Etanercept: Difference between revisions
m (Protected "Etanercept": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EJ}} + & -{{EH}} + & -{{Editor Join}} + & -{{Editor Help}} +)) |
||
| Line 19: | Line 19: | ||
}} | }} | ||
{{SI}} | {{SI}} | ||
==Overview== | ==Overview== | ||
Revision as of 02:25, 9 August 2012
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 58–76% (SC) |
| Metabolism | Reticuloendothelial system (speculative) |
| Elimination half-life | 70–132 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C2224H3475N621O698S36 |
| Molar mass | 51234.9 g/mol |
|
WikiDoc Resources for Etanercept |
|
Articles |
|---|
|
Most recent articles on Etanercept |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Etanercept at Clinical Trials.gov Clinical Trials on Etanercept at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Etanercept
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Etanercept Discussion groups on Etanercept Patient Handouts on Etanercept Directions to Hospitals Treating Etanercept Risk calculators and risk factors for Etanercept
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Etanercept |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Etanercept is a recombinant human soluble tumor necrosis factor-alpha (TNFα) receptor fusion protein. It is a large molecule, with a molecular weight of 150 kDa., that binds to TNFα and decreases its role in disorders involving excess inflammation in humans and other animals, including autoimmune diseases such as ankylosing spondylitis, juvenile rheumatoid arthritis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and, potentially, in a variety of other disorders mediated by excess TNFα. This therapeutic potential is based on the fact that TNF-alpha is the "master regulator" (as coined by Marc Feldmann, Phd, and Ravinder N. Maini BCh, recipients of the 2003 Lasker Award for their anti-TNF research in rheumatoid arthritis) of the inflammatory response in many organ systems. In the United States and the United Kingdom, etanercept is co-marketed by Amgen and Wyeth under the trade name Enbrel in two separate formulations, one in powder form, the other as a pre-mixed liquid.
Development
Etanercept was developed by researchers at biotechnology company Immunex, which was subsequently acquired by Amgen. It was released for commercial use in late 1998, soon after the release of infliximab (Remicade) – the first chimeric monoclonal antibody against TNFα to be marketed for clinical use. Etanercept is a dimeric molecule, and this dimeric structure is necessary for its proper therapeutic activity. During its development at Immunex Corporation an earlier monomeric version did not have sufficient biologic activity.
Mode of action
Tumor necrosis factor-alpha (TNFα) is a cytokine produced by monocytes and macrophages, two types of white blood cells. It mediates the immune response by increasing the transport of white blood cells to sites of inflammation, and through additional molecular mechanisms which initiate and amplify inflammation. Inhibition of its action by etanercept reduces the inflammatory response which is especially useful for treating autoimmune diseases.
There are two types of TNF receptors: those found embedded in white blood cells that respond to TNF by releasing other cytokines, and soluble TNF receptors which are used to deactivate TNF and blunt the immune response. In addition, TNF receptors are found on the surface of virtually all nucleated cells (red blood cells, which are not nucleated, do not contain TNF receptors on their surface). Etanercept mimics the inhibitory effects of naturally occurring soluble TNF receptors, the difference being that etanercept, because it is a fusion protein rather than a simple TNF receptor, has a greatly extended half-life in the bloodstream, and therefore a more profound and long-lasting biologic effect than than a naturally occurring soluble TNF receptor.
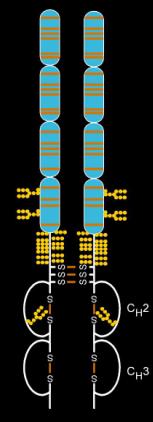
Structure
Etanercept is made from the combination of two naturally occurring soluble human 75-kilodalton TNF receptors linked to an Fc portion of an IgG1. The effect is an artificially engineered dimeric fusion protein.
Administration
Enbrel is marketed as a lyophylized powder in 25mg vials which must be reconstituted with a diluent and then injected subcutaneously, typically by the patient at home. Because patients with arthritis found the reconstitution procedure difficult, it was made available as pre-filled 50mg/ml syringes in late 2004 and a single-use 50mg autoinjector "pen" was brought to market in mid-2006. It cannot be administered orally, because the digestive system would destroy the drug. FDA approved dose is 25 mg BIW (twice weekly) or 50 mg QW (once weekly).
Safety
According to the product labeling of infliximab, etanercept, and adalimumab, these drugs are in the class of immunosuppressants. After a number of studies and reports of adverse reactions in patients receiving anti-TNF alpha therapy (including serious and sometimes fatal blood disorders, infections, rare reports of lymphoma and solid tissue cancers, rare reports of serious liver injury, and rare reports of demyelinating central nervous system disorders), rare reports of congestive heart failure, the U.S. Food and Drug Administration issued a warning to doctors appearing in the respective product labeling of these drugs instructing them to screen and monitor potential patients more carefully. Although these three agents are all biologic anti-TNF therapeutics, their methods of administration, dosing, and side effect profiles are somewhat different. These differences may be accounted for by fundamental differences in their biologic structure. Both infliximab and adalimumab fix complement, and have the ability to lyze cells. While potentially contributing to their therapeutic efficacy in disorders such as Crohn's Disease (for which both of these monoclonal antibodies are now FDA-approved), these mABs also carry black-box warnings which are not shared by etanercept. In addition infliximab has a higher propensity for the development of anaphylaxis, perhaps as a result both of its chimeric structure and its intravenous route of administration.
Sales
Enbrel is the most widely used anti-TNF biologic drug in the field of rheumatology with more patients taking this drug for that indication than either Remicade (infliximab) or Humira (adalimumab). Remicade (infliximab) is the most widely used anti-TNF biologic drug when all FDA approved indications (uses) of the drugs are considered, including Crohn's disease and ulcerative colitis, two autoimmune diseases for which etanercept does not have an FDA approved indication. In addition to their labeled indications, there are multiple published, peer-reviewed scientific studies suggesting potential uses for off-label indications, for which the FDA has not verified either safety or efficacy. Certolizumab pegol is a fourth biologic anti-TNF therapeutic which is currently awaiting FDA-approval in the U.S., for an initial indication of Crohn's Disease.
Similar agents
External links
See also
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- TNF-alpha inhibitors
- Amgen
- Immunosuppressive agents
- Recombinant proteins