Goserelin: Difference between revisions
No edit summary |
Rabin Bista (talk | contribs) No edit summary |
||
| (17 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|authorTag={{RB}} | |||
|genericName=Goserelin acetate | |||
|aOrAn=a | |||
|drugClass=[[Gonadotropin-releasing hormone agonist|Gonadotropin-releasing Hormone (GnRH) agonist]] | |||
|indicationType=treatment | |||
|indication=locally confined [[Prostate cancer|carcinoma of the prostate]], and palliative treatment of [[Prostate cancer|advanced carcinoma of the prostate]] | |||
|adverseReactions=[[hot flashes]], [[sexual dysfunction]], [[Erectile dysfunction|decreased erections]] and [[Urinary system|lower urinary tract symptoms]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult=====Indications==== | |||
=====Stage B2-C Prostatic Carcinoma===== | |||
* ZOLADEX is indicated for use in combination with [[flutamide]] for the management of locally confined Stage T2b-T4 (Stage B2-C) [[carcinoma of the prostate]]. Treatment with ZOLADEX and flutamide should start 8 weeks prior to initiating radiation therapy and continue during radiation therapy | |||
======Dosage====== | |||
* When ZOLADEX is given in combination with radiotherapy and [[flutamide]] for patients with Stage T2b-T4 (Stage B2-C) [[prostatic carcinoma]], treatment should be started 8 weeks prior to initiating [[radiotherapy]] and should continue during radiation therapy. A treatment regimen using one ZOLADEX 3.6 mg depot, followed in 28 days by one ZOLADEX 10.8 mg depot, should be administered. | |||
=====Prostatic Carcinoma===== | |||
* ZOLADEX is indicated in the palliative treatment of [[Prostate cancer|advanced carcinoma of the prostate]]. | |||
* In controlled studies of patients with [[Prostate cancer|advanced prostatic cancer]] comparing ZOLADEX 3.6 mg to [[orchiectomy]], the long-term endocrine responses and objective responses were similar between the two treatment arms. Additionally, duration of survival was similar between the two treatment arms in a major comparative trial. | |||
* In controlled studies of patients with [[Prostate cancer|advanced prostatic cancer]], ZOLADEX 10.8 mg implant produced pharmacodynamically similar effect in terms of suppression of serum [[testosterone]] to that achieved with ZOLADEX 3.6 mg implant. Clinical outcome similar to that produced with the use of the ZOLADEX 3.6 mg implant administered every 28 days is predicted with the ZOLADEX 10.8 mg implant administered every 12 weeks. | |||
* The automatic safety feature of the syringe aids in the prevention of needlestick injury. | |||
======Dosage====== | |||
* For the management of [[Prostate cancer|advanced prostate cancer]], ZOLADEX is intended for long-term administration unless clinically inappropriate. | |||
======Renal or Hepatic Impairment====== | |||
* No dosage adjustment is necessary for patients with renal or hepatic impairment. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
{{ | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=* [[Breast cancer]], Adjuvant treatment of hormone receptor-positive, axillary lymph node-positive disease in premenopausal women<ref name="pmid19821328">{{cite journal| author=Goel S, Sharma R, Hamilton A, Beith J| title=LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. | journal=Cochrane Database Syst Rev | year= 2009 | volume= | issue= 4 | pages= CD004562 | pmid=19821328 | doi=10.1002/14651858.CD004562.pub4 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19821328 }} </ref><ref name="pmid19244174">{{cite journal| author=Hackshaw A, Baum M, Fornander T, Nordenskjold B, Nicolucci A, Monson K et al.| title=Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer. | journal=J Natl Cancer Inst | year= 2009 | volume= 101 | issue= 5 | pages= 341-9 | pmid=19244174 | doi=10.1093/jnci/djn498 | pmc=PMC2650713 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19244174 }} </ref> | |||
* [[Dysfunctional uterine bleeding]]<ref name="pmid8445603">{{cite journal| author=Vercellini P, Fedele L, Maggi R, Vendola N, Bocciolone L, Colombo A| title=Gonadotropin releasing hormone agonist for chronic anovulatory uterine bleeding and severe anemia. | journal=J Reprod Med | year= 1993 | volume= 38 | issue= 2 | pages= 127-9 | pmid=8445603 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8445603 }} </ref> | |||
* [[In vitro fertilization]]<ref name="pmid8199101">{{cite journal| author=Tapanainen JS, Hovatta O| title=Pituitary down-regulation with goserelin (Zoladex) for in vitro fertilisation. | journal=Br J Obstet Gynaecol | year= 1994 | volume= 101 Suppl 10 | issue= | pages= 27-8 | pmid=8199101 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8199101 }} </ref> | |||
* [[Precocious puberty]]<ref name="pmid10325702">{{cite journal| author=de Brito VN, Latronico AC, Arnhold IJ, Lo LS, Domenice S, Albano MC et al.| title=Treatment of gonadotropin dependent precocious puberty due to hypothalamic hamartoma with gonadotropin releasing hormone agonist depot. | journal=Arch Dis Child | year= 1999 | volume= 80 | issue= 3 | pages= 231-4 | pmid=10325702 | doi= | pmc=PMC1717869 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10325702 }} </ref> | |||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
< | <!--Guideline-Supported Use (Pediatric)--> | ||
{{ | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
| | |||
| image = Goserelin. | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications======Hypersensitivity===== | |||
* [[Anaphylactic reactions]] to ZOLADEX have been reported in the medical literature. ZOLADEX is contraindicated in those patients who have a known [[hypersensitivity]] to GnRH, GnRH agonist analogues or any of the components in ZOLADEX. | |||
=====Pregnancy===== | |||
* Expected hormonal changes that occur with ZOLADEX treatment increase the risk for pregnancy loss. ZOLADEX may cause fetal harm when administered to a pregnant woman. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus | |||
|warnings======Tumor Flare Phenomenon===== | |||
* Initially, ZOLADEX, like other GnRH agonists, causes transient increases in serum levels of [[testosterone]]. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of [[Prostate cancer|prostatic cancer]], may occasionally develop during the first few weeks of ZOLADEX treatment. A small number of patients may experience a temporary increase in [[bone pain]], which can be managed symptomatically. As with other [[GnRH agonists]], isolated cases of [[ureteral obstruction]] and [[spinal cord compression]] have been observed. If spinal cord compression or renal impairment secondary to [[ureteral obstruction]] develops, standard treatment of these complications should be instituted, and in extreme cases an immediate [[orchiectomy]]. | |||
=====Hypersensitivity===== | |||
* [[Hypersensitivity]], antibody formation and [[acute anaphylactic reactions]] have been reported with GnRH agonist analogues. | |||
* Of 115 women worldwide treated with ZOLADEX and tested for development of binding to goserelin following treatment with ZOLADEX, one patient showed low-titer binding to goserelin. On further testing of this patient's plasma obtained following treatment, her goserelin binding component was found not to be precipitated with rabbit antihuman immunoglobulin polyvalent sera. These findings suggest the possibility of antibody formation. | |||
=====Hyperglycemia and Diabetes===== | |||
* [[Hyperglycemia]] and an increased risk of developing [[diabetes]] have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or [[glycosylated hemoglobin]] (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes. | |||
=====Cardiovascular Diseases===== | |||
* Increased risk of developing [[myocardial infarction]], sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice. | |||
=====Effect on QT/QTc Interval===== | |||
* Androgen deprivation therapy may prolong the [[QT/QTc interval]]. Providers should consider whether the benefits of [[androgen deprivation]] therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes. | |||
|clinicalTrials======Clinical Trials===== | |||
* ZOLADEX has been found to be generally well tolerated in clinical trials. Adverse reactions reported in these trials were rarely severe enough to result in the patients' withdrawal from ZOLADEX treatment. As seen with other hormonal therapies, the most commonly observed adverse events during ZOLADEX therapy were due to the expected physiological effects from decreased [[testosterone]] levels. These included [[hot flashes]], [[sexual dysfunction]] and [[decreased erections]]. | |||
* [[Tumor Flare Phenomenon]]: Initially, ZOLADEX, like other GnRH agonists, causes transient increases in serum levels of testosterone. A small percentage of patients experienced a temporary worsening of signs and symptoms, usually manifested by an increase in cancer-related pain which was managed symptomatically. Isolated cases of exacerbation of disease symptoms, either ureteral obstruction or spinal cord compression, occurred at similar rates in controlled clinical trials with both ZOLADEX and orchiectomy. The relationship of these events to therapy is uncertain | |||
=====Stage B2-C Prostatic Carcinoma===== | |||
* Treatment with ZOLADEX and flutamide did not add substantially to the toxicity of radiation treatment alone. The following adverse experiences were reported during a multicenter clinical trial comparing ZOLADEX + flutamide + radiation versus radiation alone. The most frequently reported (greater than 5%) adverse experiences are listed below: | |||
: [[File:Goserelin Adv effects table 1 and 2.png|none|500px]] | |||
* Additional adverse event data was collected for the combination therapy with radiation group over both the hormonal treatment and hormonal treatment plus radiation phases of the study. Adverse experiences occurring in more than 5% of patients in this group, over both parts of the study, were [[hot flashes]] (46%), [[diarrhea]] (40%), [[nausea]] (9%), and [[skin rash]] (8%). | |||
=====Prostatic Carcinoma===== | |||
* Two controlled clinical trials using ZOLADEX 10.8 mg versus ZOLADEX 3.6 mg were conducted. During a comparative phase, patients were randomized to receive either a single 10.8 mg implant or three consecutive 3.6 mg implants every 4 weeks over weeks 0-12. During this phase, the only adverse event reported in greater than 5% of patients was hot flashes, with an incidence of 47% in the ZOLADEX 10.8 mg group and 48% in the ZOLADEX 3.6 mg group. | |||
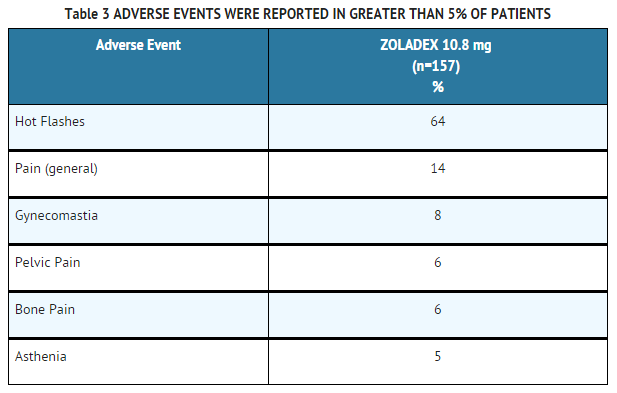
* From weeks 12-48 all patients were treated with a 10.8 mg implant every 12 weeks. During this noncomparative phase, the following adverse events were reported in greater than 5% of patients: | |||
: [[File:Goserelin adv effect tab 3.png|none|500px]] | |||
The following adverse events were reported in greater than 1%, but less than 5% of patients treated with ZOLADEX 10.8 mg implant every 12 weeks. Some of these are commonly reported in elderly patients. | |||
======WHOLE BODY====== | |||
* [[Abdominal pain]] | |||
* [[Back pain]] | |||
* [[Flu syndrome]] | |||
* [[Headache]] | |||
* [[Sepsis]] | |||
* Aggravation reaction | |||
======CARDIOVASCULAR====== | |||
* [[Angina pectoris]] | |||
* [[Cerebral ischemia]] | |||
* [[Cerebrovascular accident]] | |||
* [[Heart failure]] | |||
* [[Pulmonary embolus]] | |||
* [[Varicose veins]] | |||
======DIGESTIVE====== | |||
* [[Diarrhea]] | |||
* [[Hematemesis]] | |||
======ENDOCRINE====== | |||
* [[Diabetes mellitus]] | |||
======HEMATOLOGIC====== | |||
* [[Anemia]] | |||
======METABOLIC====== | |||
* [[Peripheral edema]] | |||
======NERVOUS SYSTEM====== | |||
* [[Dizziness]] | |||
* [[Paresthesia]] | |||
* [[Urinary retention]] | |||
======RESPIRATORY====== | |||
* [[Cough increased]] | |||
* [[Dyspnea]] | |||
* [[Pneumonia]] | |||
======SKIN====== | |||
* [[Herpes simplex]] | |||
* [[Pruritus]] | |||
======UROGENITAL===== | |||
* [[Bladder neoplasm]] | |||
* [[Breast pain]] | |||
* [[Hematuria]] | |||
* [[Impotence]] | |||
* [[Urinary frequency]] | |||
* [[Urinary incontinence]] | |||
* [[Urinary tract disorder]] | |||
* [[Urinary tract infection]] | |||
* Urination impaired | |||
* The following adverse events not already listed above were reported in patients receiving ZOLADEX 3.6 mg in other clinical trials. Inclusion does not necessarily represent a causal relationship to ZOLADEX 10.8 mg. | |||
======WHOLE BODY====== | |||
* [[Allergic reaction]] | |||
* [[Chills]] | |||
* [[Fever]] | |||
* [[Infection]] | |||
* [[Injection site reaction]] | |||
* [[Lethargy]] | |||
* [[Malaise]] | |||
======CARDIOVASCULAR====== | |||
* [[Arrhythmia]] | |||
* [[Chest pain]] | |||
* [[Hemorrhage]] | |||
* [[Hypertension]] | |||
* [[Migraine]] | |||
* [[Myocardial infarction]] | |||
* [[Palpitations]] | |||
* [[Peripheral vascular disorder]] | |||
* [[Tachycardia]] | |||
======DIGESTIVE====== | |||
* [[Anorexia]] | |||
* [[Constipation]] | |||
* [[Dry mouth]] | |||
* [[Dyspepsia]] | |||
* [[Flatulence]] | |||
* Increased appetite | |||
* [[Nausea]] | |||
* [[Ulcer]] | |||
* [[Vomiting]] | |||
======HEMATOLOGIC====== | |||
* [[Ecchymosis]] | |||
======METABOLIC====== | |||
* [[Edema]] | |||
* [[Gout]] | |||
* [[Hyperglycemia]] | |||
* Weight increase | |||
======MUSCULOSKELETAL====== | |||
* [[Arthralgia]] | |||
* [[Hypertonia]] | |||
* [[Joint disorder]] | |||
* [[Leg cramps]] | |||
* [[Myalgia]] | |||
* [[Osteoporosis]] | |||
======NERVOUS SYSTEM====== | |||
* [[Anxiety]] | |||
* [[Depression]] | |||
* [[Emotional lability]] | |||
* [[Headache]] | |||
* [[Insomnia]] | |||
* [[Nervousness]] | |||
* [[Somnolence]] | |||
* Thinking abnormal | |||
======RESPIRATORY====== | |||
* [[Bronchitis]] | |||
* [[Chronic obstructive pulmonary disease]] | |||
* [[Epistaxis]] | |||
* [[Rhinitis]] | |||
* [[Sinusitis]] | |||
* [[Upper respiratory infection]] | |||
* Voice alterations | |||
======SKIN====== | |||
* [[Acne]] | |||
* [[Alopecia]] | |||
* [[Dry skin]] | |||
* Hair disorders | |||
* [[Rash]] | |||
* [[Seborrhea]] | |||
* Skin discoloration | |||
* [[Sweating]] | |||
======SPECIAL SENSES====== | |||
* [[Amblyopia]] | |||
* [[Dry eyes]] | |||
======UROGENITAL====== | |||
* [[Breast tenderness]] | |||
* [[Decreased erections]] | |||
* [[Renal insufficiency]] | |||
* [[Sexual dysfunction]] | |||
* [[Urinary obstruction]] | |||
======Changes in Laboratory Values During Treatment====== | |||
* Plasma Enzymes: Elevation of liver enzymes ([[AST]], [[ALT]]) have been reported in female patients exposed to ZOLADEX 3.6 mg (representing less than 1% of all patients). There was no other evidence of abnormal liver function. Causality between these changes and ZOLADEX have not been established. | |||
* Lipids: In a controlled trial in females, ZOLADEX 3.6 mg implant therapy resulted in a minor, but statistically significant effect on serum lipids (i.e., increases in LDL cholesterol of 21.3 mg/dL; increases in HDL cholesterol of 2.7 mg/dL; and triglycerides increased by 8.0 mg/dL). | |||
|postmarketing=* The following adverse reactions have been identified during post-approval use of ZOLADEX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
======Hypercalcemia====== | |||
* In patients with bone metastases. | |||
======Bone Mineral Density====== | |||
* [[Osteoporosis]], decreased bone mineral density and [[bony fracture]] in men. | |||
======Changes in Blood Pressure====== | |||
* [[Hypotension]] and [[hypertension]] have been reported. These changes are usually transient, resolving either during continued therapy or after cessation of therapy. | |||
======Pituitary Apoplexy and Tumors====== | |||
* [[Pituitary apoplexy]] (a clinical syndrome secondary to infarction of the [[pituitary gland]]) and [[pituitary adenoma]] have been diagnosed. Most of the pituitary apoplexy cases occurred within 2 weeks of the first dose, and some occurred within the first hour. In these cases, pituitary apoplexy has presented as sudden [[headache]], [[vomiting]], [[visual changes]], [[ophthalmoplegia]], altered mental status, and sometimes [[cardiovascular collapse]]. Immediate medical attention has been required. Pituitary tumors have been reported. | |||
======Acne====== | |||
* Usually within one month of starting treatment. | |||
======Other Adverse Reactions====== | |||
* Psychotic disorders, [[convulsions]] and [[mood swings]]. | |||
|drugInteractions=* No formal drug-drug interaction studies have been performed. | |||
* No drug interaction studies with other drugs have been conducted with ZOLADEX. No confirmed interactions have been reported between ZOLADEX and other drugs. | |||
=====Drug/Laboratory Test Interactions===== | |||
* Administration of ZOLADEX in therapeutic doses results in suppression of the pituitary-gonadal system. Because of this suppression, diagnostic tests of pituitary-gonadotropic and gonadal functions conducted during treatment may show results which are misleading. | |||
|FDAPregCat=X | |||
|useInPregnancyFDA=* Based on mechanism of action in humans and findings of increased pregnancy loss in animal studies, ZOLADEX may cause fetal harm when administered to a pregnant woman. Expected hormone changes that occur with ZOLADEX treatment increase the risk for pregnancy loss. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. | |||
* ZOLADEX crosses the placenta in rats and rabbits following subcutaneous administration. Administration of goserelin to pregnant rats and rabbits during organogenesis resulted in increased preimplantation loss and increased resorptions. When pregnant rats received goserelin throughout gestation and lactation, there was a dose-related increase in umbilical hernia in offspring. In additional reproduction studies in rats, goserelin decreased fetus and pup survival. Human dose/exposure multiples could not be calculated from available animal data. | |||
* Actual animal doses: rat (≥ 2 mcg/kg/day for pregnancy loss; ≥ 10 mcg/kg/day for umbilical hernia in offspring); rabbits (> 20 mcg/kg/day). | |||
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known if goserelin is excreted in human milk. Goserelin is excreted in the milk of lactating rats. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ZOLADEX, a decision should be made to either discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=* Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri=* There is no need for any dosage adjustment when administering ZOLADEX 10.8 mg to geriatric patients. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=* In clinical trials with the solution formulation of goserelin, subjects with impaired renal function (creatinine clearance < 20 mL/min) had a serum elimination half-life of 12.1 hours compared to 4.2 hours for subjects with normal renal function (creatinine clearance > 70 mL/min). However, there was no evidence for any accumulation of goserelin on multiple dosing of the ZOLADEX 10.8 mg depot to subjects with impaired renal function. There was no evidence for any increase in incidence of adverse events in renally impaired patients administered the 10.8 mg depot. These data indicate that there is no need for any dosage adjustment when administering ZOLADEX 10.8 mg to subjects with impaired renal function. | |||
|useInHepaticImpair=* The total body clearances and serum elimination half-lives were similar between normal subjects and patients with moderate hepatic impairment (alanine transaminase < 3xULN and asparate aminotransferase < 3xULN) when treated with a 250 mcg subcutaneous formulation of goserelin. This pharmacokinetic study indicates that no dose adjustment is needed in patients with moderately impaired liver function. There is no pharmacokinetic data with goserelin in patients with severe hepatic insufficiency. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|othersTitle=Body Weight | |||
|useInOthers=* A decline of approximately 1 to 2.5% in the AUC after administration of a 10.8 mg depot was observed with a kilogram increase in body weight. In obese patients who have not responded clinically, testosterone levels should be monitored closely. | |||
|administration=* ZOLADEX, at a dose of 10.8 mg, should be administered subcutaneously every 12 weeks into the anterior abdominal wall below the navel line using an aseptic technique under the supervision of a physician. | |||
* While a delay of a few days is permissible, every effort should be made to adhere to the 12-week schedule | |||
=====Administration Technique===== | |||
* The proper method of administration of ZOLADEX is described in the instructions that follow. | |||
:* Put the patient in a comfortable position with the upper part of the body slightly raised. Prepare an area of the anterior abdominal wall below the navel line with an alcohol swab. | |||
:* Examine the foil pouch and syringe for damage. Remove the syringe from the opened foil pouch and hold the syringe at a slight angle to the light. Check that at least part of the ZOLADEX implant is visible. | |||
:* Grasp the blue plastic safety tab and pull away from the syringe, and discard. Remove needle cover. Unlike liquid injections, there is no need to remove air bubbles as attempts to do so may displace the ZOLADEX implant. | |||
:* Holding the syringe around the protective sleeve, using an aseptic technique, pinch the skin of the patient’s anterior abdominal wall below the navel line. With the bevel of the needle facing up, insert the needle at a 30 to 45 degree angle to the skin in one continuous deliberate motion until the protective sleeve touches the patient’s skin. | |||
* NOTE: The ZOLADEX syringe cannot be used for aspiration. If the hypodermic needle penetrates a large vessel, blood will be seen instantly in the syringe chamber. If a vessel is penetrated, withdraw the needle and inject with a new syringe elsewhere. | |||
:* Do not penetrate into muscle or peritoneum. | |||
:* To administer the ZOLADEX implant and to activate the protective sleeve, grasp the barrel at the finger grip and depress the plunger until you cannot depress it any further. If the plunger is not depressed fully, the protective sleeve will NOT activate. When the protective sleeve ‘clicks’, the protective sleeve will automatically begin to slide to cover the needle. | |||
* NOTE: The needle does not retract. | |||
:* Withdraw the needle and allow protective sleeve to slide and cover needle. Dispose of the syringe in an approved sharps collector. | |||
* NOTE: In the unlikely event of the need to surgically remove ZOLADEX, it may be localized by ultrasound. | |||
|monitoring=* Transient worsening of tumor symptoms may occur during the first few weeks of treatment with ZOLADEX, which may include ureteral obstruction and spinal cord compression. Monitor patients at risk for complications of tumor flare | |||
* Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH analogs. Monitor blood glucose level and manage according to current clinical practice. | |||
* Increased risk of myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH analogs in men. Monitor for cardiovascular disease and manage according to current clinical practice | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose=* The pharmacologic properties of ZOLADEX and its mode of administration make accidental or intentional overdosage unlikely. There is no experience of overdosage from clinical trials. Animal studies indicate that no increased pharmacologic effect occurred at higher doses or more frequent administration. Subcutaneous doses of the drug as high as 1 mg/kg/day in rats and dogs did not produce any nonendocrine related sequelae; this dose is up to 250 times the estimated human daily dose based on the body surface area. If overdosage occurs, it should be managed symptomatically. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 459437828 | |||
| IUPAC_name = N-(21-((1H-indol-3-yl)methyl)-1,1-diamino-12-(tert-butoxymethyl)-6-(2-(2-carbamoylhydrazinecarbonyl)cyclopentanecarbonyl)-15-(4-hydroxybenzyl)-18-(hydroxymethyl)-25-(1H-imidazol-5-yl)-9-isobutyl-8,11,14,17,20,23-hexaoxo-2,7,10,13,16,19,22-heptaazapentacos-1-en-24-yl)-5-oxopyrrolidine-2-carboxamide | |||
| image = Goserelin wiki str.png | |||
| image2 = Goserelin wiki str 2.png | |||
<!--Clinical data--> | |||
| tradename = Zoladex | |||
| Drugs.com = {{drugs.com|monograph|zoladex}} | |||
| MedlinePlus = a601002 | |||
| pregnancy_category = D (3.6mg) / X (10.8mg) ([[United States|USA]]) | |||
| legal_status = Rx-only | |||
| routes_of_administration = [[Implant (medicine)|implant]] | |||
<!--Pharmacokinetic data--> | |||
| protein_bound = 27.3% | |||
| elimination_half-life = 4-5 hours | |||
| excretion = renal | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 65807-02-5 | | CAS_number = 65807-02-5 | ||
| ATC_prefix = L02 | | ATC_prefix = L02 | ||
| ATC_suffix =AE03 | | ATC_suffix = AE03 | ||
| PubChem = | | PubChem = 5311128 | ||
| DrugBank = | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| C=59 | H=84| N=18| O=14 | | DrugBank = DB00014 | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4470656 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 0F65R8P09N | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D04405 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1201247 | |||
<!--Chemical data--> | |||
| C=59 | H=84 | N=18 | O=14 | |||
| molecular_weight = 1269.410 g/mol | | molecular_weight = 1269.410 g/mol | ||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChI = 1S/C59H84N18O14/c1-31(2)22-40(49(82)68-39(12-8-20-64-57(60)61)56(89)77-21-9-13-46(77)55(88)75-76-58(62)90)69-54(87)45(29-91-59(3,4)5)74-50(83)41(23-32-14-16-35(79)17-15-32)70-53(86)44(28-78)73-51(84)42(24-33-26-65-37-11-7-6-10-36(33)37)71-52(85)43(25-34-27-63-30-66-34)72-48(81)38-18-19-47(80)67-38/h6-7,10-11,14-17,26-27,30-31,38-46,65,78-79H,8-9,12-13,18-25,28-29H2,1-5H3,(H,63,66)(H,67,80)(H,68,82)(H,69,87)(H,70,86)(H,71,85)(H,72,81)(H,73,84)(H,74,83)(H,75,88)(H4,60,61,64)(H3,62,76,90)/t38-,39-,40-,41-,42-,43-,44-,45+,46-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}}= {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = BLCLNMBMMGCOAS-URPVMXJPSA-N | |||
| synonyms = D-Ser(But)<sup>6</sup>Azgly<sup>10</sup>LHRH | | synonyms = D-Ser(But)<sup>6</sup>Azgly<sup>10</sup>LHRH | ||
}} | }} | ||
== | |mechAction=* ZOLADEX is a synthetic decapeptide analogue of GnRH. ZOLADEX acts as an inhibitor of pituitary gonadotropin secretion when administered in the biodegradable formulation. | ||
* In animal and in vitro studies, administration of goserelin resulted in the regression or inhibition of growth of the hormonally sensitive dimethylbenzanthracene (DMBA)-induced rat mammary tumor and Dunning R3327 prostate tumor. | |||
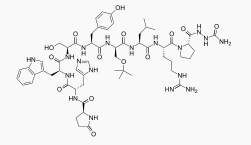
|structure=* ZOLADEX® (goserelin acetate implant) is a GnRH agonist. Goserelin acetate is chemically described as an acetate salt of [D-Ser(But)6,Azgly10]. Its chemical structure is pyro-Glu-His-Trp-Ser-Tyr-D-Ser(But)-Leu-Arg-Pro-Azgly-NH2 acetate [C59H84N18O14 ·(C2H4O2)x where x = 1 to 2.4] | |||
|PD=* Following initial administration, ZOLADEX causes an initial increase in serum [[luteinizing hormone]] (LH) and [[follicle stimulating hormone]] (FSH) levels with subsequent increases in serum levels of [[testosterone]]. Chronic administration of ZOLADEX leads to sustained suppression of pituitary gonadotropins, and serum levels of testosterone consequently fall into the range normally seen in surgically castrated men approximately 21 days after initiation of therapy. This leads to accessory sex organ regression. | |||
* In clinical trials using ZOLADEX 3.6 mg with follow-up of more than 2 years, suppression of serum testosterone to castrate levels has been maintained for the duration of therapy. | |||
|PK======Absorption===== | |||
* The pharmacokinetics of ZOLADEX have been determined in healthy male volunteers and patients. In healthy males, radiolabeled goserelin was administered as a single 250 mcg (aqueous solution) dose by the subcutaneous route. The absorption of radiolabeled drug was rapid, and the peak blood radioactivity levels occurred between 0.5 and 1.0 hour after dosing. | |||
* The overall pharmacokinetic profile of goserelin following administration of a ZOLADEX 10.8 mg depot to patients with prostate cancer was determined. The initial release of goserelin from the depot was relatively rapid resulting in a peak concentration at 2 hours after dosing. From Day 4 until the end of the 12-week dosing interval, the sustained release of goserelin from the depot produced reasonably stable systemic exposure. Mean (Standard Deviation) pharmacokinetic data are presented in Table 4. There is no clinically significant accumulation of goserelin following administration of four depots administered at 12-week intervals. | |||
: [[File:Goserelin PK table 4.png|none|500px]] | |||
* SD = standard deviation | |||
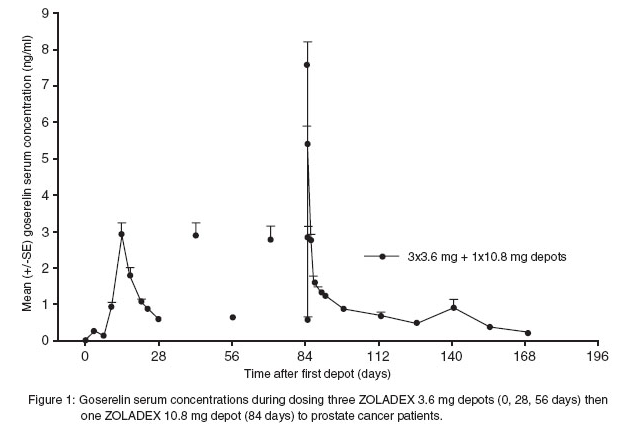
* Serum goserelin concentrations in prostate cancer patients administered three 3.6 mg depots followed by one 10.8 mg depot are displayed in Figure 1. The profiles for both formulations are primarily dependent upon the rate of drug release from the depots. For the 3.6 mg depot, mean concentrations gradually rise to reach a peak of about 3 ng/mL at around 15 days after administration and then decline to approximately 0.5 ng/mL by the end of the treatment period. For the 10.8 mg depot, mean concentrations increase to reach a peak of about 8 ng/mL within the first 24 hours and then decline rapidly up to Day 4. Thereafter, mean concentrations remain relatively stable in the range of about 0.3 to 1 ng/mL up to the end of the treatment period. | |||
: [[File:Goserelin PK figure 1.png|none|500px]] | |||
* Administration of four ZOLADEX 10.8 mg depots to patients with prostate cancer resulted in testosterone levels that were suppressed to and maintained within the range normally observed in surgically castrated men (0 – 1.73 nmol/L or 0-50 ng/dL), over the dosing interval in approximately 91% (145/160) of patients studied. In 6 of 15 patients that escaped from castrate range, serum testosterone levels were maintained below 2.0 nmol/L (58 ng/dL) and in only one of the 15 patients did the depot completely fail to maintain serum testosterone levels to within the castrate range over a 336-day period (4 depot injections). In the 8 additional patients, a transient escape was followed 14 days later by a level within the castrate range. | |||
=====Distribution===== | |||
* The apparent volume of distribution determined after subcutaneous administration of 250 mcg aqueous solution of goserelin was 44.1 ± 13.6 liters for healthy males. The plasma protein binding of goserelin was found to be 27%. | |||
=====Metabolism===== | |||
* Metabolism of goserelin, by hydrolysis of the C-terminal amino acids, is the major clearance mechanism. The major circulating component in serum appeared to be 1–7 fragment, and the major component present in urine of one healthy male volunteer was 5-10 fragment. The metabolism of goserelin in humans yields a similar but narrow profile of metabolites to that found in other species. All metabolites found in humans have also been found in toxicology species. | |||
=====Excretion===== | |||
* Clearance of goserelin following subcutaneous administration of a radiolabeled solution of goserelin was very rapid and occurred via a combination of hepatic and urinary excretion. More than 90% of a subcutaneous radiolabeled solution formulation dose of goserelin was excreted in urine. Approximately 20% of the dose recovered in urine was accounted for by unchanged goserelin. | |||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
* Subcutaneous implantation of goserelin in male and female rats once every 4 weeks for 1 year and recovery for 23 weeks at doses of about 80 and 150 mcg/kg (males) and 50 and 100 mcg/kg (females) daily resulted in an increased incidence of pituitary adenomas. An increased incidence of pituitary adenomas was also observed following subcutaneous implant of goserelin in rats at similar dose levels for a period of 72 weeks in males and 101 weeks in females. The relevance of the rat pituitary adenomas to humans has not been established. Subcutaneous implants of goserelin every 3 weeks for 2 years delivered to mice at doses of up to 2400 mcg/kg/day resulted in an increased incidence of histiocytic sarcoma of the vertebral column and femur. Human dose/exposure multiples could not be calculated from available animal data. | |||
* Mutagenicity tests using bacterial and mammalian systems for point mutations and cytogenetic effects have provided no evidence for mutagenic potential. | |||
* Administration of goserelin led to changes that were consistent with gonadal suppression in both male and female rats as a result of its endocrine action. In male rats administered 500-1000 mcg/kg/day, a decrease in weight and atrophic histological changes were observed in the [[testes]], [[epididymis]], [[seminal vesicle]] and prostate gland with complete suppression of [[spermatogenesis]]. In female rats administered 50-1000 mcg/kg/day, suppression of ovarian function led to decreased size and weight of ovaries and secondary sex organs; follicular development was arrested at the antral stage and the corpora lutea were reduced in size and number. Except for the testes, almost complete histologic reversal of these effects in males and females was observed several weeks after dosing was stopped; however, fertility and general reproductive performance were reduced in those that became pregnant after goserelin was discontinued. Fertile matings occurred within 2 weeks after cessation of dosing, even though total recovery of reproductive function may not have occurred before mating took place; and, the ovulation rate, the corresponding implantation rate, and number of live fetuses were reduced. | |||
* Based on histological examination, drug effects on reproductive organs were reversible in male and female dogs administered 107-214 mcg/kg/day goserelin when drug treatment was stopped after continuous administration for 1 year. Human dose/exposure multiples could not be calculated from available animal data. | |||
|clinicalStudies======Stage B2-C Prostatic Carcinoma===== | |||
* The effects of hormonal treatment combined with radiation were studied in 466 patients (231 ZOLADEX + flutamide + radiation, 235 radiation alone) with bulky primary tumors confined to the prostate (stage B2) or extending beyond the capsule (stage C), with or without pelvic node involvement. | |||
* In this multicentered, controlled trial, administration of ZOLADEX (3.6 mg depot) and flutamide capsules (250 mg t.i.d.) prior to and during radiation was associated with a significantly lower rate of local failure compared to radiation alone (16% vs 33% at 4 years, P<0.001). The combination therapy also resulted in a trend toward reduction in the incidence of distant metastases (27% vs 36% at 4 years, P =0.058). Median disease-free survival was significantly increased in patients who received complete hormonal therapy combined with radiation as compared to those patients who received radiation alone (4.4 vs 2.6 years, P<0.001). Inclusion of normal PSA level as a criterion for disease-free survival also resulted in significantly increased median disease-free survival in patients receiving the combination therapy (2.7 vs 1.5 years, P<0.001). | |||
=====Prostatic Carcinoma===== | |||
* In two controlled clinical trials, 160 patients with advanced prostate cancer were randomized to receive either one 3.6 mg ZOLADEX implant every four weeks or a single 10.8 mg ZOLADEX implant every 12 weeks. Mean serum testosterone suppression was similar between the two arms. PSA falls at three months were 94% in patients who received the 10.8 mg implant and 92.5% in patients that received three 3.6 mg implants. | |||
* Periodic monitoring of serum testosterone levels should be considered if the anticipated clinical or biochemical response to treatment has not been achieved. A clinical outcome similar to that produced with the use of the 3.6 mg implant administered every 28 days is predicted with ZOLADEX 10.8 mg implant administered every 12 weeks (84 days). Total testosterone was measured by the DPC Coat-A-Count radioimmunoassay method which, as defined by the manufacturers, is highly specific and accurate. Acceptable variability of approximately 20% at low testosterone levels has been demonstrated in the clinical studies performed with the ZOLADEX 10.8 mg depot. | |||
|howSupplied=* ZOLADEX 10.8 mg implant is supplied as a sterile and totally biodegradable D,L-lactic and glycolic acids copolymer (12.82-14.76 mg/dose) impregnated with goserelin acetate equivalent to 10.8 mg of goserelin in a disposable syringe device fitted with a 14-gauge x 36 +/- 0.5 mm siliconized hypodermic needle with protective sleeve [SafeSystem™ Syringe] (NDC 0310-0951-30). The unit is sterile and comes in a sealed, light- and moisture-proof, aluminum foil laminate pouch containing a desiccant capsule. | |||
|storage=* Store at room temperature (do not exceed 25°C [77°F]). | |||
|packLabel======PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 10.8 MG 3-MONTH===== | |||
: [[File:Goserelin PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====INGREDIENTS AND APPEARANCE===== | |||
: [[File:Goserelin Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo======Males===== | |||
* The use of ZOLADEX in patients at particular risk of developing ureteral obstruction or spinal cord compression should be considered carefully and the patients monitored closely during the first month of therapy. Patients with ureteral obstruction or spinal cord compression should have appropriate treatment prior to initiation of ZOLADEX therapy. | |||
* The use of GnRH agonists may cause a reduction in bone mineral density. In men, data suggest the use of a bisphosphonate in combination with a GnRH agonist may reduce bone mineral loss. | |||
* Patients should be informed that diabetes, or loss of glycemic control in patients with pre-existing [[diabetes]], has been reported during treatment with GnRH agonists, including ZOLADEX. Therefore, consideration should be given to monitoring blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving ZOLADEX. | |||
* A small increased risk of developing [[myocardial infarction]], [[sudden cardiac death]] and [[stroke]] has been reported in association with use of GnRH agonists in men. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames=* Zoladex®<ref>{{Cite web | title = Goserelin acetate | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4ff62a5a-77ba-4bf5-0497-95ccca842315 }}</ref> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Drug]] | |||
[[Category: | [[Category:Chemotherapeutic agents]] | ||
[[Category: | |||
Latest revision as of 20:26, 6 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Goserelin is a Gonadotropin-releasing Hormone (GnRH) agonist that is FDA approved for the treatment of locally confined carcinoma of the prostate, and palliative treatment of advanced carcinoma of the prostate. Common adverse reactions include hot flashes, sexual dysfunction, decreased erections and lower urinary tract symptoms.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Stage B2-C Prostatic Carcinoma
- ZOLADEX is indicated for use in combination with flutamide for the management of locally confined Stage T2b-T4 (Stage B2-C) carcinoma of the prostate. Treatment with ZOLADEX and flutamide should start 8 weeks prior to initiating radiation therapy and continue during radiation therapy
Dosage
- When ZOLADEX is given in combination with radiotherapy and flutamide for patients with Stage T2b-T4 (Stage B2-C) prostatic carcinoma, treatment should be started 8 weeks prior to initiating radiotherapy and should continue during radiation therapy. A treatment regimen using one ZOLADEX 3.6 mg depot, followed in 28 days by one ZOLADEX 10.8 mg depot, should be administered.
Prostatic Carcinoma
- ZOLADEX is indicated in the palliative treatment of advanced carcinoma of the prostate.
- In controlled studies of patients with advanced prostatic cancer comparing ZOLADEX 3.6 mg to orchiectomy, the long-term endocrine responses and objective responses were similar between the two treatment arms. Additionally, duration of survival was similar between the two treatment arms in a major comparative trial.
- In controlled studies of patients with advanced prostatic cancer, ZOLADEX 10.8 mg implant produced pharmacodynamically similar effect in terms of suppression of serum testosterone to that achieved with ZOLADEX 3.6 mg implant. Clinical outcome similar to that produced with the use of the ZOLADEX 3.6 mg implant administered every 28 days is predicted with the ZOLADEX 10.8 mg implant administered every 12 weeks.
- The automatic safety feature of the syringe aids in the prevention of needlestick injury.
Dosage
- For the management of advanced prostate cancer, ZOLADEX is intended for long-term administration unless clinically inappropriate.
Renal or Hepatic Impairment
- No dosage adjustment is necessary for patients with renal or hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Goserelin in adult patients.
Non–Guideline-Supported Use
- Breast cancer, Adjuvant treatment of hormone receptor-positive, axillary lymph node-positive disease in premenopausal women[1][2]
- Dysfunctional uterine bleeding[3]
- In vitro fertilization[4]
- Precocious puberty[5]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Goserelin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Goserelin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Goserelin in pediatric patients.
Contraindications
Hypersensitivity
- Anaphylactic reactions to ZOLADEX have been reported in the medical literature. ZOLADEX is contraindicated in those patients who have a known hypersensitivity to GnRH, GnRH agonist analogues or any of the components in ZOLADEX.
Pregnancy
- Expected hormonal changes that occur with ZOLADEX treatment increase the risk for pregnancy loss. ZOLADEX may cause fetal harm when administered to a pregnant woman. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus
Warnings
Tumor Flare Phenomenon
- Initially, ZOLADEX, like other GnRH agonists, causes transient increases in serum levels of testosterone. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of prostatic cancer, may occasionally develop during the first few weeks of ZOLADEX treatment. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically. As with other GnRH agonists, isolated cases of ureteral obstruction and spinal cord compression have been observed. If spinal cord compression or renal impairment secondary to ureteral obstruction develops, standard treatment of these complications should be instituted, and in extreme cases an immediate orchiectomy.
Hypersensitivity
- Hypersensitivity, antibody formation and acute anaphylactic reactions have been reported with GnRH agonist analogues.
- Of 115 women worldwide treated with ZOLADEX and tested for development of binding to goserelin following treatment with ZOLADEX, one patient showed low-titer binding to goserelin. On further testing of this patient's plasma obtained following treatment, her goserelin binding component was found not to be precipitated with rabbit antihuman immunoglobulin polyvalent sera. These findings suggest the possibility of antibody formation.
Hyperglycemia and Diabetes
- Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
Cardiovascular Diseases
- Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
Effect on QT/QTc Interval
- Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Adverse Reactions
Clinical Trials Experience
Clinical Trials
- ZOLADEX has been found to be generally well tolerated in clinical trials. Adverse reactions reported in these trials were rarely severe enough to result in the patients' withdrawal from ZOLADEX treatment. As seen with other hormonal therapies, the most commonly observed adverse events during ZOLADEX therapy were due to the expected physiological effects from decreased testosterone levels. These included hot flashes, sexual dysfunction and decreased erections.
- Tumor Flare Phenomenon: Initially, ZOLADEX, like other GnRH agonists, causes transient increases in serum levels of testosterone. A small percentage of patients experienced a temporary worsening of signs and symptoms, usually manifested by an increase in cancer-related pain which was managed symptomatically. Isolated cases of exacerbation of disease symptoms, either ureteral obstruction or spinal cord compression, occurred at similar rates in controlled clinical trials with both ZOLADEX and orchiectomy. The relationship of these events to therapy is uncertain
Stage B2-C Prostatic Carcinoma
- Treatment with ZOLADEX and flutamide did not add substantially to the toxicity of radiation treatment alone. The following adverse experiences were reported during a multicenter clinical trial comparing ZOLADEX + flutamide + radiation versus radiation alone. The most frequently reported (greater than 5%) adverse experiences are listed below:
- Additional adverse event data was collected for the combination therapy with radiation group over both the hormonal treatment and hormonal treatment plus radiation phases of the study. Adverse experiences occurring in more than 5% of patients in this group, over both parts of the study, were hot flashes (46%), diarrhea (40%), nausea (9%), and skin rash (8%).
Prostatic Carcinoma
- Two controlled clinical trials using ZOLADEX 10.8 mg versus ZOLADEX 3.6 mg were conducted. During a comparative phase, patients were randomized to receive either a single 10.8 mg implant or three consecutive 3.6 mg implants every 4 weeks over weeks 0-12. During this phase, the only adverse event reported in greater than 5% of patients was hot flashes, with an incidence of 47% in the ZOLADEX 10.8 mg group and 48% in the ZOLADEX 3.6 mg group.
- From weeks 12-48 all patients were treated with a 10.8 mg implant every 12 weeks. During this noncomparative phase, the following adverse events were reported in greater than 5% of patients:
The following adverse events were reported in greater than 1%, but less than 5% of patients treated with ZOLADEX 10.8 mg implant every 12 weeks. Some of these are commonly reported in elderly patients.
WHOLE BODY
- Abdominal pain
- Back pain
- Flu syndrome
- Headache
- Sepsis
- Aggravation reaction
CARDIOVASCULAR
- Angina pectoris
- Cerebral ischemia
- Cerebrovascular accident
- Heart failure
- Pulmonary embolus
- Varicose veins
DIGESTIVE
ENDOCRINE
HEMATOLOGIC
METABOLIC
NERVOUS SYSTEM
RESPIRATORY
SKIN
=UROGENITAL
- Bladder neoplasm
- Breast pain
- Hematuria
- Impotence
- Urinary frequency
- Urinary incontinence
- Urinary tract disorder
- Urinary tract infection
- Urination impaired
- The following adverse events not already listed above were reported in patients receiving ZOLADEX 3.6 mg in other clinical trials. Inclusion does not necessarily represent a causal relationship to ZOLADEX 10.8 mg.
WHOLE BODY
CARDIOVASCULAR
- Arrhythmia
- Chest pain
- Hemorrhage
- Hypertension
- Migraine
- Myocardial infarction
- Palpitations
- Peripheral vascular disorder
- Tachycardia
DIGESTIVE
- Anorexia
- Constipation
- Dry mouth
- Dyspepsia
- Flatulence
- Increased appetite
- Nausea
- Ulcer
- Vomiting
HEMATOLOGIC
METABOLIC
- Edema
- Gout
- Hyperglycemia
- Weight increase
MUSCULOSKELETAL
NERVOUS SYSTEM
- Anxiety
- Depression
- Emotional lability
- Headache
- Insomnia
- Nervousness
- Somnolence
- Thinking abnormal
RESPIRATORY
- Bronchitis
- Chronic obstructive pulmonary disease
- Epistaxis
- Rhinitis
- Sinusitis
- Upper respiratory infection
- Voice alterations
SKIN
SPECIAL SENSES
UROGENITAL
Changes in Laboratory Values During Treatment
- Plasma Enzymes: Elevation of liver enzymes (AST, ALT) have been reported in female patients exposed to ZOLADEX 3.6 mg (representing less than 1% of all patients). There was no other evidence of abnormal liver function. Causality between these changes and ZOLADEX have not been established.
- Lipids: In a controlled trial in females, ZOLADEX 3.6 mg implant therapy resulted in a minor, but statistically significant effect on serum lipids (i.e., increases in LDL cholesterol of 21.3 mg/dL; increases in HDL cholesterol of 2.7 mg/dL; and triglycerides increased by 8.0 mg/dL).
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of ZOLADEX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypercalcemia
- In patients with bone metastases.
Bone Mineral Density
- Osteoporosis, decreased bone mineral density and bony fracture in men.
Changes in Blood Pressure
- Hypotension and hypertension have been reported. These changes are usually transient, resolving either during continued therapy or after cessation of therapy.
Pituitary Apoplexy and Tumors
- Pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) and pituitary adenoma have been diagnosed. Most of the pituitary apoplexy cases occurred within 2 weeks of the first dose, and some occurred within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required. Pituitary tumors have been reported.
Acne
- Usually within one month of starting treatment.
Other Adverse Reactions
- Psychotic disorders, convulsions and mood swings.
Drug Interactions
- No formal drug-drug interaction studies have been performed.
- No drug interaction studies with other drugs have been conducted with ZOLADEX. No confirmed interactions have been reported between ZOLADEX and other drugs.
Drug/Laboratory Test Interactions
- Administration of ZOLADEX in therapeutic doses results in suppression of the pituitary-gonadal system. Because of this suppression, diagnostic tests of pituitary-gonadotropic and gonadal functions conducted during treatment may show results which are misleading.
Use in Specific Populations
Pregnancy
- Based on mechanism of action in humans and findings of increased pregnancy loss in animal studies, ZOLADEX may cause fetal harm when administered to a pregnant woman. Expected hormone changes that occur with ZOLADEX treatment increase the risk for pregnancy loss. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- ZOLADEX crosses the placenta in rats and rabbits following subcutaneous administration. Administration of goserelin to pregnant rats and rabbits during organogenesis resulted in increased preimplantation loss and increased resorptions. When pregnant rats received goserelin throughout gestation and lactation, there was a dose-related increase in umbilical hernia in offspring. In additional reproduction studies in rats, goserelin decreased fetus and pup survival. Human dose/exposure multiples could not be calculated from available animal data.
- Actual animal doses: rat (≥ 2 mcg/kg/day for pregnancy loss; ≥ 10 mcg/kg/day for umbilical hernia in offspring); rabbits (> 20 mcg/kg/day).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Goserelin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Goserelin during labor and delivery.
Nursing Mothers
- It is not known if goserelin is excreted in human milk. Goserelin is excreted in the milk of lactating rats. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ZOLADEX, a decision should be made to either discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- There is no need for any dosage adjustment when administering ZOLADEX 10.8 mg to geriatric patients.
Gender
There is no FDA guidance on the use of Goserelin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Goserelin with respect to specific racial populations.
Renal Impairment
- In clinical trials with the solution formulation of goserelin, subjects with impaired renal function (creatinine clearance < 20 mL/min) had a serum elimination half-life of 12.1 hours compared to 4.2 hours for subjects with normal renal function (creatinine clearance > 70 mL/min). However, there was no evidence for any accumulation of goserelin on multiple dosing of the ZOLADEX 10.8 mg depot to subjects with impaired renal function. There was no evidence for any increase in incidence of adverse events in renally impaired patients administered the 10.8 mg depot. These data indicate that there is no need for any dosage adjustment when administering ZOLADEX 10.8 mg to subjects with impaired renal function.
Hepatic Impairment
- The total body clearances and serum elimination half-lives were similar between normal subjects and patients with moderate hepatic impairment (alanine transaminase < 3xULN and asparate aminotransferase < 3xULN) when treated with a 250 mcg subcutaneous formulation of goserelin. This pharmacokinetic study indicates that no dose adjustment is needed in patients with moderately impaired liver function. There is no pharmacokinetic data with goserelin in patients with severe hepatic insufficiency.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Goserelin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Goserelin in patients who are immunocompromised.
Body Weight
- A decline of approximately 1 to 2.5% in the AUC after administration of a 10.8 mg depot was observed with a kilogram increase in body weight. In obese patients who have not responded clinically, testosterone levels should be monitored closely.
Administration and Monitoring
Administration
- ZOLADEX, at a dose of 10.8 mg, should be administered subcutaneously every 12 weeks into the anterior abdominal wall below the navel line using an aseptic technique under the supervision of a physician.
- While a delay of a few days is permissible, every effort should be made to adhere to the 12-week schedule
Administration Technique
- The proper method of administration of ZOLADEX is described in the instructions that follow.
- Put the patient in a comfortable position with the upper part of the body slightly raised. Prepare an area of the anterior abdominal wall below the navel line with an alcohol swab.
- Examine the foil pouch and syringe for damage. Remove the syringe from the opened foil pouch and hold the syringe at a slight angle to the light. Check that at least part of the ZOLADEX implant is visible.
- Grasp the blue plastic safety tab and pull away from the syringe, and discard. Remove needle cover. Unlike liquid injections, there is no need to remove air bubbles as attempts to do so may displace the ZOLADEX implant.
- Holding the syringe around the protective sleeve, using an aseptic technique, pinch the skin of the patient’s anterior abdominal wall below the navel line. With the bevel of the needle facing up, insert the needle at a 30 to 45 degree angle to the skin in one continuous deliberate motion until the protective sleeve touches the patient’s skin.
- NOTE: The ZOLADEX syringe cannot be used for aspiration. If the hypodermic needle penetrates a large vessel, blood will be seen instantly in the syringe chamber. If a vessel is penetrated, withdraw the needle and inject with a new syringe elsewhere.
- Do not penetrate into muscle or peritoneum.
- To administer the ZOLADEX implant and to activate the protective sleeve, grasp the barrel at the finger grip and depress the plunger until you cannot depress it any further. If the plunger is not depressed fully, the protective sleeve will NOT activate. When the protective sleeve ‘clicks’, the protective sleeve will automatically begin to slide to cover the needle.
- NOTE: The needle does not retract.
- Withdraw the needle and allow protective sleeve to slide and cover needle. Dispose of the syringe in an approved sharps collector.
- NOTE: In the unlikely event of the need to surgically remove ZOLADEX, it may be localized by ultrasound.
Monitoring
- Transient worsening of tumor symptoms may occur during the first few weeks of treatment with ZOLADEX, which may include ureteral obstruction and spinal cord compression. Monitor patients at risk for complications of tumor flare
- Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH analogs. Monitor blood glucose level and manage according to current clinical practice.
- Increased risk of myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH analogs in men. Monitor for cardiovascular disease and manage according to current clinical practice
IV Compatibility
There is limited information regarding IV Compatibility of Goserelin in the drug label.
Overdosage
- The pharmacologic properties of ZOLADEX and its mode of administration make accidental or intentional overdosage unlikely. There is no experience of overdosage from clinical trials. Animal studies indicate that no increased pharmacologic effect occurred at higher doses or more frequent administration. Subcutaneous doses of the drug as high as 1 mg/kg/day in rats and dogs did not produce any nonendocrine related sequelae; this dose is up to 250 times the estimated human daily dose based on the body surface area. If overdosage occurs, it should be managed symptomatically.
Pharmacology
Mechanism of Action
- ZOLADEX is a synthetic decapeptide analogue of GnRH. ZOLADEX acts as an inhibitor of pituitary gonadotropin secretion when administered in the biodegradable formulation.
- In animal and in vitro studies, administration of goserelin resulted in the regression or inhibition of growth of the hormonally sensitive dimethylbenzanthracene (DMBA)-induced rat mammary tumor and Dunning R3327 prostate tumor.
Structure
- ZOLADEX® (goserelin acetate implant) is a GnRH agonist. Goserelin acetate is chemically described as an acetate salt of [D-Ser(But)6,Azgly10]. Its chemical structure is pyro-Glu-His-Trp-Ser-Tyr-D-Ser(But)-Leu-Arg-Pro-Azgly-NH2 acetate [C59H84N18O14 ·(C2H4O2)x where x = 1 to 2.4]
Pharmacodynamics
- Following initial administration, ZOLADEX causes an initial increase in serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels with subsequent increases in serum levels of testosterone. Chronic administration of ZOLADEX leads to sustained suppression of pituitary gonadotropins, and serum levels of testosterone consequently fall into the range normally seen in surgically castrated men approximately 21 days after initiation of therapy. This leads to accessory sex organ regression.
- In clinical trials using ZOLADEX 3.6 mg with follow-up of more than 2 years, suppression of serum testosterone to castrate levels has been maintained for the duration of therapy.
Pharmacokinetics
Absorption
- The pharmacokinetics of ZOLADEX have been determined in healthy male volunteers and patients. In healthy males, radiolabeled goserelin was administered as a single 250 mcg (aqueous solution) dose by the subcutaneous route. The absorption of radiolabeled drug was rapid, and the peak blood radioactivity levels occurred between 0.5 and 1.0 hour after dosing.
- The overall pharmacokinetic profile of goserelin following administration of a ZOLADEX 10.8 mg depot to patients with prostate cancer was determined. The initial release of goserelin from the depot was relatively rapid resulting in a peak concentration at 2 hours after dosing. From Day 4 until the end of the 12-week dosing interval, the sustained release of goserelin from the depot produced reasonably stable systemic exposure. Mean (Standard Deviation) pharmacokinetic data are presented in Table 4. There is no clinically significant accumulation of goserelin following administration of four depots administered at 12-week intervals.
- SD = standard deviation
- Serum goserelin concentrations in prostate cancer patients administered three 3.6 mg depots followed by one 10.8 mg depot are displayed in Figure 1. The profiles for both formulations are primarily dependent upon the rate of drug release from the depots. For the 3.6 mg depot, mean concentrations gradually rise to reach a peak of about 3 ng/mL at around 15 days after administration and then decline to approximately 0.5 ng/mL by the end of the treatment period. For the 10.8 mg depot, mean concentrations increase to reach a peak of about 8 ng/mL within the first 24 hours and then decline rapidly up to Day 4. Thereafter, mean concentrations remain relatively stable in the range of about 0.3 to 1 ng/mL up to the end of the treatment period.
- Administration of four ZOLADEX 10.8 mg depots to patients with prostate cancer resulted in testosterone levels that were suppressed to and maintained within the range normally observed in surgically castrated men (0 – 1.73 nmol/L or 0-50 ng/dL), over the dosing interval in approximately 91% (145/160) of patients studied. In 6 of 15 patients that escaped from castrate range, serum testosterone levels were maintained below 2.0 nmol/L (58 ng/dL) and in only one of the 15 patients did the depot completely fail to maintain serum testosterone levels to within the castrate range over a 336-day period (4 depot injections). In the 8 additional patients, a transient escape was followed 14 days later by a level within the castrate range.
Distribution
- The apparent volume of distribution determined after subcutaneous administration of 250 mcg aqueous solution of goserelin was 44.1 ± 13.6 liters for healthy males. The plasma protein binding of goserelin was found to be 27%.
Metabolism
- Metabolism of goserelin, by hydrolysis of the C-terminal amino acids, is the major clearance mechanism. The major circulating component in serum appeared to be 1–7 fragment, and the major component present in urine of one healthy male volunteer was 5-10 fragment. The metabolism of goserelin in humans yields a similar but narrow profile of metabolites to that found in other species. All metabolites found in humans have also been found in toxicology species.
Excretion
- Clearance of goserelin following subcutaneous administration of a radiolabeled solution of goserelin was very rapid and occurred via a combination of hepatic and urinary excretion. More than 90% of a subcutaneous radiolabeled solution formulation dose of goserelin was excreted in urine. Approximately 20% of the dose recovered in urine was accounted for by unchanged goserelin.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Subcutaneous implantation of goserelin in male and female rats once every 4 weeks for 1 year and recovery for 23 weeks at doses of about 80 and 150 mcg/kg (males) and 50 and 100 mcg/kg (females) daily resulted in an increased incidence of pituitary adenomas. An increased incidence of pituitary adenomas was also observed following subcutaneous implant of goserelin in rats at similar dose levels for a period of 72 weeks in males and 101 weeks in females. The relevance of the rat pituitary adenomas to humans has not been established. Subcutaneous implants of goserelin every 3 weeks for 2 years delivered to mice at doses of up to 2400 mcg/kg/day resulted in an increased incidence of histiocytic sarcoma of the vertebral column and femur. Human dose/exposure multiples could not be calculated from available animal data.
- Mutagenicity tests using bacterial and mammalian systems for point mutations and cytogenetic effects have provided no evidence for mutagenic potential.
- Administration of goserelin led to changes that were consistent with gonadal suppression in both male and female rats as a result of its endocrine action. In male rats administered 500-1000 mcg/kg/day, a decrease in weight and atrophic histological changes were observed in the testes, epididymis, seminal vesicle and prostate gland with complete suppression of spermatogenesis. In female rats administered 50-1000 mcg/kg/day, suppression of ovarian function led to decreased size and weight of ovaries and secondary sex organs; follicular development was arrested at the antral stage and the corpora lutea were reduced in size and number. Except for the testes, almost complete histologic reversal of these effects in males and females was observed several weeks after dosing was stopped; however, fertility and general reproductive performance were reduced in those that became pregnant after goserelin was discontinued. Fertile matings occurred within 2 weeks after cessation of dosing, even though total recovery of reproductive function may not have occurred before mating took place; and, the ovulation rate, the corresponding implantation rate, and number of live fetuses were reduced.
- Based on histological examination, drug effects on reproductive organs were reversible in male and female dogs administered 107-214 mcg/kg/day goserelin when drug treatment was stopped after continuous administration for 1 year. Human dose/exposure multiples could not be calculated from available animal data.
Clinical Studies
Stage B2-C Prostatic Carcinoma
- The effects of hormonal treatment combined with radiation were studied in 466 patients (231 ZOLADEX + flutamide + radiation, 235 radiation alone) with bulky primary tumors confined to the prostate (stage B2) or extending beyond the capsule (stage C), with or without pelvic node involvement.
- In this multicentered, controlled trial, administration of ZOLADEX (3.6 mg depot) and flutamide capsules (250 mg t.i.d.) prior to and during radiation was associated with a significantly lower rate of local failure compared to radiation alone (16% vs 33% at 4 years, P<0.001). The combination therapy also resulted in a trend toward reduction in the incidence of distant metastases (27% vs 36% at 4 years, P =0.058). Median disease-free survival was significantly increased in patients who received complete hormonal therapy combined with radiation as compared to those patients who received radiation alone (4.4 vs 2.6 years, P<0.001). Inclusion of normal PSA level as a criterion for disease-free survival also resulted in significantly increased median disease-free survival in patients receiving the combination therapy (2.7 vs 1.5 years, P<0.001).
Prostatic Carcinoma
- In two controlled clinical trials, 160 patients with advanced prostate cancer were randomized to receive either one 3.6 mg ZOLADEX implant every four weeks or a single 10.8 mg ZOLADEX implant every 12 weeks. Mean serum testosterone suppression was similar between the two arms. PSA falls at three months were 94% in patients who received the 10.8 mg implant and 92.5% in patients that received three 3.6 mg implants.
- Periodic monitoring of serum testosterone levels should be considered if the anticipated clinical or biochemical response to treatment has not been achieved. A clinical outcome similar to that produced with the use of the 3.6 mg implant administered every 28 days is predicted with ZOLADEX 10.8 mg implant administered every 12 weeks (84 days). Total testosterone was measured by the DPC Coat-A-Count radioimmunoassay method which, as defined by the manufacturers, is highly specific and accurate. Acceptable variability of approximately 20% at low testosterone levels has been demonstrated in the clinical studies performed with the ZOLADEX 10.8 mg depot.
How Supplied
- ZOLADEX 10.8 mg implant is supplied as a sterile and totally biodegradable D,L-lactic and glycolic acids copolymer (12.82-14.76 mg/dose) impregnated with goserelin acetate equivalent to 10.8 mg of goserelin in a disposable syringe device fitted with a 14-gauge x 36 +/- 0.5 mm siliconized hypodermic needle with protective sleeve [SafeSystem™ Syringe] (NDC 0310-0951-30). The unit is sterile and comes in a sealed, light- and moisture-proof, aluminum foil laminate pouch containing a desiccant capsule.
Storage
- Store at room temperature (do not exceed 25°C [77°F]).
Images
Drug Images
{{#ask: Page Name::Goserelin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 10.8 MG 3-MONTH
INGREDIENTS AND APPEARANCE
{{#ask: Label Page::Goserelin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Males
- The use of ZOLADEX in patients at particular risk of developing ureteral obstruction or spinal cord compression should be considered carefully and the patients monitored closely during the first month of therapy. Patients with ureteral obstruction or spinal cord compression should have appropriate treatment prior to initiation of ZOLADEX therapy.
- The use of GnRH agonists may cause a reduction in bone mineral density. In men, data suggest the use of a bisphosphonate in combination with a GnRH agonist may reduce bone mineral loss.
- Patients should be informed that diabetes, or loss of glycemic control in patients with pre-existing diabetes, has been reported during treatment with GnRH agonists, including ZOLADEX. Therefore, consideration should be given to monitoring blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving ZOLADEX.
- A small increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice
Precautions with Alcohol
- Alcohol-Goserelin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Zoladex®[6]
Look-Alike Drug Names
There is limited information regarding Goserelin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Goel S, Sharma R, Hamilton A, Beith J (2009). "LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women". Cochrane Database Syst Rev (4): CD004562. doi:10.1002/14651858.CD004562.pub4. PMID 19821328.
- ↑ Hackshaw A, Baum M, Fornander T, Nordenskjold B, Nicolucci A, Monson K; et al. (2009). "Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer". J Natl Cancer Inst. 101 (5): 341–9. doi:10.1093/jnci/djn498. PMC 2650713. PMID 19244174.
- ↑ Vercellini P, Fedele L, Maggi R, Vendola N, Bocciolone L, Colombo A (1993). "Gonadotropin releasing hormone agonist for chronic anovulatory uterine bleeding and severe anemia". J Reprod Med. 38 (2): 127–9. PMID 8445603.
- ↑ Tapanainen JS, Hovatta O (1994). "Pituitary down-regulation with goserelin (Zoladex) for in vitro fertilisation". Br J Obstet Gynaecol. 101 Suppl 10: 27–8. PMID 8199101.
- ↑ de Brito VN, Latronico AC, Arnhold IJ, Lo LS, Domenice S, Albano MC; et al. (1999). "Treatment of gonadotropin dependent precocious puberty due to hypothalamic hamartoma with gonadotropin releasing hormone agonist depot". Arch Dis Child. 80 (3): 231–4. PMC 1717869. PMID 10325702.
- ↑ "Goserelin acetate".