Drug-induced hepatitis: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | __NOTOC__ | ||
{{Drug-induced hepatitis}} | |||
{{CMG}} | {{CMG}} | ||
==Overview== | |||
More than 900 drugs have been implicated in causing liver injury<ref name="isbn0-8385-1551-7">{{cite book |author=Friedman, Scott E.; Grendell, James H.; McQuaid, Kenneth R. |title=Current diagnosis & treatment in gastroenterology |publisher=Lang Medical Books/McGraw-Hill |location=New York |year=2003 |pages=p664-679 |isbn=0-8385-1551-7 |oclc= |doi=}}</ref> and it is the most common reason for a drug to be withdrawn from the market. Chemicals often cause [[subclinical]] injury to liver which manifests only as abnormal [[Liver function tests|liver enzyme tests]]. Drug induced liver injury is responsible for 5% of all hospital admissions and 50% of all [[acute liver failure]]s.<ref name="isbn1-56053-618-7">{{cite book |author=McNally, Peter F. |title=GI/Liver Secrets: with STUDENT CONSULT Access |publisher=C.V. Mosby |location=Saint Louis |year= |pages= |isbn=1-56053-618-7 |oclc= |doi=}}</ref><ref>{{cite journal |author=Ostapowicz G, Fontana RJ, Schiødt FV, ''et al'' |title=Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States |journal=Ann. Intern. Med. |volume=137 |issue=12 |pages=947–54 |year=2002 |pmid=12484709 |doi=}}</ref> | More than 900 drugs have been implicated in causing liver injury<ref name="isbn0-8385-1551-7">{{cite book |author=Friedman, Scott E.; Grendell, James H.; McQuaid, Kenneth R. |title=Current diagnosis & treatment in gastroenterology |publisher=Lang Medical Books/McGraw-Hill |location=New York |year=2003 |pages=p664-679 |isbn=0-8385-1551-7 |oclc= |doi=}}</ref> and it is the most common reason for a drug to be withdrawn from the market. Chemicals often cause [[subclinical]] injury to liver which manifests only as abnormal [[Liver function tests|liver enzyme tests]]. Drug induced liver injury is responsible for 5% of all hospital admissions and 50% of all [[acute liver failure]]s.<ref name="isbn1-56053-618-7">{{cite book |author=McNally, Peter F. |title=GI/Liver Secrets: with STUDENT CONSULT Access |publisher=C.V. Mosby |location=Saint Louis |year= |pages= |isbn=1-56053-618-7 |oclc= |doi=}}</ref><ref>{{cite journal |author=Ostapowicz G, Fontana RJ, Schiødt FV, ''et al'' |title=Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States |journal=Ann. Intern. Med. |volume=137 |issue=12 |pages=947–54 |year=2002 |pmid=12484709 |doi=}}</ref> | ||
Revision as of 19:08, 20 November 2012
Template:Drug-induced hepatitis Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
More than 900 drugs have been implicated in causing liver injury[1] and it is the most common reason for a drug to be withdrawn from the market. Chemicals often cause subclinical injury to liver which manifests only as abnormal liver enzyme tests. Drug induced liver injury is responsible for 5% of all hospital admissions and 50% of all acute liver failures.[2][3]
Pathophysiology
A- Pathophysiologic mechanisms
The pathophysiologic mechanisms of hepatotoxicity are still being explored and include both hepatocellular and extracellular mechanisms. The following are some of the mechanisms that have been described:
Apoptosis of hepatocytes
Activation of the apoptotic pathways by the tumor necrosis factor-alpha receptor of Fas may trigger the cascade of intercellular caspases, which results in programmed cell death.
Bile duct injury
Toxic metabolites excreted in bile may cause injury to the bile duct epithelium.
Cytolytic T-cell activation
Covalent binding of a drug to the P450 enzyme acts as an immunogen, activating T cells and cytokines and stimulating a multifaceted immune response.
Disruption of the hepatocyte
Covalent binding of the drug to intracellular proteins can cause a decrease in ATP levels, leading to actin disruption. Disassembly of actin fibrils at the surface of the hepatocyte causes blebs and rupture of the membrane.
Disruption of the transport proteins
Drugs that affect transport proteins at the canalicular membrane can interrupt bile flow. Loss of villous processes and interruption of transport pumps such as multidrug resistance–associated protein 3 prevent the excretion of bilirubin, causing cholestasis.
Mitochondrial disruption
Certain drugs inhibit mitochondrial function by a dual effect on both beta-oxidation energy production by inhibiting the synthesis of nicotinamide adenine dinucleotide and flavin adenine dinucleotide, resulting in decreased ATP production.
B- Drug toxicity mechanisms
The classic division of drug reactions is into at least 2 major groups,
- Drugs that directly affect the liver
- Drugs that mediate an immune response.
Hypersensitivity
Phenytoin is a classic, if not common, cause of hypersensitivity reactions. The response is characterized by fever, rash, and eosinophilia and is an immune-related response with a typical short latency period of 1-4 weeks.
Idiosyncratic drug reactions
Idiosyncratic drug reactions can be subdivided into those that are classified as hypersensitivity or immunoallergic and those that are metabolic-idiosyncratic.
Intrinsic or predictable drug reactions
Drugs that fall into this category cause reproducible injuries in animals, and the injury is dose related. The *injury can be due to the drug itself or to a metabolite. Acetaminophen is a classic example of a known intrinsic or predictable hepatotoxin at supertherapeutic doses. Another classic example is carbon tetrachloride.
Metabolic-idiosyncratic
This type of reaction occurs through an indirect metabolite of the offending drug. Unlike intrinsic hepatotoxins, the response rate is variable and can occur within a week or up to one year later. It occurs in a minority of patients taking the drug, and no clinical manifestations of hypersensitivity are noted. INH toxicity is considered to fall into this class. Not all drugs fall neatly into one of these categories, and overlapping mechanisms may occur with some drugs (e.g., halothane).
Drug metabolism in liver
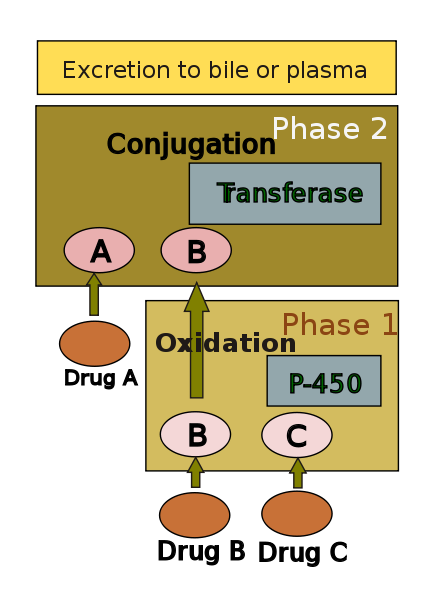
The human body identifies almost all drugs as foreign substances (i.e. xenobiotics) and subjects them to various chemical processes (i.e. metabolism) to make them suitable for elimination. This involves chemical transformations to (a) reduce fat solubility and (b) to change biological activity. Although almost all tissue in the body have some ability to metabolize chemicals, smooth endoplasmic reticulum in liver is the principal "metabolic clearing house" for both endogenous chemicals (e.g., cholesterol, steroid hormones, fatty acids, and proteins), and exogenous substances (e.g. drugs).[4] The central role played by liver in the clearance and transformation of chemicals also makes it susceptible to drug induced injury.
Drug metabolism is usually divided into two phases: phase 1 and phase 2. Phase 1 reaction is thought to prepare a drug for phase 2. However many compounds can be metabolised by phase 2 directly. Phase 1 reaction involves oxidation, reduction, hydrolysis, hydration and many other rare chemical reactions. These processes tend to increase water solubility of the drug and can generate metabolites which are more chemically active and potentially toxic. Most of phase 2 reactions take place in cytosol and involve conjugation with endogenous compounds via transferase enzymes. Chemically active phase 1 products are rendered relatively inert and suitable for elimination by this step.
A group of enzymes located in the endoplasmic reticulum, known as cytochrome P-450, is the most important family of metabolizing enzymes in the liver. Cytochrome P-450 is the terminal oxidase component of an electron transport chain. It is not a single enzyme, rather consists of a family of closely related 50 isoforms, six of them metabolize 90% of drugs.[5][6] There is a tremendous diversity of individual P-450 gene products and this heterogeneity allows the liver to perform oxidation on a vast array of chemicals (including almost all drugs) in phase 1. Three important characteristics of the P450 system have roles in drug induced toxicity:
- 1. Genetic diversity:
Each of the P-450 proteins is unique and accounts to some extent for the variation in drug metabolism between individuals. Genetic variations (polymorphism) in CYP450 metabolism should be considered when patients exhibit unusual sensitivity or resistance to drug effects at normal doses. Such polymorphism is also responsible for variable drug response among patients of differing ethnic backgrounds.
| Potent inducers | Potent inhibitors | Substrates |
|---|---|---|
| Rifampicin, Carbamazepine, Phenobarbital, Phenytoin, (St John's wort), |
Amiodarone, cimetidine, ciprofloxacin, fluconazole, fluoxetine, erythromycin, isoniazid, diltiazem |
Caffeine, clozapine, omeprazole, losartan, theophylline |
- 2. Change in enzyme activity:
Many substances can influence P-450 enzyme mechanism. Drugs interact with the enzyme family in several ways.[9] Drugs that modify Cytochrome P-450 enzyme are referred to as either inhibitors or inducers. Enzyme inhibitors block the metabolic activity of one or several P-450 enzymes. This effect usually occurs immediately. On the other hand inducers increase P-450 activity by increasing its synthesis. Depending on inducing drug's half life, there is usually a delay before enzyme activity increases.[6]
- 3. Competitive inhibition:
Some drugs may share the same P-450 specificity and thus competitively block their bio transformation. This may lead to accumulation of drugs metabolised by the enzyme. This type of drug interaction may also reduce the rate of generation of toxic substrate.
Patterns of injury
| Type of injury: | Hepatocellular | Cholestatic | Mixed |
|---|---|---|---|
| ALT | ≥ Twofold rise | Normal | ≥ Twofold rise |
| ALP | Normal | ≥ Twofold rise | ≥ Twofold rise |
| ALT: ALP ratio | High, ≥5 | Low, ≤2 | 2-5 |
| Examples[10] | Acetaminophen Allopurinol Amiodarone HAART NSAID |
Anabolic steroid Chlorpromazine Clopidogrel Erythromycin Hormonal contraception |
Amitryptyline, Enalapril Carbamazepine Sulphonamide Phenytoin |
Chemicals produce a wide variety of clinical and pathological hepatic injury. Biochemical markers (i.e. alanine transferase, alkaline phosphatase and bilirubin) are often used to indicate liver damage. Liver injury is defined as rise in either (a) ALT level more than three times of upper limit of normal (ULN), (b) ALP level more than twice ULN, or (c) total bilirubin level more than twice ULN when associated with increased ALT or ALP.[11][10] Liver damage is further characterized into hepatocellular (predominantly initial Alanine transferase elevation) and cholestatic (initial alkaline phosphatase rise) types. However they are not mutually exclusive and mixed type of injuries are often encountered.
Specific histo-pathological patterns of liver injury from drug induced damage are discussed below.
Zonal Necrosis
This is the most common type of drug induced liver cell necrosis where the injury is largely confined to a particular zone of the liver lobule. It may manifest as very high level of ALT and severe disturbance of liver function leading to acute liver failure.
In this pattern hepatocellular necrosis is associated with infiltration of inflammatory cells. There can be three types of drug induced hepatitis. (A) viral hepatitis type picture is the commonest, where histological features are similar to acute viral hepatitis. (B) in the focal or non specific hepatitis scattered foci of cell necrosis may accompany lymphocytic infiltrate. (C) chronic hepatitis type is very similar to autoimmune hepatitis clinically, serologically as well as histologically.
- Causes:
- (a) Viral hepatitis like: Halothane, Isoniazid, Phenytoin
- (b) Focal hepatitis: Aspirin
- (c) Chronic hepatitis: Methyldopa, Diclofenac
Liver injury leads to impairment of bile flow and clinical picture is predominated by itching and jaundice. Histology may show inflammation (cholestatic hepatitis) or it can be bland without any parenchymal inflammation. In rare occasions it can produce features similar to primary biliary cirrhosis due to progressive destruction of small bile ducts (Vanishing duct syndrome).
- Causes:
- (a) Bland: Oral contraceptive pills, anabolic steroid, Androgens
- (b) Inflammatory: Allopurinol, Co-amoxiclav, Carbamazepine
- (c) Ductal: Chlorpromazine, flucloxacillin
Hepatotoxicity may manifest as triglyceride accumulation which leads to either small droplet (microvesicular) or large droplet (macrovesicular) fatty liver. There is a separate type of steatosis where phospholipid accumulation leads to a pattern similar to the diseases with inherited phospholipid metabolism defects (e.g. Tay-Sachs disease)
- Causes:
- (a) Microvesicular: Aspirin (Reye's syndrome), Ketoprofen, Tetracycline
- (b) Macrovesicular: Acetamenophen, methotrexate
- (c) Phospholipidosis: Amiodarone, Total parenteral nutrition
Drug induced hepatic granulomas are usually associated with granulomas in other tissues and patients typically have features of systemic vasculitis and hypersensitivity. More than 50 drugs have been implicated.
- Causes:
- Allopurinol, Phenytoin, Isoniazid, Quinine, Penicillin, Quinidine
Vascular lesions
They result from injury to the vascular endothelium.
- Causes:
- Venoocclusive disease: Chemotherapeutic agents, bush tea
- Peliosis hepatis: anabolic steroid
- Hepatic vein thrombosis: Oral contraceptives
Neoplasms have been described with prolonged exposure to some medications or toxins. Hepatocellular carcinoma, angiosarcoma and liver adenomas are the ones usually reported.
Risk Factors
- Age: Apart from accidental exposure, hepatic drug reactions are rare in children. Elderly persons are at increased risk of hepatic injury because of decreased clearance, drug-to-drug interactions, reduced hepatic blood flow, variation in drug binding, and lower hepatic volume. In addition, poor diet, infections, and multiple hospitalizations are important reasons for drug-induced hepatotoxicity.
- Alcohol ingestion: Alcoholic persons are susceptible to drug toxicity because alcohol induces liver injury and cirrhotic changes that alter drug metabolism. Alcohol causes depletion of glutathione (hepatoprotective) stores that make the person more susceptible to toxicity by drugs.
- Drug formulation: Long-acting drugs may cause more injury than shorter-acting drugs.
- Gender: Although the reasons are unknown, hepatic drug reactions are more common in females.
- Genetic factors: A unique gene encodes each P-450 protein. Genetic differences in the P-450 enzymes can result in abnormal reactions to drugs, including idiosyncratic reactions. Debrisoquine is an antiarrhythmic drug that undergoes poor metabolism because of abnormal expression of P-450-II-D6. This can be identified by polymerase chain reaction amplification of mutant genes. This has led to the possibility of future detection of persons who can have abnormal reactions to a drug.
- Host factors that may enhance susceptibility to drugs, possibly inducing liver disease:
- AIDS - Dapsone, trimethoprim-sulfamethoxazole
- Diabetes mellitus - Methotrexate, niacin
- Fasting or malnutrition - Acetaminophen
- Female - Halothane, nitrofurantoin, sulindac
- Hepatitis C - Ibuprofen, ritonavir, flutamide
- Large body mass index / obesity - Halothane
- Male - Amoxicillin-clavulanic acid (Augmentin)
- Old age - Acetaminophen, halothane, INH, amoxicillin-clavulanic acid
- Preexisting liver disease - Niacin, tetracycline, methotrexate
- Renal failure - Tetracycline, allopurinol
- Young age - Salicylates, valproic acid
- Liver disease: In general, patients with chronic liver disease are not uniformly at increased risk of hepatic injury. Although the total cytochrome P450 is reduced, some may be affected more than others. The modification of doses in persons with liver disease should be based on the knowledge of the specific enzyme involved in the metabolism. Patients with HIV infection who are co-infected with hepatitis B or C virus are at increased risk for hepatotoxic effects when treated with antiretroviral therapy. Similarly, patients with cirrhosis are at increased risk of decompensation by toxic drugs.
- Other comorbidities: Persons with AIDS, persons who are malnourished, and persons who are fasting may be susceptible to drug reactions because of low glutathione stores.
- Race: Some drugs appear to have different toxicities based on race. For example, blacks and Hispanics may be more susceptible to isoniazid (INH) toxicity. The rate of metabolism is under the control of P450 enzymes and can vary from individual to individual.
List of Drugs that can cause Hepatitis
Drug that Effect Cytochrome P450
Inducers
- Carbamazepine
- Ethanol
- Glucocorticoids
- Griseofulvin
- Omeprazole - Induces P450 1A2
- Phenobarbital
- Phenytoin
- Primidone
- Quinine
- Rifampin
Inhibitors
- Amiodarone
- Cimetidine
- Erythromycin
- Grape fruit
- Isoniazid
- Ketoconazole
- Metronidazole
- Omeprazole - Inhibits P450 2C8
- Quinidine
- Sulfonamides
Drugs withdrawn for hepatotoxicity
Troglitazone, bromfenac, trovafloxacin, ebrotidine, nimesulide, nefazodone and ximelagatran.[12] [13]
Diagnosis
History and symptoms
- fever
- jaundice
- nausea
- rash or itchy red hives on skin
- vomiting
- decreased appetite
- flu-like symptoms
- joint pain
- sore muscles
Diagnostic Tests
- CBC
- Alkaline phosphatase
- AST / serum glutamic oxaloacetic transaminase (SGOT)
- ALT / serum glutamic pyruvate transaminase (SGPT)
- Albumin
- Gammaglobulin
- Prothrombin time after vitamin K
- Anti-mitochondrial antibody (AMA)
- Anti smooth muscle antibody (ASMA)
- Urinalysis
- Electrolytes
- Drug levels
- Ultrasonography
- CT
- MRI
- Liver biopsy
Differential Diagnosis
- Acute viral hepatitis
- Alcoholic liver disease
- Autoimmune hepatitis
- Budd-Chiari syndrome
- Cholangitis
- Cholecystitis
- Cholestatic liver disease
- Coagulation disorders
- Hemochromatosis
- Malignancy
- Pregnancy-related conditions of liver
- Shock liver
- Wilson disease
Treatment
There is no specific treatment for drug-induced hepatitis other than stopping the drug that is causing the problem.
Bed rest during the acute phase of the disease is essential part of the therapy, when the symptoms are most severe. If significant nausea and vomiting occur, fluids given through a vein may be needed.
Patients with acute hepatitis should avoid physical exertion, alcohol, acetaminophen, and any other substances that are harmful to the liver.
References
- ↑ Friedman, Scott E.; Grendell, James H.; McQuaid, Kenneth R. (2003). Current diagnosis & treatment in gastroenterology. New York: Lang Medical Books/McGraw-Hill. pp. p664–679. ISBN 0-8385-1551-7.
- ↑ McNally, Peter F. GI/Liver Secrets: with STUDENT CONSULT Access. Saint Louis: C.V. Mosby. ISBN 1-56053-618-7.
- ↑ Ostapowicz G, Fontana RJ, Schiødt FV; et al. (2002). "Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States". Ann. Intern. Med. 137 (12): 947–54. PMID 12484709.
- ↑ Donald Blumenthal; Laurence Brunton; Keith Parker; Lazo, John S.; Iain Buxton. Goodman and Gilman's Pharmacological Basis of Therapeutics Digital Edition. McGraw-Hill Professional. ISBN 0-07-146804-8.
- ↑ Skett, Paul; Gibson, G. Gordon (2001). Introduction to drug metabolism. Cheltenham, UK: Nelson Thornes Publishers. ISBN 0-7487-6011-3.
- ↑ 6.0 6.1 6.2 Lynch T, Price A (2007). "The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects". American family physician. 76 (3): 391–6. PMID 17708140.
- ↑ Jessica R. Oesterheld; Kelly L. Cozza; Armstrong, Scott. Concise Guide to Drug Interaction Principles for Medical Practice: Cytochrome P450s, Ugts, P-Glycoproteins. Washington, DC: American Psychiatric Association. pp. 167–396. ISBN 1-58562-111-0.
- ↑ Flockhart DA. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine (2007). http://medicine.iupui.edu/flockhart/table.htm. Accessed [29-09-2007]
- ↑ Michalets EL (1998). "Update: clinically significant cytochrome P-450 drug interactions". Pharmacotherapy. 18 (1): 84–112. PMID 9469685.
- ↑ 10.0 10.1 Mumoli N, Cei M, Cosimi A (2006). "Drug-related hepatotoxicity". N. Engl. J. Med. 354 (20): 2191–3, author reply 2191-3. PMID 16710915.
- ↑ Bénichou C (1990). "Criteria of drug-induced liver disorders. Report of an international consensus meeting". J. Hepatol. 11 (2): 272–6. PMID 2254635.
- ↑ PMID 17230599
- ↑ Shah RR (1999). "Drug-induced hepatotoxicity: pharmacokinetic perspectives and strategies for risk reduction". Adverse drug reactions and toxicological reviews. 18 (4): 181–233. PMID 10687025.