Digoxin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Synonyms / Brand Names: Digox; Lanoxin
Disclaimer
WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs. Please read our full disclaimer here.
Black Box Warning
FDA Package Insert for Digoxin contains no information regarding Black Box Warning.
Overview
Digoxin is a cardiac glycoside drug that is FDA approved for the treatment of atrial fibrillation, atrial flutter, and heart failure. Common adverse reactions include dizziness, headache, mental disorder, nausea, and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Heart failure
- Dosing Information
- Loading dose: Oral10-15 mcg/kg half of dose administered initially, and the other two quarters are given every 6-8 hours twice.
Intravenous 8-12 mcg/kg half of the dose is given initially, and the other two quarters are given each 6-8 hours in two doses.
- Maintenance dose: Oral3.4-5.1 mcg/kg/day once daily.
Intravenous 2.4-3.6 mcg/kh/day once daily.
Atrial fibrillation
- Dosing Information
- Loading dose: Oral 10-15 mcg/kg PO half of dose administered initially, and the other two quarters are given every 6-8 hours twice.
Intravenous 8-12 mcg/kg half of the dose is given initially, and the other two quarters are given each 6-8 hours in two doses.
- Maintenance dose: Oral 3.4-5.1 mcg/kg/day PO once daily.
Intravenous 2.4-3.6 mcg/kh/day once daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Heart failure
- Dosing Information
- Loading dose
- 5-10 years old: 20-45 mcg/kg Administer half the total loading dose initially, then ¼ the loading dose every 6 to 8 hours twice.
- >10 year old: 10-15 mcg/kg Administer half the total loading dose initially, then ¼ the loading dose every 6 to 8 hours twice.
- Maintenence dose:
- 2-5 years: 10-15 mcg/kg/day.
- 5-10 years: 7-10 mcg/kg/day.
- More than 10 years: 3-5 mcg/kg/day.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Supraventricular tachycardia, Recurrent; Prophylaxis
- Dosage information
Fetal tachycardia - Supraventricular tachycardia
- Dosage information
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
Ventricular Fibrillation in Patients With Accessory AV Pathway (Wolff-Parkinson-White Syndrome)
Patients with Wolff-Parkinson-White syndrome who develop atrial fibrillation are at high risk of ventricular fibrillation. Treatment of these patients with digoxin leads to greater slowing of conduction in the atrioventricular node than in accessory pathways, and the risks of rapid ventricular response leading to ventricular fibrillation are thereby increased.
Sinus Bradycardia and Sino-atrial Block
Digoxin may cause severe sinus bradycardia or sino-atrial block particularly in patients with pre-existing sinus node disease and may cause advanced or complete heart block in patients with pre-existing incomplete AV block. Consider insertion of a pacemaker before treatment with digoxin.
Digoxin Toxicity
Signs and symptoms of digoxin toxicity include anorexia, nausea, vomiting, visual changes and cardiac arrhythmias [first-degree, second-degree (Wenckebach), or third-degree heart block (including asystole); atrial tachycardia with block; AV dissociation; accelerated junctional (nodal) rhythm; unifocal or multiform ventricular premature contractions (especially bigeminy or trigeminy); ventricular tachycardia; and ventricular fibrillation]. Toxicity is usually associated with digoxin levels greater than 2 ng/mL although symptoms may also occur at lower levels. Low body weight, advanced age or impaired renal function, hypokalemia, hypercalcemia, or hypomagnesemia may predispose to digoxin toxicity. Obtain serum digoxin levels in patients with signs or symptoms of digoxin therapy and interrupt or adjust dose if necessary [see Adverse Reactions (6) and Overdosage (10)]. Assess serum electrolytes and renal function periodically. The earliest and most frequent manifestation of digoxin toxicity in infants and children is the appearance of cardiac arrhythmias, including sinus bradycardia. In children, the use of digoxin may produce any arrhythmia. The most common are conduction disturbances or supraventricular tachyarrhythmias, such as atrial tachycardia (with or without block) and junctional (nodal) tachycardia. Ventricular arrhythmias are less common. Sinus bradycardia may be a sign of impending digoxin intoxication, especially in infants, even in the absence of first-degree heart block. Any arrhythmias or alteration in cardiac conduction that develops in a child taking digoxin should initially be assumed to be a consequence of digoxin intoxication.
Misidentification of Digoxin Toxicity
Given that adult patients with heart failure have some symptoms in common with digoxin toxicity, it may be difficult to distinguish digoxin toxicity from heart failure. Misidentification of their etiology might lead the clinician to continue or increase digoxin dosing, when dosing should actually be suspended. When the etiology of these signs and symptoms is not clear, measure serum digoxin levels.
Risk of Ventricular Arrhythmias During Electrical Cardioversion
It may be desirable to reduce the dose of or discontinue digoxin for 1-2 days prior to electrical cardioversion of atrial fibrillation to avoid the induction of ventricular arrhythmias, but physicians must consider the consequences of increasing the ventricular response if digoxin is decreased or withdrawn. If digitalis toxicity is suspected, elective cardioversion should be delayed. If it is not prudent to delay cardioversion, the lowest possible energy level should be selected to avoid provoking ventricular arrhythmias.
Risk of Ischemia in Patients With Acute Myocardial Infarction
Digoxin is not recommended in patients with acute myocardial infarction because digoxin may increase myocardial oxygen demand and lead to ischemia.
Vasoconstriction in Patients With Myocarditis
Digoxin can precipitate vasoconstriction and may promote production of pro-inflammatory cytokines; therefore, avoid use in patients with myocarditis.
Decreased Cardiac Output in Patients With Preserved Left Ventricular Systolic Function
Patients with heart failure associated with preserved left ventricular ejection fraction may experience decreased cardiac output with use of digoxin. Such disorders include restrictive cardiomyopathy, constrictive pericarditis, amyloid heart disease, and acute cor pulmonale. Patients with idiopathic hypertrophic subaortic stenosis may have worsening of the outflow obstruction due to the inotropic effects of digoxin. Patients with amyloid heart disease may be more susceptible to digoxin toxicity at therapeutic levels because of an increased binding of digoxin to extracellular amyloid fibrils. Digoxin should generally be avoided in these patients, although it has been used for ventricular rate control in the subgroup of patients with atrial fibrillation.
Reduced Efficacy in Patients With Hypocalcemia
Hypocalcemia can nullify the effects of digoxin in humans; thus, digoxin may be ineffective until serum calcium is restored to normal. These interactions are related to the fact that digoxin affects contractility and excitability of the heart in a manner similar to that of calcium.
Altered Response in Thyroid Disorders and Hypermetabolic States
Hypothyroidism may reduce the requirements for digoxin. Heart failure and/or atrial arrhythmias resulting from hypermetabolic or hyperdynamic states (e.g., hyperthyroidism, hypoxia, or arteriovenous shunt) are best treated by addressing the underlying condition. Atrial arrhythmias associated with hypermetabolic states are particularly resistant to digoxin treatment. Patients with beri beri heart disease may fail to respond adequately to digoxin if the underlying thiamine deficiency is not treated concomitantly.
Adverse Reactions
Clinical Trials Experience
Nervous System
- Visual disturbances (blurred or yellow vision), headache, weakness, dizziness, apathy, confusion, and mental disturbances (such as anxiety, depression, delirium, and hallucination).
Cardiovascular
- Arrhythmia( first-degree, second-degree (Wenckebach), or third-degree heart block (including asystole); atrial tachycardia with block; AV dissociation; accelerated junctional (nodal) rhythm; unifocal or multiform ventricular premature contractions (especially bigeminy or trigeminy); ventricular tachycardia; and ventricular fibrillation).
Gastrointestinal
Miscellaneous
- Gynecomastia, thrombocytopenia and maculopapular rash.
Infant and Children
The side effects of digoxin in infants and children differ from those seen in adults in several respects. Although digoxin may produce anorexia, nausea, vomiting, diarrhea, and CNS disturbances in young patients, these are rarely the initial symptoms of overdosage. Rather, the earliest and most frequent manifestation of excessive dosing with digoxin in infants and children is the appearance of cardiac arrhythmias, including sinus bradycardia. In children, the use of digoxin may produce any arrhythmia. The most common are conduction disturbances or supraventricular tachyarrhythmias, such as atrial tachycardia (with or without block) and junctional (nodal) tachycardia. Ventricular arrhythmias are less common. Sinus bradycardia may be a sign of impending digoxin intoxication, especially in infants, even in the absence of first-degree heart block. Any arrhythmia or alteration in cardiac conduction that develops in a child taking digoxin should be assumed to be caused by digoxin, until further evaluation proves otherwise.
Postmarketing Experience
FDA Package Insert for digoxin tablet contains no information regarding 'Postmarketing Experience'.
Drug Interactions
- Potassium-depleting diuretics
- Calcium
- Quinidine, verapamil, amiodarone, propafenone, indomethacin, itraconazole, alprazolam, and spironolactone
- Erythromycin, clarithromycin, and tetracycline
- Propantheline and diphenoxylate
- Antacids, kaolin-pectin, sulfasalazine, neomycin, cholestyramine, certain anticancer drugs, and metoclopramide
- Rifampin
- Thyroxine
- Sympathomimetics
- Succinylcholine
- Calcium Channel Blockers
- Beta blockers
Potassium-depleting diuretics
Potassium-depleting diuretics are a major contributing factor to digitalis toxicity.
Calcium
Calcium, particularly if administered rapidly by the intravenous route, may produce serious arrhythmias in digitalized patients.
Quinidine, verapamil, amiodarone, propafenone, indomethacin, itraconazole, alprazolam, and spironolactone
Quinidine, verapamil, amiodarone, propafenone, indomethacin, itraconazole, alprazolam, and spironolactone raise the serum digoxin concentration due to a reduction in clearance and/or in volume of distribution of the drug, with the implication that digitalis intoxication may result.
Erythromycin, clarithromycin, and tetracycline
Erythromycin and clarithromycin (and possibly othermacrolide antibiotics) and tetracycline may increase digoxin absorption in patients who inactivate digoxin by bacterial metabolism in the lower intestine, so that digitalis intoxication may result (see Pharmacology).
Propantheline and diphenoxylate
Decrease gut motility, which may increase digoxin absorption.
Antacids, kaolin-pectin, sulfasalazine, neomycin, cholestyramine, certain anticancer drugs, and metoclopramide
May interfere with intestinal digoxin absorption, resulting in unexpectedly low serum concentrations.
Rifampin
May decrease serum digoxin concentration, especially in patients with renal dysfunction, by increasing the non-renal clearance of digoxin.
Thyroxine
Thyroid administration to a digitalized, hypothyroid patient may increase the dose requirement of digoxin.
Sympathomimetics
Concomitant use of digoxin and sympathomimetics increases the risk of cardiac arrhythmias.
Succinylcholine
Succinylcholine may cause a sudden extrusion of potassium from muscle cells, and may thereby cause arrhythmias in digitalized patients.
Calcium Channel Blockers
Use with digoxin may be useful in combination to control atrial fibrillation, their additive effects on AV node conduction can result in advanced or complete heart block.
Beta blockers
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. Digoxin concentrations are increased by about 15% when digoxin and carvedilol are administered concomitantly. Therefore, increased monitoring of digoxin is recommended when initiating, adjusting, or discontinuing carvedilol.
Use in Specific Populations
Pregnancy
Animal reproduction studies have not been conducted with digoxin. It is also not known whether digoxin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Digoxin should be given to a pregnant woman only if clearly needed.
Labor and Delivery
(Description)
Nursing Mothers
Studies have shown that digoxin concentrations in the mother’s serum and milk are similar. However, the estimated exposure of a nursing infant to digoxin via breastfeeding will be far below the usual infant maintenance dose. Therefore, this amount should have no pharmacologic effect upon the infant. Nevertheless, caution should be exercised when digoxin is administered to a nursing woman.
Pediatric Use
Newborn infants display considerable variability in their tolerance to digoxin. Premature and immature infants are particularly sensitive to the effects of digoxin, and the dosage of the drug must not only be reduced but must be individualized according to their degree of maturity. Digitalis glycosides can cause poisoning in children due to accidental ingestion.
Geriatric Use
The majority of clinical experience gained with digoxin has been in the elderly population. This experience has not identified differences in response or adverse effects between the elderly and younger patients. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, which should be based on renal function, and it may be useful to monitor renal function
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Carcinogenesis, Mutagenesis, Impairment of Fertility
(Description)
Immunocompromised Patients
(Description)
Miscellaneous
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
| File:Digoxin structure 2.svg | |
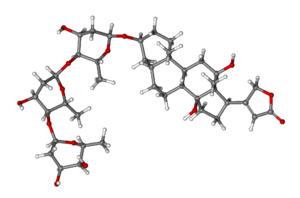 | |
| Clinical data | |
|---|---|
| Trade names | Lanoxin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682301 |
| Pregnancy category | |
| Routes of administration | Oral, Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60 to 80% (Oral) |
| Protein binding | 25% |
| Metabolism | Hepatic (16%) |
| Elimination half-life | 36 to 48 hours (patients with normal renal function) 3.5 to 5 days (patients with impaired renal function) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C41H64O14 |
| Molar mass | 780.938 g/mol |
| 3D model (JSmol) | |
| Melting point | 249.3 °C (480.74 °F) |
| Solubility in water | 0.0648 mg/mL (20 °C) |
| |
| |
| (verify) | |
Mechanism of Action
Mechanism of Action
All of digoxin’s actions are mediated through its effects on Na-K ATPase. This enzyme, the “sodium pump,” is responsible for maintaining the intracellular milieu throughout the body by moving sodium ions out of and potassium ions into cells. By inhibiting Na-K ATPase, digoxin
- causes increased availability of intracellular calcium in the myocardium and conduction system, with consequent increased inotropy, increased automaticity, and reduced conduction velocity
- indirectly causes parasympathetic stimulation of the autonomic nervous system, with consequent effects on the sino-atrial (SA) and atrioventricular (AV) nodes
- reduces catecholamine reuptake at nerve terminals, rendering blood vessels more sensitive to endogenous or exogenous catecholamines
- increases baroreceptor sensitization, with consequent increased carotid sinus nerve activity and enhanced sympathetic withdrawal for any given increment in mean arterial pressure
- increases (at higher concentrations) sympathetic outflow from the central nervous system (CNS) to both cardiac and peripheral sympathetic nerves
allows (at higher concentrations) progressive efflux of intracellular potassium, with consequent increase in serum potassium levels.
The cardiologic consequences of these direct and indirect effects are an increase in the force and velocity of myocardial systolic contraction (positive inotropic action), a slowing of the heart rate (negative chronotropic effect), decreased conduction velocity through the AV node, and a decrease in the degree of activation of the sympathetic nervous system and renin-angiotensin system (neurohormonal deactivating effect).
Structure
Digoxin is one of the cardiac (or digitalis) glycosides, a closely related group of drugs having in common specific effects on the myocardium. These drugs are found in a number of plants. Digoxin is extracted from the leaves of Digitalis lanata. The term “digitalis” is used to designate the whole group of glycosides. The glycosides are composed of 2 portions: a sugar and a cardenolide (hence “glycosides”).
Digoxin is described chemically as (3β,5β,12β)-3-[(O-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1→4)-O-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1→4)-2,6-dideoxy-β-D-ribo-hexopyranosyl)oxy]-12,14-dihydroxy-card-20(22)-enolide. Its molecular formula is C41H64O14, its molecular weight is 780.95, and its structural formula is:
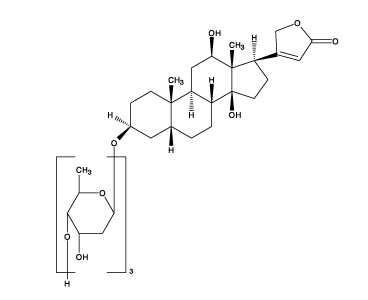 |
Digoxin exists as clear to white odorless crystals or white, odorless crystalline powder that melts with decomposition above 230°C. The drug is practically insoluble in water and in ether; slightly soluble in diluted (50%) alcohol and in chloroform; and freely soluble in pyridine.
Digoxin injection USP is a sterile solution of digoxin for intravenous or intramuscular injection. Each mL contains: digoxin 0.25 mg, propylene glycol 40% (v/v), anhydrous ethanol 10% (v/v), dibasic sodium phosphate 0.3% (w/v) and anhydrous citric acid 0.08% (w/v) to adjust pH between 6.8 and 7.2, and water for injection. Dilution is not required.
Pharmacodynamics
The times to onset of pharmacologic effect and to peak effect of preparations of digoxin are shown in Table 7.
 |
Hemodynamic Effects: Short- and long-term therapy with the drug increases cardiac output and lowers pulmonary artery pressure, pulmonary capillary wedge pressure, and systemic vascular resistance in patients with heart failure. These hemodynamic effects are accompanied by an increase in the left ventricular ejection fraction and a decrease in end-systolic and end-diastolic dimensions.
ECG Changes: The use of therapeutic doses of digoxin may cause prolongation of thePR interval and depression of theST segment on the electrocardiogram. Digoxin may produce false positive ST-T changes on the electrocardiogram during exercise testing. These electrophysiologic effects are not indicative of toxicity. Digoxin does not significantly reduce heart rate during exercise.
Pharmacokinetics
Note: the following data are from studies performed in adults, unless otherwise stated.
Comparisons of the systemic availability and equivalent doses for oral preparations of digoxin are shown in Table 6 [see Dosage and Administration].
Distribution: Following drug administration, a 6 to 8 hour tissue distribution phase is observed. This is followed by a much more gradual decline in the serum concentration of the drug, which is dependent on the elimination of digoxin from the body. The peak height and slope of the early portion (absorption/distribution phases) of the serum concentration-time curve are dependent upon the route of administration and the absorption characteristics of the formulation. Clinical evidence indicates that the early high serum concentrations do not reflect the concentration of digoxin at its site of action, but that with chronic use, the steady-state post-distribution serum concentrations are in equilibrium with tissue concentrations and correlate with pharmacologic effects. In individual patients, these post-distribution serum concentrations may be useful in evaluating therapeutic and toxic effects [seeDosage and Administration (2.1)].
Digoxin is concentrated in tissues and therefore has a large apparent volume of distribution (approximately 475 to 500 L). Digoxin crosses both the blood-brain barrier and the placenta. At delivery, the serum digoxin concentration in the newborn is similar to the serum concentration in the mother. Approximately 25% of digoxin in the plasma is bound to protein. Serum digoxin concentrations are not significantly altered by large changes in fat tissue weight, so that its distribution space correlates best with lean (i.e., ideal) body weight, not total body weight.
Metabolism: Only a small percentage (13%) of a dose of digoxin is metabolized in healthy volunteers. The urinary metabolites, which include dihydrodigoxin, digoxigenin bisdigitoxoside, and their glucuronide and sulfate conjugates are polar in nature and are postulated to be formed via hydrolysis, oxidation, and conjugation. The metabolism of digoxin is not dependent upon the cytochrome P-450 system, and digoxin is not known to induce or inhibit thecytochrome P-450 system.
Excretion: Elimination of digoxin follows first-order kinetics (that is, the quantity of digoxin eliminated at any time is proportional to the total body content). Following intravenous administration to healthy volunteers, 50 to 70% of a digoxin dose is excreted unchanged in the urine. Renal excretion of digoxin is proportional to creatinine clearance and is largely independent of urine flow. In healthy volunteers with normal renal function, digoxin has a half-life of 1.5 to 2 days. The half-life in anuric patients is prolonged to 3.5 to 5 days. Digoxin is not effectively removed from the body by dialysis, exchange transfusion, or during cardiopulmonary bypass because most of the drug is bound to extravascular tissues.
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
- National Drug Code (NDC):
- Storage:
- Manufactured by:
- Distributed by:
Images
Drug Images
Drug Name: |
Package and Label Display Panel
(Package Images)
(Display Panel Images)
Patient Information
Patient Information from FDA
- Advise patients that digoxin is a cardiac glycoside used to treat heart failure and heart arrhythmias.
- Instruct patients to take this medication as directed by their physician.
- Advise patients that many drugs can interact with digoxin. Instruct patients to inform their doctor and pharmacist if they are taking any over the counter medications, including herbal *medication, or are started on a new prescription.
- Advise patient that blood tests will be necessary to ensure that their digoxin dose is appropriate for them.
- Advise patients to contact their doctor or a health care professional if they experience nausea, vomiting, persistent diarrhea, confusion, weakness, or visual disturbances (including *blurred vision, green-yellow color disturbances, halo effect) as these could be signs that the dose of digoxin may be too high.
- Advise parents or caregivers that the symptoms of having too high digoxin doses may be difficult to recognize in infants and pediatric patients. Symptoms such as weight loss, failure to thrive in infants, abdominal pain, and behavioral disturbances may be indications of digoxin toxicity.
- Suggest to the patient to monitor and record their heart rate and blood pressure daily.
- Instruct women of childbearing potential who become or are planning to become pregnant to consult a physician prior to initiation or continuing therapy with digoxin.
Patient Information from NLM
(Link to patient information page)
Precautions with Alcohol
Alcohol-Digoxin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Brand Names®
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Price
References
- ↑ Pfammatter, JP.; Stocker, FP. (1998). "Re-entrant supraventricular tachycardia in infancy: current role of prophylactic digoxin treatment". Eur J Pediatr. 157 (2): 101–6. PMID 9504781. Unknown parameter
|month=ignored (help) - ↑ Sanatani, S.; Potts, JE.; Reed, JH.; Saul, JP.; Stephenson, EA.; Gibbs, KA.; Anderson, CC.; Mackie, AS.; Ro, PS. (2012). "The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants". Circ Arrhythm Electrophysiol. 5 (5): 984–91. doi:10.1161/CIRCEP.112.972620. PMID 22962431. Unknown parameter
|month=ignored (help) - ↑ Lilja, H.; Karlsson, K.; Lindecrantz, K.; Sabel, KG. (1984). "Treatment of intrauterine supraventricular tachycardia with digoxin and verapamil". J Perinat Med. 12 (3): 151–4. PMID 6502442.
- Pages with script errors
- Pages with citations using unsupported parameters
- Pages with broken file links
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to watched fields