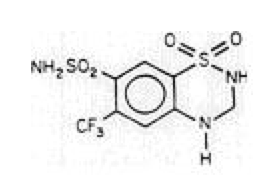
Hydroflumethiazide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Hydroflumethiazide is a thiazide-like diuretic that is FDA approved for the {{{indicationType}}} of hypertension and edema associated with congestive heart failure, hepatic cirrhosis, corticosteroid and estrogen therapy, and renal dysfunction. Common adverse reactions include hyponatremia, hypokalemia, hyperglycemia, orthostatic hypotension, photosensitivity, loss of appetite, nausea, vomiting, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Therapy should be individualized according to patient response. This therapy should be titrated to gain maximal therapeutic response, as well as the minimal dose possible to maintain that therapeutic response.
Edema
- Dosing Information
- Initial Dosage
- 50 to 200 mg daily, in 1 or 2 divided doses
- Maintenance Dosage
- 25 to 50 mg on alternate days or intermittently
Hypertension
- Dosing Information
- 25 to 50 mg daily in 1 or 2 divided doses, either alone, or in conjunction with other antihypertensive agents
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydroflumethiazide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroflumethiazide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Edema
- Dosing Information
- A suggested initial dose for children is 1 mg/kg of body weight daily, reduced for maintenance.
Hypertension
- Dosing Information
- A suggested initial dose for children is 1 mg/kg of body weight daily, reduced for maintenance.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydroflumethiazide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroflumethiazide in pediatric patients.
Contraindications
- Hypersensitivity to this or other sulfonamide-derived drugs
Warnings
- Hydroflumethiazide should be used with caution in severe renal disease. In patients with renal disease, thiazide may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
- Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
- Thiazides may be additive or potentiative of the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic blocking drugs.
- Sensitivity reactions may occur in patients with a history of allergy or bronchial asthma.
- The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
Precautions
- All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance, namely, hyponatremia. hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Medication such as digitalis may also influence serum electrolytes. Warning signs, irrespective of cause, are: dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
- Hypokalemia may develop with thiazides as with any other potent diuretic, especially with brisk diuresis. when severe cirrhosis is present, or during concomitant use of corticosteroids, including ACTH.
- Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Digitalis therapy may exaggerate the metabolic effects of hypokalemia, especially with respect to myocardial activity.
- Any chloride deficit is generally mild and usually does not require specific treatment, except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather. Appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
- Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
- Insulin requirements in diabetic patients may be increased, decreased, or unchanged. Latent diabetes mellitus may become manifested during thiazide administration.
- Thiazide drugs may increase the responsiveness to tubocurarine.
- The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.
- Thiazides may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
- If progressive renal impairment becomes evident, as indicated by rising nonprotein nitrogen or blood urea nitrogen, a careful reappraisal of therapy is necessary with consideration given to withholding or discontinuing diuretic therapy.
- Thiazides may decrease serum PBI levels without signs of thyroid disturbance.
- Lithium generally should not be given with diuretics, because they reduce its renal clearance and increase the risk of lithium toxicity. Read circulars for lithium preparations before use of such concomitant therapy with Hydroflumethiazide.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Hydroflumethiazide in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Hydroflumethiazide in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydroflumethiazide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Hydroflumethiazide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Hydroflumethiazide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Hydroflumethiazide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Hydroflumethiazide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Hydroflumethiazide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydroflumethiazide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Hydroflumethiazide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Hydroflumethiazide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydroflumethiazide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydroflumethiazide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Hydroflumethiazide in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Hydroflumethiazide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Hydroflumethiazide in the drug label.
Pharmacology
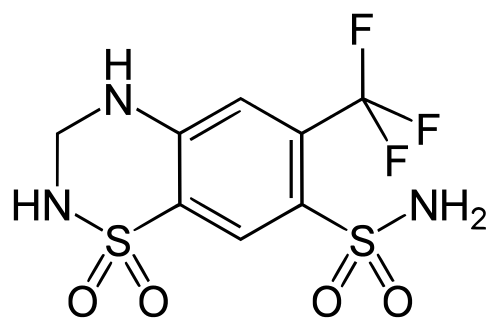
| |
Hydroflumethiazide
| |
| Systematic (IUPAC) name | |
| 1,1-Dioxo-6-(trifluoromethyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide | |
| Identifiers | |
| CAS number | |
| ATC code | C03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 331.29 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Hydroflumethiazide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Hydroflumethiazide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Hydroflumethiazide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Hydroflumethiazide in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Hydroflumethiazide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Hydroflumethiazide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Hydroflumethiazide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Hydroflumethiazide in the drug label.
Precautions with Alcohol
- Alcohol-Hydroflumethiazide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Saluron®[1]
Look-Alike Drug Names
- N/A[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Saluron (hydroflumethiazide) Tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Hydroflumethiazide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Hydroflumethiazide |Label Name=Hydroflumethiazide11.png
}}
{{#subobject:
|Label Page=Hydroflumethiazide |Label Name=Hydroflumethiazide11.png
}}