery 6 to 8 hours [9] OR 3 to 6 mg rectallyevery 3 to 4 hours.
- Pain (Moderate to Severe), Opioid-tolerant: do not use hydromorphone HP if the amount of hydromorphone required cannot be delivered accurately with this formulation; never administer hydromorphone HP injection to opioid-naïve patients; use hydromorphone HP only if higher concentration and lower total volume are required.
- Pain (Moderate to Severe), Opioid-tolerant: conversion from hydromorphone to hydromorphone HP: initiate with an equipotent dose based on previous hydromorphone requirement and administer IV/IM/subQ in divided doses; titrate gradually based on response and tolerability [6]
- Pain (Moderate to Severe), Opioid-tolerant: conversion from other opioids: initiate with one-half of the estimated daily hydromorphone dose based on previous 24-hour analgesic requirement; administer IV/IM/subQ in divided doses; may titrate dose gradually based on response and tolerability.
- Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: individualize dose; after all other extended-release or around-the-clock opioids have been discontinued, initial dose selection must take into account patient's prior analgesic treatment experience and risk factors for addiction, abuse, and misuse; due to substantial inter-patient variability in relative potency of different opioid products, when converting it is recommended to underestimate a patient's 24-hour oral hydromorphone requirements and provide rescue mediation as needed.
- Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Exalgo(R)) conversion from oral immediate-release (IR) hydromorphone: initiate dose equal to total daily hydromorphone requirement orally once daily; may titrate by increments of 4 to 8 mg every 3 to 4 days as needed.
- Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Exalgo) conversion from other oral opioids: initiate with one-half of the estimated daily hydromorphone dose orally once daily using the following conversion factors to Exalgo(R): hydromorphone (1), codeine (0.06), hydrocodone (0.4), methadone (0.6), morphine (0.2), oxycodone (0.4), oxymorphone (0.6)); provide rescue medication as needed and titrate by increments of 4 to 8 mg every 3 to 4 days as needed.
- Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Palladone(R)) conversion from other oral opioids: initiate with one-half of the estimated daily hydromorphone dose orally once daily using the following conversion factors to Palladone(R): hydromorphone (1), codeine (0.04), hydrocodone (0.22), methadone (0.38), morphine (0.12), oxycodone (0.25); provide rescue medication as needed and titrate by increments of 4 to 8 mg every 3 to 4 days as needed.
- Pain (Severe), in opioid-tolerant patients requiring a long-term daily around-the-clock opioid analgesic: (Exalgo(R) or Palladone(R)) conversion from transdermal fentanyl: starting 18 hours after the removal of the fentanyl patch, initiate with one-half of the 24-hour hydromorphone dose orally once daily, using a conversion factor of 25 mcg/hr fentanyl transdermal patch to 12 mg of hydromorphone; provide rescue medication as needed and titrate by increments of 4 to 8 mg every 3 to 4 days as needed
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of hydromorphone in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of hydromorphone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy not established in pediatric patients.
- Pain, When opioid analgesics are appropriate: adolescents, 1 to 4 mg orally every 4 to 6 hr as needed has been used.
- Pain, When opioid analgesics are appropriate: children, 0.05 to 0.1 mg/kg/dose orally every 6 hr; MAX 5 mg/dose.
- Pain, When opioid analgesics are appropriate: adolescents, initial (opiate-naive patients) 0.2 to 0.6 mg IV (slow) every 2 to 3 hours as needed; usual, 1 to 2 mg/dose IM/IV/subQ every 4 to 6 hours.
- Pain, When opioid analgesics are appropriate: children, 0.015 mg/kg/dose IV every 4 to 6 hours.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of hydromorphone in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of hydromorphone in pediatric patients.
Contraindications
- hydromorphone is contraindicated in:
- Opioid non-tolerant patients. Fatal respiratory depression could occur in patients who are not opioid tolerant.
- Patients with significant respiratory depression
- Patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment.
- Patients with known or suspected paralytic ileus.
- Patients who have had surgical procedures and/or underlying disease resulting in narrowing of the gastrointestinal tract, or have “blind loops” of the gastrointestinal tract or gastrointestinal obstruction.
- Patients with hypersensitivity (e.g., anaphylaxis) to hydromorphone or sulfite-containing medications [see Warnings and Precautions].
Warnings
|
Warning: addiction, abuse, and misuse; life-threatening respiratory depression; accidental ingestion; neonatal opioid withdrawal syndrome; and interaction with alcohol
See full prescribing information for complete Boxed Warning.
|
- Addiction, Abuse, and Misuse
- hydromorphone contains hydromorphone, a Schedule II controlled substance. As an opioid, hydromorphone exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence]. As modified-release products such as hydromorphone deliver the opioid over an extended period of time, there is a greater risk for overdose and death due to the larger amount of hydromorphone present.
- Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed hydromorphone and in those who obtain the drug illicitly. Addiction can occur at recommended doses and if the drug is misused or abused.
- Assess each patient’s risk for opioid addiction, abuse, or misuse prior to prescribing hydromorphone, and monitor all patients receiving hydromorphone for the development of these behaviors or conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of hydromorphone for the proper management of pain in any given patient. Patients at increased risk may be prescribed modified-release opioid formulations such as hydromorphone, but use in such patients necessitates intensive counseling about the risks and proper use of hydromorphone along with intensive monitoring for signs of addiction, abuse, and misuse.
- Abuse or misuse of hydromorphone by crushing, chewing, snorting, or injecting the dissolved product will result in the uncontrolled delivery of hydromorphone and can result in overdose and death [see Overdosage].
- Opioid agonists such as hydromorphone are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
- Consider these risks when prescribing or dispensing hydromorphone. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on the proper disposal of unused drug [see Patient Counseling Information].
- Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
- Life-threatening Respiratory Depression
- Serious, life-threatening, or fatal respiratory depression has been reported with the use of modified-release opioids, even when used as recommended. Respiratory depression from opioid use, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see Overdosage]. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
- While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of hydromorphone, the risk is greatest during the initiation of therapy or following a dose increase. Closely monitor patients for respiratory depression when initiating therapy with hydromorphone and following dose increases.
- To reduce the risk of respiratory depression, proper dosing and titration of hydromorphone are essential [see Dosage and Administration].
- Overestimating the hydromorphone dose when converting patients from another opioid product can result in fatal overdose with the first dose.
- Accidental ingestion of even one dose of hydromorphone, especially by children, can result in respiratory depression and death due to an overdose of hydromorphone.
- Neonatal Opioid Withdrawal Syndrome
- Prolonged use of hydromorphone during pregnancy can result in withdrawal signs in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available.
- Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn.
- Interactions with Central Nervous System Depressants
- Hypotension, profound sedation, coma, respiratory depression, and death may result if hydromorphone is used concomitantly with alcohol or other central nervous system (CNS) depressants (e.g., sedatives, anxiolytics, hypnotics, neuroleptics, other opioids).
- When considering the use of hydromorphone in a patient taking a CNS depressant, assess the duration use of the CNS depressant and the patient’s response, including the degree of tolerance that has developed to CNS depression. Additionally, evaluate the patient’s use of alcohol or illicit drugs that cause CNS depression. If the decision to begin hydromorphone is made, start with 1/3 to 1/2 the calculated starting dose of hydromorphone, monitor patients for signs of sedation and respiratory depression, and consider using a lower dose of the concomitant CNS depressant [see Drug Interactions].
- Use in Ederly, Cachectic, and Debilitated Patients
- Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients. Monitor such patients closely, particularly when initiating and titrating hydromorphone and when hydromorphone is given concomitantly with other drugs that depress respiration [see Warnings and Precautions].
- Use in Patients with Chronic Pulmonary Disease
- Monitor patients with significant chronic obstructive pulmonary disease or cor pulmonale, and patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression for respiratory depression, particularly when initiating therapy and titrating with hydromorphone, as in these patients, even usual therapeutic doses of hydromorphone may decrease respiratory drive to the point of apnea [see Warnings and Precautions]. Consider the use of alternative non-opioid analgesics in these patients if possible.
- Hypotensive Effect
- hydromorphone may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is an increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g. phenothiazines or general anesthetics) [see Drug Interactions]. Monitor these patients for signs of hypotension after initiating or titrating the dose of hydromorphone.
- Use in Patients with Head Injury or Increased Intracranial Pressure
- Monitor patients taking hydromorphone who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors) for signs of sedation and respiratory depression, particularly when initiating therapy with hydromorphone. hydromorphone may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of hydromorphone in patients with impaired consciousness or coma.
- Use in Patients with Gastrointestinal Conditions
- hydromorphone is contraindicated in patients with paralytic ileus. Avoid the use of hydromorphone in patients with other GI obstruction.
- Because the hydromorphone capsule is nondeformable and does not appreciably change in shape in the GI tract, hydromorphone is contraindicated in patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic, for example: esophageal motility disorders, small bowel inflammatory disease, “short gut” syndrome due to adhesions or decreased transit time, past history of peritonitis, cystic fibrosis, chronic intestinal pseudoobstruction, or Meckel’s diverticulum). There have been reports of obstructive symptoms in patients with known strictures or risk of strictures, such as previous GI surgery, in association with the ingestion of drugs in nondeformable extended-release formulations.
- It is possible that hydromorphone capsules may be visible on abdominal x-rays under certain circumstances, especially when digital enhancing techniques are utilized.
- The hydromorphone in hydromorphone may cause spasm of the sphincter of Oddi. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
- Sulfites
- hydromorphone contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
- Use in Patients with Convulsive or Seizure Disorders
- The hydromorphone in hydromorphone may aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings. Monitor patients with a history of seizure disorders for worsened seizure control during hydromorphone therapy.
- Avoidance of Withdrawal
- Avoid the use of mixed agonist/antagonist (i.e., pentazocine, nalbuphine, and butorphanol) or partial agonists (buprenorphine) analgesics in patients who have received or are receiving a course of therapy with a full opioid agonist analgesic, including hydromorphone . In these patients, mixed agonists/antagonist and partial agonist analgesics may reduce the analgesic effect and/or may precipitate withdrawal symptoms [see Drug Interactions].
- When discontinuing hydromorphone , gradually taper the dose [see Dosage and Administration (2.3)]. Do not abruptly discontinue hydromorphone .
- Driving and Operating Machinery
- hydromorphone may impair the mental and/or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of hydromorphone and know how they will react to the medication.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions are discussed elsewhere in the labeling:
- Addiction, Abuse, and Misuse [see Warnings and Precautions]
- Life Threatening Respiratory Depression [see Warnings and Precautions]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions]
- Interactions with Other CNS Depressants [see Warnings and Precautions]
- Hypotensive Effect [see Warnings and Precautions]
- Gastrointestinal Effects [see Warnings and Precautions]
- Seizures [see Warnings and Precautions]
- Clinical Trial Experience
- The safety of hydromorphone was evaluated in double-blind clinical trials involving 612 patients with moderate to severe pain. An open-label extension study involving 143 patients with cancer pain was conducted to evaluate the safety of hydromorphone when used for longer periods of time in higher doses than in the controlled trials. Patients were treated with doses averaging 40 to 50 mg of hydromorphone per day (ranging between 12 and 500 mg/day) for several months (range 1 to 52 weeks).
- Serious adverse reactions which may be associated with hydromorphone therapy in clinical use are similar to those of other opioid analgesics, including respiratory depression, apnea, respiratory arrest, and to a lesser degree, circulatory depression, hypotension, shock or cardiac arrest [see OVERDOSAGE].
- Adverse Events Reported in Controlled Trials
- Table 2 lists treatment emergent signs and symptoms that were reported in at least 2% of patients in the placebo-controlled trials for which the rate of occurrence was greater for those treated with hydromorphone 12 mg capsules than those treated with placebo.
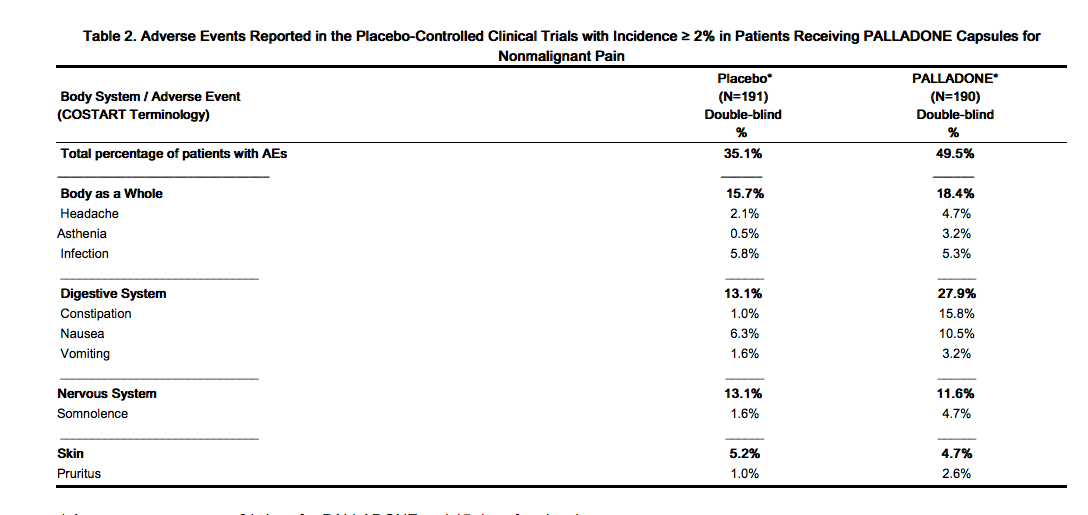
- Average exposure was 21 days for hydromorphone and 15 days for placebo.
- Adverse Events Observed in Clinical Trials
- hydromorphone has been administered to 785 individuals during completed clinical trials. The conditions and duration of exposure to hydromorphone varied greatly, and included open-label and double-blind studies, uncontrolled and controlled studies, inpatient and outpatient studies, fixed dose and titration studies. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing.
- These categories are used in the listing below. The frequencies represent the proportion of 785 patients from these trials who experienced that event while receiving hydromorphone . All adverse events included in this tabulation occurred in at least one patient. Events are classified by body system and listed using the following definitions: frequent adverse events - those occurring in at least 1/100 patients; adverse events occurring with an incidence less than 1% are considered infrequent. These adverse events are not necessarily related to hydromorphone treatment and in most cases were observed at a similar frequency in placebo-treated patients in the controlled studies.
- Frequent Adverse Events
- Body as a Whole: headache, [[]asthenia]], pain, abdominal pain, fever, chest pain, infection, chills, malaise, neck pain, carcinoma, accidental injury
- Cardiovascular System: vasodilatation, tachycardia, migraine
- Digestive System: nausea, constipation, vomiting, diarrhea, dyspepsia, anorexia, dry mouth, nausea and vomiting, dysphagia, flatulence
- Hemic and Lymphatic System: anemia, leukopenia
- Metabolic and Nutritional Disorders: peripheral edema, dehydration, edema, generalized edema, hypokalemia, weight loss
- Musculoskeletal: arthralgia, bone pain, leg cramps, myalgia
- Nervous System: somnolence, dizziness, nervousness, confusion, insomnia, anxiety, depression, hypertonia, hypesthesia, paresthesia, tremor, thinking abnormal, hallucinations, speech disorder, agitation, amnesia, tinnitus, abnormal gait
- Respiratory System: dyspnea, cough increased, rhinitis, pharyngitis, pneumonia, epistaxis, hiccup, hypoxia, pleural effusion
- Skin and Appendages: pruritus, sweating, rash
- Special Senses: amblyopia, taste perversion
- Urogenital System: dysuria, urinary incontinence
- Infrequent Adverse Events
- Body as a Whole: face edema, ascites, allergic reaction, cellulitis, overdose, hypothermia, neoplasm, photosensitivity reaction, sepsis, flank pain
- Cardiovascular System: hypertension, hypotension, syncope, deep thrombophlebitis, arrhythmia, postural hypotension, atrial fibrillation, pallor, bradycardia, electrocardiogram abnormal, myocardial infarction, palpitation, angina pectoris, congestive heart failure, QT interval prolonged, supraventricular tachycardia, thrombosis, cardiomegaly, hemorrhage
- Digestive System: fecal impaction, intestinal obstruction, abnormal stools, fecal incontinence, hepatic failure, increased appetite, cholangitis, cholecystitis, colitis, enterocolitis, hepatomegaly, jaundice, liver function tests abnormal, biliary spasm, ileus, eructation, rectal hemorrhage, esophagitis, glossitis, melena, mouth ulceration, gastrointestinal hemorrhage, tongue edema
- Endocrine: adrenal cortex insufficiency
- Hemic and Lymphatic System: ecchymosis, thrombocytopenia, leukocytosis, lymphadenopathy, agranulocytosis, lymphoma like reaction, pancytopenia, petechia
- Metabolic and Nutritional Disorders: hyperglycemia, hyponatremia, cachexia, hypercalcemia, hypomagnesemia, cyanosis, diabetes mellitus, gout, respiratory acidosis, elevated liver enzymes, thirst
- Musculoskeletal: myasthenia
- Nervous System: abnormal dreams, emotional lability, paranoid reaction, sleep disorder euphoria, incoordination, stupor, ataxia, convulsion, hallucination, hostility, myoclonus, psychosis, vertigo, withdrawal syndrome, apathy, delirium, dementia, drug dependence, nystagmus, twitching, depersonalization, aphasia, cerebrovascular accident, circumoral parasthesia, seizure, hyperkinesia, hypotonia, increased salivation, neuralgia
- Respiratory System: hypoventilation, apnea, atelectasis, hemoptysis, asthma, hyperventilation, pulmonary embolus, laryngismus
- Skin and Appendages: urticaria, maculopapular rash, alopecia
- Special Senses: abnormal vision, diplopia, dry eyes, lacrimation disorder, hyperacusis
- Urogenital: urinary retention, hematuria, impotence, urinary frequency, urination impaired, dysmenorrhea, creatinine increased, urinary urgency
- Additional Adverse Events From Non-U.S. Experience
- Addiction, blurred vision, drowsiness, dysphoria, sedation, seizure, physical dependence, biliary spasm, and ileus
Postmarketing Experience
There is limited information regarding Hydromorphone (oral) Postmarketing Experience in the drug label.
Drug Interactions
- Alcohol
- Concomitant use of alcohol with hydromorphone can result in an increase of hydromorphone plasma levels and potentially fatal overdose of hydromorphone. Instruct patients not to consume alcoholic beverages or use prescription or non-prescription products containing alcohol while on hydromorphone therapy [see Clinical Pharmacology].
- CNS Depressants
- The concomitant use of hydromorphone with other CNS depressants including sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and alcohol can increase the risk of respiratory depression, profound sedation, coma and death. Monitor patients receiving CNS depressants and hydromorphone for signs of respiratory depression, sedation and hypotension.
- When combined therapy with any of the above medications is considered, the dose of one or both agents should be reduced [see Dosage and Administration and Warnings and Precautions].
- Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics
- Mixed agonist/antagonist (pentazocine, nalbuphine, and butorphanol) and partial agonist (buprenorphine) analgesics may reduce the analgesic effect of hydromorphone and/or may precipitate withdrawal symptoms in these patients. Avoid the use of mixed agonist/antagonist analgesics in patients receiving hydromorphone .
- Monoamine Oxidase Inhibitors (MAOIs)
- The effects of opioid analgesics may be potentiated by MAOIs. hydromorphone is not recommended for use in patients who have received MAOIs within 14 days. If concurrent therapy with an MAOI and hydromorphone is unavoidable, monitor patients for increased respiratory and central nervous system depression.
- Anticholinergics
- Anticholinergics or other medications with anticholinergic activity when used concurrently with hydromorphone may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. Monitor patients for signs of urinary retention or reduced gastric motility when hydromorphone is used concurrently with anticholinergic drugs.
Use in Specific Populations
Pregnancy
- Fetal/neonatal adverse reactions
- Prolonged use of opioid analgesics during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth. Observe newborns for symptoms of neonatal opioid withdrawal syndrome, such as poor feeding, diarrhea, irritability, tremor, rigidity, and seizures, and manage accordingly [see Warnings and Precautions (5.3)].
- Teratogenic Effects - Pregnancy Category C
- There are no adequate and well-controlled studies in pregnant women. hydromorphone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- hydromorphone was not teratogenic in female rats given oral doses up to 10 mg/kg or female rabbits given oral doses up to 50 mg/kg during the major period of organ development. Estimated exposures in the female rat and rabbit were approximately 3-fold and 6-fold higher than a 32 mg human daily oral dose based on exposure (AUC0-24h).
- hydromorphone administration to pregnant Syrian hamsters and CF-1 mice during major organ development revealed teratogenicity likely the result of maternal toxicity associated with sedation and hypoxia. In Syrian hamsters given single subcutaneous doses from 14 to 278 mg/kg during organogenesis (gestation days 8 to10), doses ≥ 19 mg/kg hydromorphone produced skull malformations (exencephaly and cranioschisis).
- Continuous infusion of hydromorphone (5 mg/kg, s.c.) via implanted osmotic mini pumps during organogenesis (gestation days 7 to10) produced soft tissue malformations (cryptorchidism, cleft palate, malformed ventricals and retina), and skeletal variations (supraoccipital, checkerboard and split sternebrae, delayed ossification of the paws and ectopic ossification sites). The malformations and variations observed in the hamsters and mice were at doses approximately 3-fold higher and <1-fold lower, respectively, than a 32 mg human daily oral dose on a body surface area basis.
- Nonteratogenic Effect
- In a rat pre- and post-natal study, an increase in pup mortality and a decrease in pup body weight which was associated with maternal toxicity was observed at doses of 2 and 5 mg/kg/day. The maternal no effect level for hydromorphone was 0.5 mg/kg/day which is <1-fold lower than a 32 mg human daily oral dose on a body surface area. hydromorphone had no effect on pup development or reproduction when given to female rats during the pre-natal and postnatal periods up to a dose of 5 mg/kg which is equivalent to a 32 mg human daily oral dose on a body surface area basis.
- Neonates born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Approaches to the treatment of the syndrome have included supportive care and, if indicated, drugs such as paregoric or phenobarbital.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydromorphone (oral) in women who are pregnant.
Labor and Delivery
- hydromorphone is not for use in women during and immediately prior to labor, where shorter acting analgesics or other analgesic techniques are more appropriate [see Indications and Usage (1)]. Occasionally, opioid analgesics may prolong labor by temporarily reducing the strength, duration, and frequency of uterine contractions. However, these effects are not consistent and may be offset by an increased rate of cervical dilatation which tends to shorten labor.
- Opioids cross the placenta and may produce respiratory depression and psychophysiologic effects in neonates. Closely observe neonates whose mothers received opioid analgesics during labor for signs of respiratory depression. An opioid antagonist, such as naloxone, should be available for reversal of opioid-induced respiratory depression in the neonate in such situations.
Nursing Mothers
- Low concentrations of hydromorphone have been detected in human milk in clinical trials. Withdrawal symptoms can occur in breastfeeding infants when maternal administration of an opioid analgesic is stopped. Nursing should not be undertaken while a patient is receiving hydromorphone since hydromorphone is excreted in the milk.
Pediatric Use
- The safety and effectiveness of hydromorphone in pediatric patients below the age of 18 have not been established.
Geriatic Use
- Elderly patients have been shown to be more sensitive to the adverse effects of opioids compared to the younger population. Of the total number of subjects in clinical studies of hydromorphone , 22% were 65 and over, and 6% were 75 and over. Dosages should be adjusted according to the clinical situation. As with all opioids, the starting dose should be reduced to 1/3 to 1/2 of the usual dosage in debilitated patients. Respiratory depression is the chief hazard in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration. Therefore, closely monitor elderly patients for respiratory and central nervous system depression when prescribing hydromorphone , particularly during initiation and titration.
Gender
There is no FDA guidance on the use of Hydromorphone (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydromorphone (oral) with respect to specific racial populations.
Renal Impairment
- In patients with mild to moderate renal impairment, based on calculated creatinine clearance, the concentrations of hydromorphone in plasma were slightly higher than in subjects with normal renal function.
Hepatic Impairment
- hydromorphone was not studied in patients with severe hepatic impairment and are not recommended for use in such patients. Care in initial dose selection and careful observation are recommended in patients with evidence of mild to moderate hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydromorphone (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydromorphone (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Hydromorphone (oral) Administration in the drug label.
Monitoring
There is limited information regarding Hydromorphone (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Hydromorphone (oral) and IV administrations.
Overdosage
- Clinical Presentation
- Acute overdosage with opioids can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes pulmonary edema, bradycardia, hypotension and death. Marked mydriasis rather than miosis may be seen due to severe hypoxia in overdose situations.
- Treatment of Overdose
- In case of overdose, priorities are the re-establishment of a patent and protected airway and institution of assisted or controlled ventilation if needed. Employ other supportive measures (including oxygen, vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life support techniques.
- The opioid antagonists, such as naloxone and naltrexone, are specific antidotes to respiratory depression resulting from opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to hydromorphone overdose. Such agents should be administered cautiously to patients who are known, or suspected to be, physically dependent on hydromorphone . In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute withdrawal syndrome.
- Because the duration of reversal would be expected to be less than the duration of action of hydromorphone in hydromorphone , carefully monitor the patient until spontaneous respiration is reliably reestablished. hydromorphone will continue to release hydromorphone adding to the hydromorphone load for up to 24 hours after administration, necessitating prolonged monitoring for at least 24 to 48 hours beyond the overdose. If the response to opioid antagonists is suboptimal or not sustained, additional antagonist should be given as directed in the product’s prescribing information.
- In an individual physically dependent on opioids, administration of an opioid receptor antagonist may precipitate an acute withdrawal. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
Pharmacology
Mechanism of Action
- Hydromorphone, a semi-synthetic morphine derivative, is a hydrogenated ketone of morphine. Hydromorphone is principally an agonist of mu-receptors, showing a weak affinity for к-receptors. Comparing relative binding affinity for mu- and к-opioid receptors, Hydromorphone binds more specifically to mu- receptors than structurally related morphine. As an opioid agonist, the principle therapeutic action of Hydromorphone is analgesia. The precise mechanism of action of opioid analgesics is not known but the effects are thought to be mediated through opioid-specific receptors located predominantly in the central nervous system (CNS). Interaction with the mu-opioid receptor subtype is believed to be responsible for most of Hydromorphone’s clinical effects. There is no intrinsic limit to the analgesic effect of Hydromorphone. Clinically, however, dosage limitations are imposed by the adverse effects, primarily respiratory depression, sedation, nausea, and vomiting, which can result from high doses.
Structure
- Hydromorphone extended-release capsules are for oral use and contain Hydromorphone hydrochloride, a mu-opioid.
- Hydromorphone hydrochloride USP is 4,5α-epoxy-3-hydroxy-17-methlymorphinan-6-one hydrochloride. Hydromorphone hydrochloride is a white or almost white crystalline powder that is freely soluble in water, very slightly soluble in ethanol (96%), and practically insoluble in methylene chloride. Its empirical formula is C17H19NO3•HCl. The compound has the following structural formula:
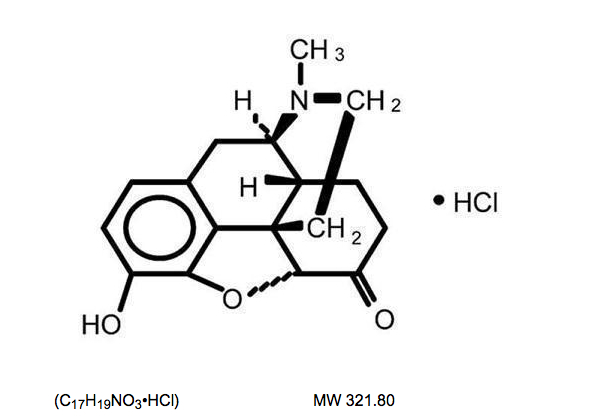
(C17H19NO3•HCl) MW 321.80
- Hydromorphone also contains the following inactive ingredients: ammonio methacrylate copolymer type B, ethylcellulose, and stearyl alcohol, synthetic black iron oxide, gelatin, red iron oxide (12 mg & 16 mg only), FD&C Blue No. 2 (24 mg only), and titanium dioxide.
Pharmacodynamics
- CNS Depressant/Alcohol Interaction
- Additive pharmacodynamic effects may be expected when Hydromorphone is used in conjunction with alcohol, other opioids, legal or illicit drugs that cause central nervous system depression.
- Effects on the Central Nervous System
- Hydromorphone produces dose-related respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
- Hydromorphone depresses the cough reflex by direct effect on the cough center in the medulla.
- Hydromorphone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomic. Marked mydriasis, rather than miosis, may be seen due to severe hypoxia in overdose situations.
- Effects on the Gastrointestinal Tract and Other Smooth Muscle
- Gastric, biliary and pancreatic secretions are decreased by Hydromorphone. Hydromorphone causes a reduction in motility associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm. The end result is constipation. Hydromorphone also can cause an increase in biliary tract pressure as a result of spasm of the sphincter of Oddi.
- Effects on the Cardiovascular System
- Hydromorphone produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Release of histamine may be induced by Hydromorphone and can contribute to opioid-induced hypotension. Manifestations of histamine release or peripheral vasodilation may include pruritus, flushing, red eyes, and sweating.
- Effects on the Endocrine System
- Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
- Effects on the Immune System
- Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Pharmacokinetics
- Absorption
- Hydromorphone is an extended-release formulation of Hydromorphone. Administration of a single Hydromorphone dose is characterized by biphasic absorption, a relatively rapid rise to an initial peak concentration, followed by a second broader peak with therapeutic plasma concentrations maintained over the 24-hour dosing interval. The absolute bioavailability of Hydromorphone from Hydromorphone has not been determined. Under conditions of multiple dosing, the bioavailability of a once-daily dose of Hydromorphone is equivalent to the same total daily dose of immediate-release Hydromorphone given in divided doses every 6 hours. Dose proportionality has been established in terms of Cmax and AUC for the 12 mg and 24 mg dosage strengths. Dosage form proportionality on a dose-adjusted basis has been demonstrated for three 12 mg capsules to one 32 mg capsule.
- In a study comparing 12 mg Hydromorphone dosed every 24 hours to 3 mg of immediate-release Hydromorphone dosed every 6 hours in healthy human subjects, the two treatments were found to be equivalent in terms of extent of absorption (AUC) (see Figure 1). The extended-release characteristics of Hydromorphone resulted in lower steady-state peak levels (Cmax), higher trough levels (Cmin), and an approximately twofold to threefold reduction in the fluctuation seen with the immediate-release Hydromorphone tablets.
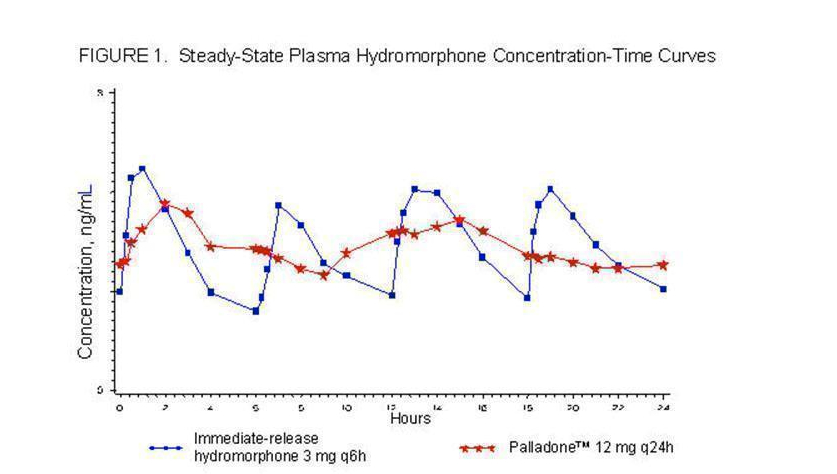
- Steady-state plasma concentrations with Hydromorphone were achieved within 2 to 3 days after initiation of dosing. This is consistent with the mean apparent terminal elimination half-life for Hydromorphone of approximately 18.6 hours. Hydromorphone did not accumulate significantly after multiple dosing with once-daily administration.
- Food had no significant effect on the peak (Cmax), AUC or the elimination of Hydromorphone from Hydromorphone (see Figure 2).
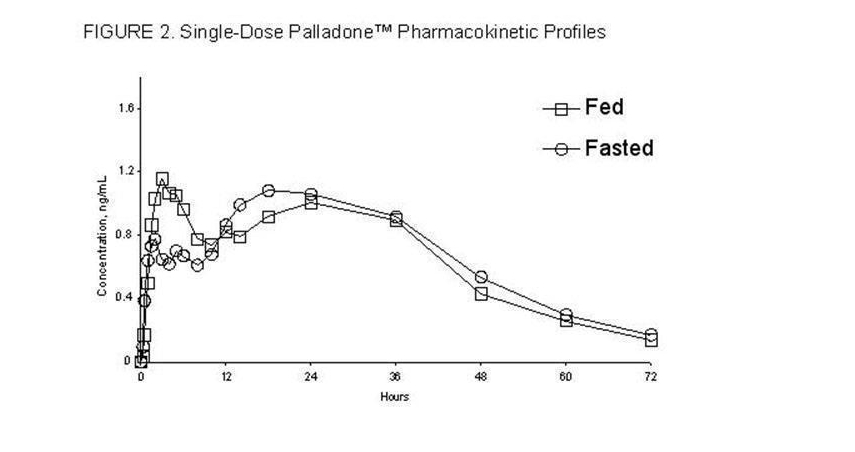
- Food Effect
- The pharmacokinetics of Hydromorphone is not affected by food as indicated by bioequivalence when administered under fed and fasting conditions. Therefore, Hydromorphone may be administered without regard to meals.
- Distribution
- Following intravenous administration of Hydromorphone, the reported volume of distribution is 295 L (4 L/kg). Hydromorphone is approximately 20% bound to human plasma proteins.
- Metabolism
- Hydromorphone is metabolized by direct conjugation, or by 6-keto reduction followed by conjugation. Following absorption, Hydromorphone is metabolized to the major metabolites Hydromorphone-3-glucuronide, Hydromorphone-3-glucoside and dihydroisomorphine-6-glucuronide. Also observed were the less prevalent metabolites, dihydroisomorphine-6-glucoside, dihydromorphine and dihydroisomorphine.
- Hydromorphone metabolites have been found in plasma, urine and in human hepatocyte test systems. However, it is not known whether Hydromorphone is metabolized by the cytochrome P450 enzyme system. Hydromorphone is a poor inhibitor of human recombinant CYP isoforms including CYP1A2, 2A6, 2C8, 2D6, and 3A4 with an IC50 > 50 µM. Therefore, Hydromorphone is not expected to inhibit the metabolism of other drugs metabolized by these CYP isoforms.
- Specific Populations
- Geriatric Patients
- Age-related increases in exposure in clinical studies were observed between geriatric and younger adult subjects. Greater sensitivity of some older individuals cannot be excluded. Dosages should be adjusted according to the clinical situation.
- Pediatric Patients
- The safety and effectiveness of Hydromorphone have not been established in patients below the age of 18.
- Gender
- Pharmacokinetics of Hydromorphone from Hydromorphone is comparable in men and women.
- Race
- The pharmacokinetics of Hydromorphone in African Americans and Caucasians in the clinical population were comparable.
- Hepatic Impairment
- Hydromorphone was not studied in patients with severe hepatic insufficiency and is not recommended for use in such patients. Start patients with moderate hepatic impairment on 25% of the usual dose of Hydromorphone and closely monitor for respiratory and central nervous system depression during dose titration. Consider alternate analgesic therapy for patients with severe hepatic impairment [see Dosage and Administration and Specific Populations].
- Renal Impairment
- In patients with mild renal impairment, based on calculated creatinine clearance, the concentrations of Hydromorphone in plasma were slightly higher than in subjects with normal renal function. Start patients with moderate renal impairment on 50% of the usual Hydromorphone dose for patients with normal renal function and closely monitor for respiratory and central nervous system depression during dose titration. As Hydromorphone is only intended for once-daily administration, consider use of an alternate analgesic that may permit more flexibility with the dosing interval in patients with severe renal impairment [see Dosage and Administration and Use in Specific Populations].
- Drug Interaction/Alcohol Interaction
- A pharmacokinetic study in healthy subjects showed that co-ingestion of a 12 mg Hydromorphone capsule with 240 mL (8 ounces) of 40% (80 proof) alcohol resulted in an average peak Hydromorphone concentration approximately six times greater than when taken with water. One subject in this study experienced a 16-fold increase when the drug was ingested with 40% alcohol compared with water. In certain subjects, 8 ounces of 4% alcohol (equivalent to 2/3 of a typical serving of beer) resulted in almost twice the peak plasma Hydromorphone concentration than when the drug was ingested with water.
- This pharmacokinetic study was an open-label, four-arm, crossover design study and included twenty-four healthy adult subjects who were tested under fasted conditions and 24 healthy adult subjects who were tested under standardized fed conditions. Subjects were pretreated with naltrexone to block the opiate effects, and then administered one of the following four treatments:
- Group A Hydromorphone , 12 mg + 240 mL of 40% ethanol
- Group B Hydromorphone , 12 mg + 240 mL of 20% ethanol
- Group C Hydromorphone , 12 mg + 240 mL of 4% ethanol
- Group D Hydromorphone , 12 mg + 240 mL of water
- Plasma was sampled and analyzed for Hydromorphone concentration at appropriate intervals. Each subject received each of the four treatments, thereby acting as his or her own control (Group D).
- The effects of alcohol co-ingestion were more marked in the fasted state and are summarized below.
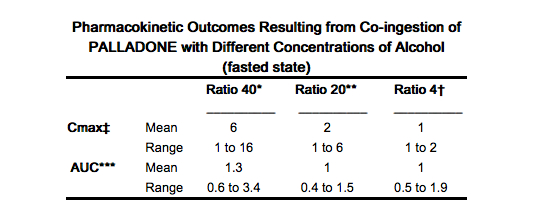
- Ratio of values when co-ingested with 240 mL of 40% ethanol compared to co-ingestion with 240 mL of water, i.e. if the peak plasma concentration was 6 ng/mL when administered with alcohol and 1 ng/mL when administered with water, this ratio would be 6
- Ratio of values (as above) when co-ingested with 240 mL of 20% ethanol compared to co-ingestion with 240 mL of water
- †Ratio of values (as above) when co-ingested with 240 mL of 4% ethanol compared to co-ingestion with 240 mL of water
- ‡Peak plasma concentration
- Measure of total drug exposure
- In the fed state, the mean peak plasma concentration ratio (40% alcohol:water) was 3.5 with a maximum of 6.
- In summary, the study showed that ingesting Hydromorphone with alcohol in clinically relevant amounts results in significantly higher peak plasma concentrations of Hydromorphone. The effect is more pronounced with increasing concentrations of alcohol and in a fasted state.
- The effects of co-ingestion of smaller volumes and with other concentrations of alcohol have not been studied.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis: No carcinogenicity studies have been conducted in animals.
- Hydromorphone was negative in the in vitro bacterial reverse mutation assay and in the in vivo mouse micronucleus assay. Hydromorphone was negative in the mouse lymphoma assay in the absence of metabolic activation, but was positive in the mouse lymphoma assay in the presence of metabolic activation. Morphinone, an impurity, tested as a besylate salt was negative in the in vitro bacterial reverse mutation assay and negative in the in vivo mouse micronucleus assay. Morphinone was positive in the Chinese Hamster Ovary Cell Chromosomal Aberration test in the absence and presence of metabolic activation.
- Hydromorphone did not affect fertility in rats at oral doses up to 5 mg/kg which is equivalent to a 32 mg human daily oral dose on a body surface area basis.
- Mutagenesis
- Hydromorphone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay). Hydromorphone was not clastogenic in either the in vitro human lymphocyte chromosome aberration assay or the in vivo mouse micronucleus assay.
- Impairment of Fertility: Hydromorphone given orally to rats during the mating period caused a slight but statistically significant reduction in implantations at 6.25 mg/kg/day (~1.2 times the human exposure following to 32 mg/day).
Clinical Studies
- The efficacy of Hydromorphone was established in a double-blind, randomized, parallel group, multicenter, placebo-controlled, four-week trial of patients with pain that was present for at least one month. The majority of these patients experienced moderate to severe pain due to musculoskeletal disorders while maintained on one or more opioid analgesics, often in addition to non-opioid analgesics. * Two hundred twenty-one patients with chronic moderate to severe pain were randomized to receive once daily 12 mg Hydromorphone capsule or placebo after they had demonstrated that they needed approximately 12 mg of immediate-release Hydromorphone (in addition to non-opioid medication) around-the-clock to improve their pain control. Patients randomized to Hydromorphone maintained adequate analgesia for a significantly longer period of time (P<0.0001) than patients randomized to placebo.
How Supplied
- 2 mg: cinnamon-colored capsules imprinted with “P-XL” on the cap and “12 mg” on the body and available as follows:
- 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-008-06
- 20 capsules, Unit Dose (2 X 10) ......…………………………. NDC 42858-008-11
- 16 mg: pink-colored capsules imprinted with “P-XL” on the cap and “16 mg” on the body and available as follows:
- 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-027-06
- 20 capsules, Unit Dose (2 X 10) ......…………………………. NDC 42858-027-11
- 24 mg: blue-colored capsules imprinted with “P-XL” on the cap and “24 mg” on the body and available as follows:
- 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-039-06
- 20 capsules, Unit Dose (2 X 10) ......…………………………. NDC 42858-039-11
- 32 mg: white capsules imprinted with “P-XL” on the cap and “32 mg” on the body and available as follows:
- 60 capsules, opaque plastic bottle ......…………………………. NDC 42858-204-06
- 20 capsules, Unit Dose (2 X 10) ......……………………….… NDC 42858-204-11
Storage
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
- Avoid temperatures above 40°C (104°F) [See USP Excessive Heat]
- Dispense in a tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Hydromorphone (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Hydromorphone (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
- Addiction, Abuse, and Misuse
- Inform patients that the use of Hydromorphone , even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose or death [see Warnings and Precautions]. Instruct patients not to share Hydromorphone with others and to take steps to protect Hydromorphone from theft or misuse.
- Life-threatening Respiratory Depression
- Inform patients of the risk of life-threatening of respiratory depression, including information that the risk is greatest when starting Hydromorphone or when the dose is increased, and that it can occur even at recommended doses [see Warnings and Precautions (5.2)]. Advise patients how to recognize respiratory depression and to seek medical attention if breathing difficulties develop.
- Accidental Ingestion
- Inform patients that accidental ingestion, especially in children, may result in respiratory depression or death [see Warnings and Precautions]. Instruct patients to take steps to store Hydromorphone securely and to dispose of unused Hydromorphone by flushing the capsules down the toilet.
- Neonatal Opioid Withdrawal Syndrome
- Inform female patients of reproductive potential that prolonged use of Hydromorphone during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated [see Warnings and Precautions].
- Interactions with Alcohol and other CNS Depressants
- Inform patients that potentially serious additive effects may occur if Hydromorphone is used with alcohol or other CNS depressants, and not to use such drugs unless supervised by a health care provider.
- Important Administration Instructions
- Instruct patients how to properly take Hydromorphone , including the following:
- Swallowing Hydromorphone whole
- Not crushing, chewing, splitting or dissolving the capsule
- Using Hydromorphone exactly as prescribed to reduce the risk of life-threatening adverse reactions (e.g., respiratory depression)
- Not discontinuing Hydromorphone without first discussing the need for a tapering regimen with the prescriber
- Gastrointestinal Blockage
- Advise patients that people with certain stomach or intestinal problems such as narrowing of the intestines or previous surgery may be at higher risk of developing a blockage. Symptoms include abdominal distension, abdominal pain, severe constipation, or vomiting. Instruct patients to contact their healthcare provider immediately if they develop these symptoms.
- Hypotension
- Inform patients that Hydromorphone may cause orthostatic hypotension and syncope. Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position).
- Driving or Operating Heavy Machinery
- Inform patients that Hydromorphone may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how they will react to the medication.
- Constipation
- Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention.
- Anaphylaxis
- Inform patients that anaphylaxis has been reported with ingredients contained in Hydromorphone . Advise patients how to recognize such a reaction and when to seek medical attention.
- Pregnancy
- Advise female patients that Hydromorphone can cause fetal harm and to inform the prescriber if they are pregnant or plan to become pregnant.
- Disposal
- Advise patients to flush the unused capsules down the toilet when Hydromorphone is no longer needed.
Marketed by: Rhodes Pharmaceuticals L.P. Coventry, RI 02816 Manufactured by: The PF Laboratories Inc. Totowa, New Jersey 07512 U.S. Patent Numbers 5,958,452; 5,965,161; 5,968,551, 6,294,195, 6,335,033; 6,706,281 303260-0A Revision: April 2014 Medication Guide PALLADONE (pal-a-d-own) (Hydromorphone hydrochloride) extended-release capsules, CII
PALLADONE is:
- A strong prescription pain medicine that contains an opioid (narcotic) that is used to manage pain severe enough to require daily around-the-clock, long-term treatment with an opioid, in people who are already regularly using opioid pain medicine, when other pain treatments such as non-opioid pain medicines or immediate-release opioid medicines do not treat your pain well enough or you cannot tolerate them.
- A long-acting (extended-release) opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death.
- Not for use to treat pain that is not around-the-clock.
Important information about PALLADONE:
- Get emergency help right away if you take too much PALLADONE (overdose). When you first start taking PALLADONE, when your dose is changed, or if you take too much (overdose), serious or life threatening breathing problems that can lead to death may occur.
- Never give anyone your PALLADONE. They could die from taking it. Store PALLADONE away from children and in a safe place to prevent stealing or abuse. Selling or giving away PALLADONE is against the law.
Do not take PALLADONE if you have:
- Severe asthma, trouble breathing, or other lung problems.
- A bowel blockage or have narrowing of the stomach or intestines.
Before taking PALLADONE, tell your healthcare provider if you have a history of:
- Head injury, seizures
- Liver, kidney, thyroid problems
- Allergy to sulfites
- Problems urinating
- Pancreas or gallbladder problems
- Abuse of street or prescription drugs, alcohol addiction, or mental health problems
- Tell your healthcare provider if you are:
- Pregnant or planning to become pregnant. Prolonged use of PALLADONE during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- Breastfeeding. PALLADONE passes into breast milk and may harm your baby.
- Taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking PALLADONE with certain other medicines can cause serious side effects.
- When taking PALLADONE:
- Do not change your dose. Take PALLADONE exactly as prescribed by your healthcare provider.
- Take your prescribed dose every 24 hours, at the same time every day. Do not take more than your prescribed dose in 24 hours. If you miss a dose, take your next dose at your usual time the next day.
- Swallow PALLADONE whole. Do not cut, break, chew, crush, dissolve, snort, or inject PALLADONE because this may cause you to overdose and die.
- Call your healthcare provider if the dose you are taking does not control your pain.
- Do not stop taking PALLADONE without talking to your healthcare provider.
- After you stop taking PALLADONE, flush any unused tablets down the toilet.
- While taking PALLADONE DO NOT:
- Drive or operate heavy machinery until you know how PALLADONE affects you. PALLADONE can make you sleepy, dizzy, or lightheaded.
- Drink alcohol or use prescription or over-the-counter medicines containing alcohol. Using products containing alcohol during treatment with PALLADONE may cause you to overdose and die.
- The possible side effects of PALLADONE are:
- Constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain. Call your healthcare provider if you have any of these symptoms and they are severe.
- Get emergency medical help if you have:
- Trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue or throat, extreme drowsiness, light-headedness when changing positions, or you are feeling faint.
- These are not all the possible side effects of PALLADONE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov
- Manufactured for: Rhodes Pharmaceuticals L.P., or call 1-888-827-0616
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: April 2014
Precautions with Alcohol
Alcohol-Hydromorphone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Hydromorphone (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Hydromorphone (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Coda BA, Rudy AC, Archer SM, Wermeling DP (July 2003). "Pharmacokinetics and bioavailability of single-dose intranasal Hydromorphone hydrochloride in healthy volunteers". Anesth. Analg. 97 (1): 117–23, table of contents. doi:10.1213/01.ANE.0000066311.40978.4F. PMID 12818953.
- ↑ Vallner JJ, Stewart JT, Kotzan JA, Kirsten EB, Honigberg IL (April 1981). "Pharmacokinetics and bioavailability of Hydromorphone following intravenous and oral administration to human subjects". J Clin Pharmacol. 21 (4): 152–6. doi:10.1002/j.1552-4604.1981.tb05693.x. PMID 6165742.
{{#subobject:
|Page Name=Hydromorphone (oral) |Pill Name=No_image.jpg |Drug Name=Hydromorphone |Pill Ingred=Ammonio methacrylate copolymer type b, ethylcellulose, steryl alcohol, synthetic black iron oxide, titanium dioxide|+sep=; |Pill Imprint=P-XL;32;mg |Pill Dosage=32 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22.00 |Pill Scoring=1 |Pill Image= |Drug Author=Purdue Pharma L.P. |NDC=42858-039-06
}}
{{#subobject:
|Label Page=Hydromorphone (oral) |Label Name=Hydromorphone label.png
}}