Fat embolism syndrome pathophysiology: Difference between revisions
Feham Tariq (talk | contribs) |
|||
| (31 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
==Overview== | ==Overview== | ||
Fat embolism syndrome (FES) is the presence of fat globules in the circulation post traumatic insult which can lodge into the small sized capillaries in the lung, brain and skin leading to devastating clinical manifestations. The two widely accepted theories which explain the pathophysiology of FES are mechanical and biochemical theory. The mechanical theory proposes that there is mechanical obstruction by fat cells from the [[bone marrow]] in the end-capillaries after trauma. Biochemical theory attributes the clinical manifestations of FES to the pro inflammatory effect of fat [[Embolism|emboli]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
Two major theories have been described to explain the pathophysiology of fat embolism syndrome(FES):<ref name="pmid12650535">{{cite journal| author=Parisi DM, Koval K, Egol K| title=Fat embolism syndrome. | journal=Am J Orthop (Belle Mead NJ) | year= 2002 | volume= 31 | issue= 9 | pages= 507-12 | pmid=12650535 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12650535 }} </ref> | Two major theories have been described to explain the pathophysiology of fat embolism syndrome(FES):<ref name="pmid12650535">{{cite journal| author=Parisi DM, Koval K, Egol K| title=Fat embolism syndrome. | journal=Am J Orthop (Belle Mead NJ) | year= 2002 | volume= 31 | issue= 9 | pages= 507-12 | pmid=12650535 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12650535 }} </ref><ref name="pmid8316420">{{cite journal| author=Robert JH, Hoffmeyer P, Broquet PE, Cerutti P, Vasey H| title=Fat embolism syndrome. | journal=Orthop Rev | year= 1993 | volume= 22 | issue= 5 | pages= 567-71 | pmid=8316420 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8316420 }} </ref> | ||
*Mechanical theory | *Mechanical theory | ||
*Biochemical theory | *Biochemical theory | ||
===Mechanical theory=== | |||
The theory proposes that there is mechanical obstruction by fat cells from the bone marrow in the end-capillaries after trauma. | The theory proposes that there is mechanical obstruction by fat cells from the [[bone marrow]] in the end-capillaries after trauma. | ||
*Post traumatic insult, the fat cells travel via venous sinusoids to the capillaries. | *Post traumatic insult, the fat cells travel via [[Vein|venous]] sinusoids to the [[Capillary|capillaries]]. | ||
*These cells have potent prothrombotic and proinflammatory potential. | *These cells have potent prothrombotic and [[Inflammation|proinflammatory]] potential. | ||
*They trigger rapid aggregation of platelets and accelerated fibrin generation as they travel through the venous system, eventually lodging in the pulmonary arterial circulation. | *They trigger rapid aggregation of [[Platelet|platelets]] and accelerated [[fibrin]] generation as they travel through the venous system, eventually lodging in the [[Lung|pulmonary]] [[Artery|arterial]] [[Circulatory system|circulation]]. | ||
*Pulmonary capillary obstruction leads to interstitial hemorrhage and edema, alveolar collapse, and reactive hypoxemic vasoconstriction. | *[[Lung|Pulmonary]] capillary obstruction leads to [[interstitial]] [[Bleeding|hemorrhage]] and [[edema]], [[Alveolus|alveolar]] collapse, and reactive hypoxemic [[vasoconstriction]]. | ||
* Massive fat emboli may also lead to macrovascular obstruction and shock. | * Massive fat [[Embolism|emboli]] may also lead to macrovascular obstruction and shock. | ||
*Fat cells may also enter the arterial circulation via a patent foramen ovale or directly through the pulmonary capillary bed, causing the characteristic neurological and dermatologic findings of FES | *Fat cells may also enter the [[Artery|arterial]] circulation via a [[patent foramen ovale]] or directly through the [[Lung|pulmonary]] capillary bed, causing the characteristic [[Neurology|neurological]] and dermatologic findings of FES. | ||
===Biochemical theory=== | |||
This theory attributes the clinical manifestations of FES to the pro inflammatory effect of fat emboli.<ref name="pmid16990064">{{cite journal| author=Husebye EE, Lyberg T, Røise O| title=Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms. | journal=Injury | year= 2006 | volume= 37 Suppl 4 | issue= | pages= S8-18 | pmid=16990064 | doi=10.1016/j.injury.2006.08.036 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16990064 }} </ref><ref name="pmid9686075">{{cite journal| author=Estèbe JP| title=[From fat emboli to fat embolism syndrome]. | journal=Ann Fr Anesth Reanim | year= 1997 | volume= 16 | issue= 2 | pages= 138-51 | pmid=9686075 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9686075 }} </ref><ref name="pmid7753543">{{cite journal| author=Hofmann S, Huemer G, Kratochwill C, Koller-Strametz J, Hopf R, Schlag G et al.| title=[Pathophysiology of fat embolisms in orthopedics and traumatology]. | journal=Orthopade | year= 1995 | volume= 24 | issue= 2 | pages= 84-93 | pmid=7753543 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7753543 }} </ref> | This theory attributes the clinical manifestations of FES to the pro inflammatory effect of fat [[Embolism|emboli]].<ref name="pmid16990064">{{cite journal| author=Husebye EE, Lyberg T, Røise O| title=Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms. | journal=Injury | year= 2006 | volume= 37 Suppl 4 | issue= | pages= S8-18 | pmid=16990064 | doi=10.1016/j.injury.2006.08.036 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16990064 }} </ref><ref name="pmid9686075">{{cite journal| author=Estèbe JP| title=[From fat emboli to fat embolism syndrome]. | journal=Ann Fr Anesth Reanim | year= 1997 | volume= 16 | issue= 2 | pages= 138-51 | pmid=9686075 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9686075 }} </ref><ref name="pmid7753543">{{cite journal| author=Hofmann S, Huemer G, Kratochwill C, Koller-Strametz J, Hopf R, Schlag G et al.| title=[Pathophysiology of fat embolisms in orthopedics and traumatology]. | journal=Orthopade | year= 1995 | volume= 24 | issue= 2 | pages= 84-93 | pmid=7753543 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7753543 }} </ref> | ||
* Tissue lipases break down the fat in the bone marrow, forming high levels of | * Tissue lipases break down the fat in the bone marrow, forming high levels of the following toxic intermediaries:<ref name="pmid621762">{{cite journal| author=Nixon JR, Brock-Utne JG| title=Free fatty acid and arterial oxygen changes following major injury: a correlation between hypoxemia and increased free fatty acid levels. | journal=J Trauma | year= 1978 | volume= 18 | issue= 1 | pages= 23-6 | pmid=621762 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=621762 }} </ref><ref name="pmid4330876">{{cite journal| author=Baker PL, Pazell JA, Peltier LF| title=Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. | journal=J Trauma | year= 1971 | volume= 11 | issue= 12 | pages= 1026-30 | pmid=4330876 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4330876 }} </ref> | ||
* | ** '''Free fatty acids''': | ||
* In the lung, toxic injury to pneumocytes and pulmonary endothelial cells causes vasogenic and cytotoxic edema as well as hemorrhage. | Free [[fatty acid]]<nowiki/>s are released into the [[Circulatory system|circulation]] after hydrolysis and deposit into the end capillaries of the [[lung]], manifesting as [[acute respiratory distress syndrome]].<ref name="pmid3560274">{{cite journal| author=Schnaid E, Lamprey JM, Viljoen MJ, Joffe BI, Seftel HC| title=The early biochemical and hormonal profile of patients with long bone fractures at risk of fat embolism syndrome. | journal=J Trauma | year= 1987 | volume= 27 | issue= 3 | pages= 309-11 | pmid=3560274 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3560274 }} </ref><ref name="pmid11833059">{{cite journal| author=Meininger G, Hadigan C, Laposata M, Brown J, Rabe J, Louca J et al.| title=Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. | journal=Metabolism | year= 2002 | volume= 51 | issue= 2 | pages= 260-6 | pmid=11833059 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11833059 }} </ref> | ||
* Acute lung injury or acute respiratory distress syndrome | |||
* '''Cytokines''': | |||
Patients with FES are also found to have high levels of certain [[Cytokine|cytokines]] such as [[tumor necrosis factor alpha]], [[phospholipase A2]], interleukin 1 and 6.<ref name="pmid17428199">{{cite journal| author=Kao SJ, Yeh DY, Chen HI| title=Clinical and pathological features of fat embolism with acute respiratory distress syndrome. | journal=Clin Sci (Lond) | year= 2007 | volume= 113 | issue= 6 | pages= 279-85 | pmid=17428199 | doi=10.1042/CS20070011 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17428199 }} </ref><ref name="pmid23423626">{{cite journal| author=Prakash S, Sen RK, Tripathy SK, Sen IM, Sharma RR, Sharma S| title=Role of interleukin-6 as an early marker of fat embolism syndrome: a clinical study. | journal=Clin Orthop Relat Res | year= 2013 | volume= 471 | issue= 7 | pages= 2340-6 | pmid=23423626 | doi=10.1007/s11999-013-2869-y | pmc=3676609 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23423626 }} </ref> | |||
* '''C-reactive proteins''': | |||
The elevation in [[c-reactive protein]]<nowiki/>s is responsible for [[lipid]] agglutintion in FES which results in microvasculature obstruction and stagnant blood flow. | |||
* Eventually, these intermediate products lead to end-organ dysfunction. | |||
* In the lung, toxic injury to [[Pneumocyte|pneumocytes]] and pulmonary [[Endothelium|endothelial]] cells causes [[Vasogenic edema|vasogenic]] and [[Cytotoxicity|cytotoxic]] edema as well as [[Bleeding|hemorrhage]]. | |||
* Acute lung injury or [[acute respiratory distress syndrome]] results from damaged [[Lung|pulmonary]] [[endothelium]] that triggers a proinflammatory [[cytokine]] cascade. | |||
===Pathogenesis of clinical manifestations=== | |||
Following pathological sequence of events occur after an orthopedic trauma such as long bone [[Bone fracture|fracture]]. | |||
{{Family tree/start}} | |||
{{Family tree | | | | A01 |-|-|-| A02 | |A01= Microvascular obstruction and free fatty acids(FFA) mediated endothelial injury leading to proinflammatory cytokine release(IL-1,IL-6,TNF-alpha)| A02= Acute respiratory distress syndrome}} | |||
{{Family tree/end}} | |||
<br> | |||
{{Family tree/start}} | |||
{{Family tree | | | | A01 |-|-|-| A02 | |A01= Arterial hypoxemia and cerebral vascular injury from FFA intermediates | A02= Encephalopathy and focal neurological deficits}} | |||
{{Family tree/end}} | |||
<br> | |||
{{Family tree/start}} | |||
{{Family tree | | | | A01 |-|-|-| A02 | |A01= Vascular stasis,microinfarction and FFA mediated endothelial damage leading to rupture of thin-walled capillaries| A02= Petechiae}} | |||
{{Family tree/end}} | |||
<br> | |||
{{Family tree/start}} | |||
{{Family tree | | | | A01 |-|-|-| A02 | |A01= Elevated tissue factor, excess thrombin and fibrin generation, aggregation of platelets and consumption of coagulation products| A02= DIC, thrombocytopenia and anemia}} | |||
{{Family tree/end}} | |||
===Video=== | ===Video=== | ||
{{#ev:youtube|eeAQ2akVjL8}} | {{#ev:youtube|eeAQ2akVjL8}} | ||
==Genetics== | |||
*There is no genetic association of FES. | |||
==Gross pathology== | |||
There is no characteristic gross pathology associated with fat embolism. | |||
==Microscopic pathology== | |||
Hematoxylin and eosin staining shows the following changes in the [[Lung|lungs]], [[Kidney|kidneys]] and [[brain]]:<ref>{{cite journal|doi=10.1042/CS2007001}}</ref> | |||
'''Lung:''' | |||
*[[Alveolus|Alveolar]] [[Bleeding|haemorrhagic]] [[edema]] | |||
*Fat droplet deposition | |||
*[[Fibrin]] [[Thrombus|thrombi]] | |||
*Multiple fat droplets | |||
Immunohistochemical staining shows the following changes: | |||
*Raised levels of iNOS in the [[Alveolus|alveolar]] [[Macrophage|macrophages]]. | |||
'''Kidney:''' | |||
*Hematoxylin and eosin staining shows fat deposits in the [[Glomerulus|glomeruli]]. | |||
'''Brain:''' | |||
*Fat droplets are seen in vessels. | |||
[[File:Fat embolism.JPEG.jpg|thumb|centre|300px|Microscopic picture of fat embolism.By Boris L Kanen, Ruud JLF Loffeld - Pancreatitis with an unusual fatal complication following endoscopic retrograde cholangiopancreaticography: a case report. Journal of Medical Case Reports. 2008;2:215. doi:10.1186/1752-1947-2-215, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=4979542]] | |||
== | ===Gallery=== | ||
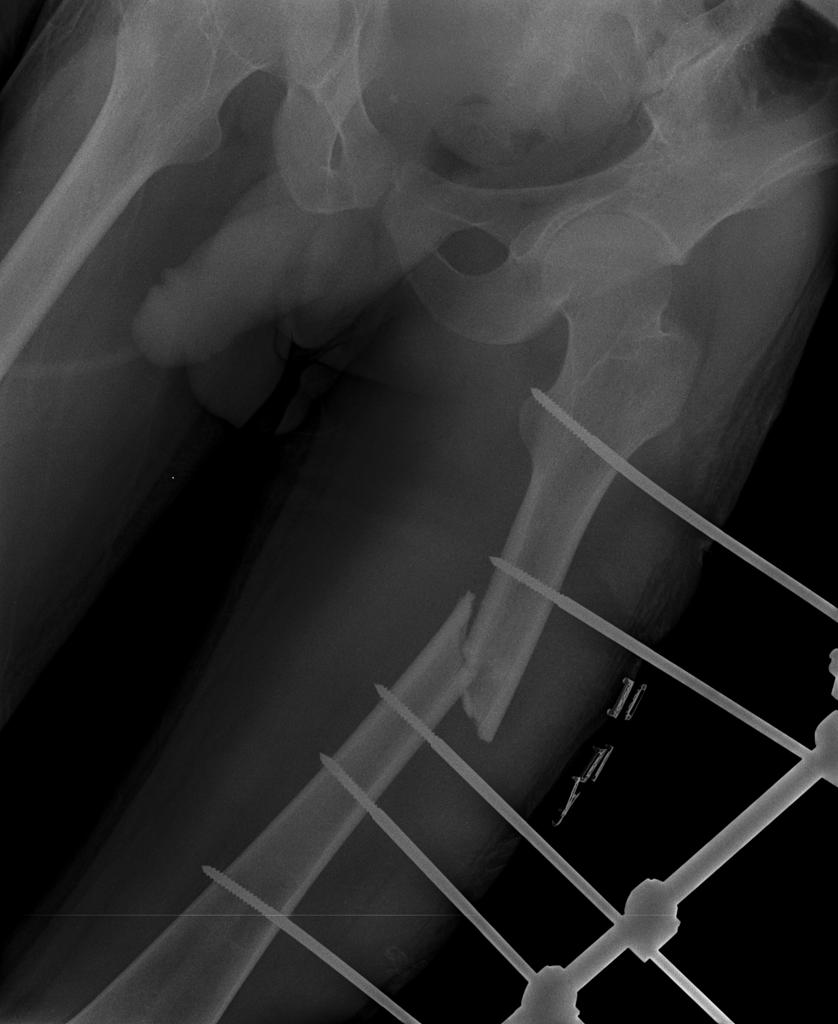
[[File:Cerebral-fat-embolism-1.jpg|thumb|centre|300px|Displaced femoral midshaft fracture. | |||
Case courtesy of Dr Rajesh Shanklesha, Radiopaedia.org, rID: 29523]] | |||
==References== | ==References== | ||
Latest revision as of 17:15, 7 December 2018
|
Fat embolism syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Fat embolism syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Fat embolism syndrome pathophysiology |
|
Risk calculators and risk factors for Fat embolism syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Feham Tariq, MD [2]
Overview
Fat embolism syndrome (FES) is the presence of fat globules in the circulation post traumatic insult which can lodge into the small sized capillaries in the lung, brain and skin leading to devastating clinical manifestations. The two widely accepted theories which explain the pathophysiology of FES are mechanical and biochemical theory. The mechanical theory proposes that there is mechanical obstruction by fat cells from the bone marrow in the end-capillaries after trauma. Biochemical theory attributes the clinical manifestations of FES to the pro inflammatory effect of fat emboli.
Pathophysiology
Two major theories have been described to explain the pathophysiology of fat embolism syndrome(FES):[1][2]
- Mechanical theory
- Biochemical theory
Mechanical theory
The theory proposes that there is mechanical obstruction by fat cells from the bone marrow in the end-capillaries after trauma.
- Post traumatic insult, the fat cells travel via venous sinusoids to the capillaries.
- These cells have potent prothrombotic and proinflammatory potential.
- They trigger rapid aggregation of platelets and accelerated fibrin generation as they travel through the venous system, eventually lodging in the pulmonary arterial circulation.
- Pulmonary capillary obstruction leads to interstitial hemorrhage and edema, alveolar collapse, and reactive hypoxemic vasoconstriction.
- Massive fat emboli may also lead to macrovascular obstruction and shock.
- Fat cells may also enter the arterial circulation via a patent foramen ovale or directly through the pulmonary capillary bed, causing the characteristic neurological and dermatologic findings of FES.
Biochemical theory
This theory attributes the clinical manifestations of FES to the pro inflammatory effect of fat emboli.[3][4][5]
- Tissue lipases break down the fat in the bone marrow, forming high levels of the following toxic intermediaries:[6][7]
- Free fatty acids:
Free fatty acids are released into the circulation after hydrolysis and deposit into the end capillaries of the lung, manifesting as acute respiratory distress syndrome.[8][9]
- Cytokines:
Patients with FES are also found to have high levels of certain cytokines such as tumor necrosis factor alpha, phospholipase A2, interleukin 1 and 6.[10][11]
- C-reactive proteins:
The elevation in c-reactive proteins is responsible for lipid agglutintion in FES which results in microvasculature obstruction and stagnant blood flow.
- Eventually, these intermediate products lead to end-organ dysfunction.
- In the lung, toxic injury to pneumocytes and pulmonary endothelial cells causes vasogenic and cytotoxic edema as well as hemorrhage.
- Acute lung injury or acute respiratory distress syndrome results from damaged pulmonary endothelium that triggers a proinflammatory cytokine cascade.
Pathogenesis of clinical manifestations
Following pathological sequence of events occur after an orthopedic trauma such as long bone fracture.
| Microvascular obstruction and free fatty acids(FFA) mediated endothelial injury leading to proinflammatory cytokine release(IL-1,IL-6,TNF-alpha) | Acute respiratory distress syndrome | ||||||||||||||||||||||||
| Arterial hypoxemia and cerebral vascular injury from FFA intermediates | Encephalopathy and focal neurological deficits | ||||||||||||||||||||||||
| Vascular stasis,microinfarction and FFA mediated endothelial damage leading to rupture of thin-walled capillaries | Petechiae | ||||||||||||||||||||||||
| Elevated tissue factor, excess thrombin and fibrin generation, aggregation of platelets and consumption of coagulation products | DIC, thrombocytopenia and anemia | ||||||||||||||||||||||||
Video
{{#ev:youtube|eeAQ2akVjL8}}
Genetics
- There is no genetic association of FES.
Gross pathology
There is no characteristic gross pathology associated with fat embolism.
Microscopic pathology
Hematoxylin and eosin staining shows the following changes in the lungs, kidneys and brain:[12]
Lung:
- Alveolar haemorrhagic edema
- Fat droplet deposition
- Fibrin thrombi
- Multiple fat droplets
Immunohistochemical staining shows the following changes:
- Raised levels of iNOS in the alveolar macrophages.
Kidney:
- Hematoxylin and eosin staining shows fat deposits in the glomeruli.
Brain:
- Fat droplets are seen in vessels.
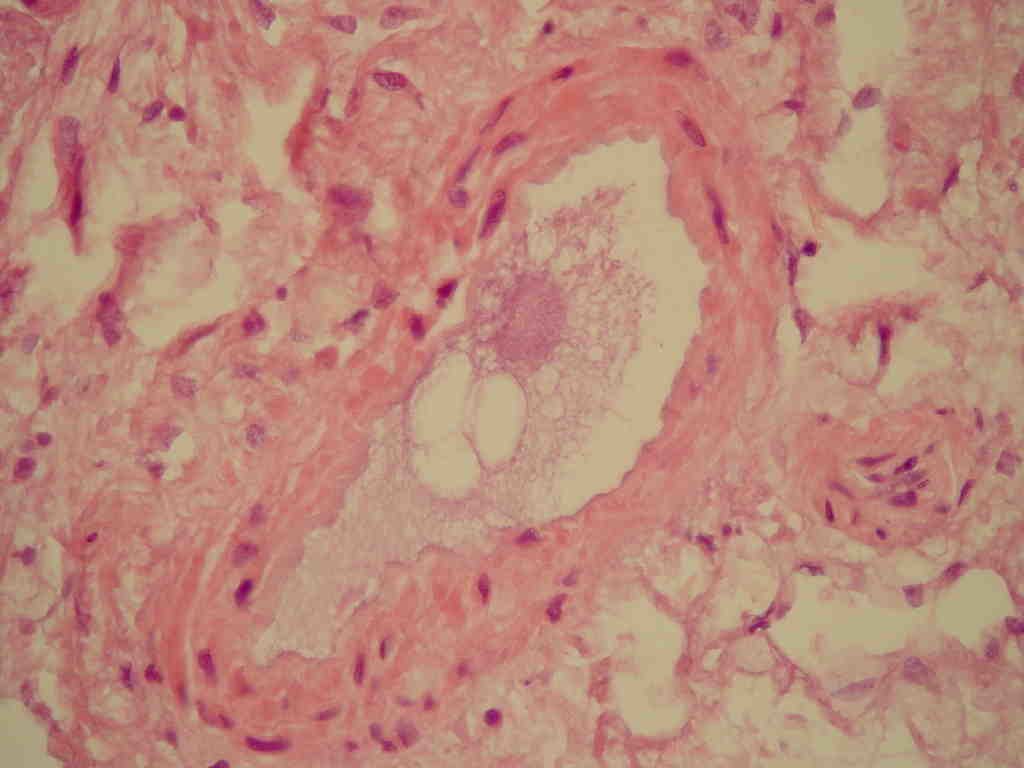
Gallery
References
- ↑ Parisi DM, Koval K, Egol K (2002). "Fat embolism syndrome". Am J Orthop (Belle Mead NJ). 31 (9): 507–12. PMID 12650535.
- ↑ Robert JH, Hoffmeyer P, Broquet PE, Cerutti P, Vasey H (1993). "Fat embolism syndrome". Orthop Rev. 22 (5): 567–71. PMID 8316420.
- ↑ Husebye EE, Lyberg T, Røise O (2006). "Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms". Injury. 37 Suppl 4: S8–18. doi:10.1016/j.injury.2006.08.036. PMID 16990064.
- ↑ Estèbe JP (1997). "[From fat emboli to fat embolism syndrome]". Ann Fr Anesth Reanim. 16 (2): 138–51. PMID 9686075.
- ↑ Hofmann S, Huemer G, Kratochwill C, Koller-Strametz J, Hopf R, Schlag G; et al. (1995). "[Pathophysiology of fat embolisms in orthopedics and traumatology]". Orthopade. 24 (2): 84–93. PMID 7753543.
- ↑ Nixon JR, Brock-Utne JG (1978). "Free fatty acid and arterial oxygen changes following major injury: a correlation between hypoxemia and increased free fatty acid levels". J Trauma. 18 (1): 23–6. PMID 621762.
- ↑ Baker PL, Pazell JA, Peltier LF (1971). "Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism". J Trauma. 11 (12): 1026–30. PMID 4330876.
- ↑ Schnaid E, Lamprey JM, Viljoen MJ, Joffe BI, Seftel HC (1987). "The early biochemical and hormonal profile of patients with long bone fractures at risk of fat embolism syndrome". J Trauma. 27 (3): 309–11. PMID 3560274.
- ↑ Meininger G, Hadigan C, Laposata M, Brown J, Rabe J, Louca J; et al. (2002). "Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution". Metabolism. 51 (2): 260–6. PMID 11833059.
- ↑ Kao SJ, Yeh DY, Chen HI (2007). "Clinical and pathological features of fat embolism with acute respiratory distress syndrome". Clin Sci (Lond). 113 (6): 279–85. doi:10.1042/CS20070011. PMID 17428199.
- ↑ Prakash S, Sen RK, Tripathy SK, Sen IM, Sharma RR, Sharma S (2013). "Role of interleukin-6 as an early marker of fat embolism syndrome: a clinical study". Clin Orthop Relat Res. 471 (7): 2340–6. doi:10.1007/s11999-013-2869-y. PMC 3676609. PMID 23423626.
- ↑ . doi:10.1042/CS2007001. Missing or empty
|title=(help)