Diabetic retinopathy: Difference between revisions
| Line 126: | Line 126: | ||
* Blood pressure measurement, | * Blood pressure measurement, | ||
* [[BMI]] calculation | * [[BMI]] calculation | ||
* Monitoring [[ | * Monitoring fasting [[blood sugar]] | ||
* Measuring | * Measuring HbA1c, | ||
* The history of any visual symptoms and changes in the vision | * The history of any visual symptoms and changes in the vision | ||
* | * Visual acuity test | ||
* | * Fundoscopic examination using ophthalmoscopy or slit lamp bio-microscopy | ||
The personnel performing the examination require considerable training accreditation. | The personnel performing the examination require considerable training accreditation. | ||
| Line 139: | Line 139: | ||
[[Cotton wool spots]] are an abnormal finding on [[fundoscope|fundoscopic]] exam of the [[retina]]. They appear as puffy white patches on the [[retina]]. They are caused by damage to nerve fibers. The nerve fibers are damaged by swelling in the surface layer of the retina. The cause of this swelling is due to the reduced [[axonal]] transport (and hence backlog of intracellular products) within the nerves because of the ischemia.{{clr}} | [[Cotton wool spots]] are an abnormal finding on [[fundoscope|fundoscopic]] exam of the [[retina]]. They appear as puffy white patches on the [[retina]]. They are caused by damage to nerve fibers. The nerve fibers are damaged by swelling in the surface layer of the retina. The cause of this swelling is due to the reduced [[axonal]] transport (and hence backlog of intracellular products) within the nerves because of the ischemia.{{clr}} | ||
===Flame Hemorrhages=== | ===Flame Hemorrhages=== | ||
[[Image:Flame-hemorrhage.jpg|thumb|left|A dark red flame hemorrhage in the retina of a diabetic is shown at the arrow: Credit: University of Michigan Kellogg Eye Center]] Flame hemorrhages are flame shaped hemorrhages located in the superficial nerve fiber layer of the retina that appear dark dark red on fundoscopic examination. Flame hemorrhages are caused by leakage from arterioles due to ischemic damage or from veins that are ischemic or in under high pressure.{{clr}} | [[Image:Flame-hemorrhage.jpg|thumb|left|A dark red flame hemorrhage in the retina of a diabetic is shown at the arrow: Credit: University of Michigan Kellogg Eye Center]] [[Flame hemorrhages]] are flame shaped hemorrhages located in the superficial nerve fiber layer of the retina that appear dark dark red on fundoscopic examination. Flame hemorrhages are caused by leakage from arterioles due to ischemic damage or from veins that are ischemic or in under high pressure.{{clr}} | ||
===Dot Hemorrhages=== | ===Dot Hemorrhages=== | ||
Revision as of 15:54, 14 December 2012
For patient information click here
| Diabetic retinopathy | |
| ICD-10 | H36 (E10.3 E11.3 E12.3 E13.3 E14.3) |
|---|---|
| ICD-9 | 250.5 |
| DiseasesDB | 29372 |
|
Diabetic retinopathy Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Diabetic retinopathy On the Web |
|
American Roentgen Ray Society Images of Diabetic retinopathy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Priyamvada Singh, M.B.B.S. [2]; Cafer Zorkun, M.D., Ph.D. [3]; Afsaneh Morteza, MD-MPH [4]
Overview
Diabetic retinopathy is the most severe form of the several kinds of ocular complications caused by diabetes.It is an ocular manifestation of systemic disease which affects up to 80% of all diabetics who have had diabetes for 15 years or more. It is the leading cause of non traumatic blindness in adults. People with untreated diabetes are 25 times more at risk for blindness than the general population.Despite these intimidating statistics, research indicates that at least 90% of these new cases could be reduced if there was proper and vigilant treatment and monitoring of the eyes.
Historical Perspective
Although diabetes was a well known disease since 2nd AD, no one ever linked this disorder to the eye. In 1946, the french scientist, Appolinaire Bouchardat reported vision loss in the absence of cataract in patients with diabetes. After the introduction of ophthalmoscopes in 1985, Edward Jaeger firstly described the diabetic macular changes in the form of yellowish spots that permeated retina. These observations were challenged as there were no proofs whether macular changes were directly related to diabetes, or they were caused by hypertension and atherosclerosis. The debate continued until the beginning of the 20th century, when Arthur James Ballantyne suggested that diabetic retinopathy represents a unique form of vasculopathy and his work showed for the first time the role of capillary wall alterations in the development of diabetic retinopathy.[1]
Pathophysiology
The retina is a multicellular photon sensor, a unique component of the central nervous system, which is structured on the vessels.
Promoted by the observations that there is a selective loss of pericytes early in diabetic retinopathy, many researchers were attracted to these cells as the origin of the disease.[2]. Pericytes are enigmatic cells, which are regular components of all human tissues and organs. In contrast to arteries and arterioles where the coverage consists of the smooth muscle cells, the capillary system is individually covered by the pericytes. Pericytes are codependent on the endothelial cells. Normal pericytes have a contractile function that helps to regulate capillary blood flow. The loss of pericytes, due to diabetic inflammation, is followed by the loss of capillary endothelial cells. Apoptosis of the pericytes, leads to the disappearance of both types of cells. Since neurons in the retina have high metabolic requirements, the hypoxia that results from extensive retinal capillary cell death is a probable stimulus for the increased expression of molecules that enhance the breakdown of the blood–retinal barrier and lead to vascular proliferation or angiogenesis. [3] Angiogenesis is a complex process, characterized by a cascade of events:
1: Initial vasodilatation of existing vessels
2: Increased vascular permeability and degradation of the surrounding matrix,
3: Migration and tube forming of the activated and proliferating endothelial cells
4: Maturation and remodeling of these new vessels takes place to form a vascular network.
These new blood vessels are abnormal and fragile. They grow along the retina and along the surface of the vitreous. By themselves, these blood vessels do not cause symptoms or vision loss. However, they have thin, fragile walls, and they ultimately leak blood. Retinal damage can result from persistent vitreous haemorrhage. On the other hand, contraction of associated fibrous tissue formed by proliferative disease tissue can result in deformation of the retina and tractional retinal detachment. The detachment may tear the retina (rhegmatogenous) or may not (non-rhegmatogenous). The non-rhegmatogenous retinal detachment is worse and is characterized by the
1: Confined retina
2: A taut and shiny appearance
3: Concave retina toward the pupil
4: Disappearance of the sub retinal fluid shifting
The cascade of these events causes vision loss.[4]
Recent studies which are focused on the neural component of the retina, have shown that diabetic neuropathy of the neuroglial cells may play an important role in the pathophysiology of disease.[5]
Classification
The disease is classified according to types of lesions detected on fundoscopy into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Diabetic retinopathy affect both eyes in parallel. NPDR is subdivided into mild and moderate-to-severe forms.
Mild NPDR
- Microaneurysms
- Dot and blot hemorrhages
- Hard (intra-retinal) exudate
Moderate-to-severe NPDR
Is mild NPDR plus:
- Cotton-wool spots
- Venous beading and loops
- Intraretinal microvascular abnormalities ( IRMA )
PDR
- Neovascularization of the retina, optic disc or iris
- Fibrous tissue adherent to vitreous face of retina
- Retinal detachment
- Vitreous hemorrhage
- Pre-retinal hemorrhage
Some people develop a condition called macular edema. It occurs when the damaged blood vessels leak fluid and lipids onto the macula. The fluid makes the macula swell, which blurs vision.
Epidemiology and Demographics
The prevalence of retinopathy is strongly related to the duration of diabetes. Between 40 to 45 percent of Americans diagnosed with diabetes have some stage of diabetic retinopathy. [6] After 20 years of diabetes, nearly all patients with type 1 diabetes and >60% of patients with type 2 diabetes have some degree of retinopathy. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy, 3.6% of patients with type 1 diabetes and 1.6% of type 2 diabetes were legally blind. In the type 1 diabetes, 86% of blindness was attributable to diabetic retinopathy. The cumulative incidence of any retinopathy in type 1 diabetes was 97%. In the type 2 diabetic patients, where other eye diseases were common, one-third of the cases of legal blindness were due to diabetic retinopathy. [7]
Genetics
There are evidence that not every diabetic patients has the same risk of diabetic retinopathy. Studies have shown that polymorphisms of Tumor Necrosis Factor Alpha play an important role in the risk of patients with diabetic retinopathy.[8]
Risk Factors
All patients with diabetes mellitus are at risk of developing diabetic retinopathy. This includes those with Type I diabetes and those with Type II diabetes. During pregnancy, diabetic retinopathy may also be a problem for women with diabetes. It is recommended that all pregnant women with diabetes have dilated eye examinations each trimester to protect their vision.
Screening
All patients with type 2 diabetes should be visited by an ophthalmologist prior to diabetes diagnosis.
Symptoms
- When the disease first starts, there are no symptoms.
- Blurred vision and slow vision loss over time
- Eye floaters and spots
- Shadows or missing areas of vision
- Trouble seeing at night
- Fluctuating vision
- Blurry and/or distorted vision
- Other symptoms are related to diabetic ocular disease
After the hemorrhage due to PDR, most of the symptoms occurs. The first time, it may not be very severe. In most cases, it will leave just a few specks of blood, or spots, floating in a person's visual field, though the spots often go away after a few hours.These spots are often followed within a few days or weeks by a much greater leakage of blood, which blurs vision. In extreme cases, a person will only be able to recognize the light.
-
Normal vision. Courtesy NIH National Eye Institute
-
The same view with diabetic retinopathy.
Physical Examination
- History taking is the first step.
- Blood pressure measurement,
- BMI calculation
- Monitoring fasting blood sugar
- Measuring HbA1c,
- The history of any visual symptoms and changes in the vision
- Visual acuity test
- Fundoscopic examination using ophthalmoscopy or slit lamp bio-microscopy
The personnel performing the examination require considerable training accreditation. On fundoscopic the physician is looking for cotton wool spots, flame hemorrhages, dot-blot hemorrhages and boat hemorrhages.
Cotton Wool Spots
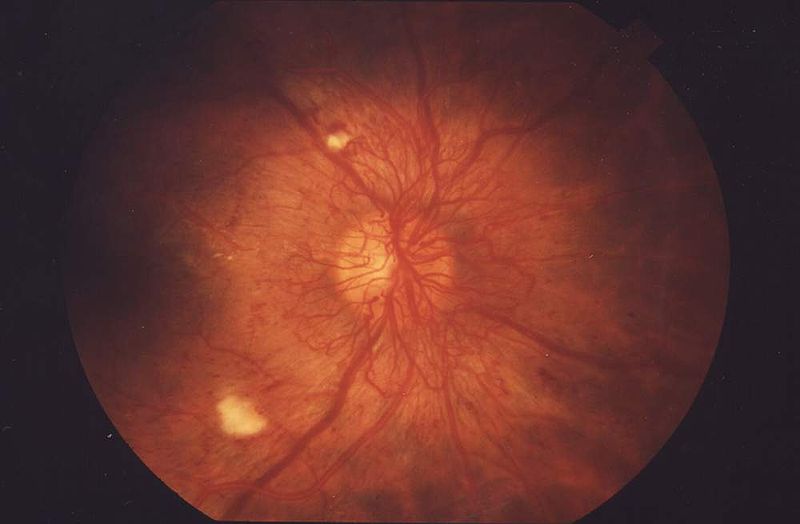
Cotton wool spots are an abnormal finding on fundoscopic exam of the retina. They appear as puffy white patches on the retina. They are caused by damage to nerve fibers. The nerve fibers are damaged by swelling in the surface layer of the retina. The cause of this swelling is due to the reduced axonal transport (and hence backlog of intracellular products) within the nerves because of the ischemia.
Flame Hemorrhages
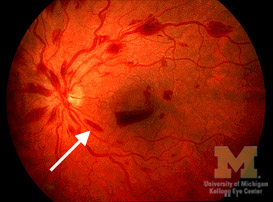
Flame hemorrhages are flame shaped hemorrhages located in the superficial nerve fiber layer of the retina that appear dark dark red on fundoscopic examination. Flame hemorrhages are caused by leakage from arterioles due to ischemic damage or from veins that are ischemic or in under high pressure.
Dot Hemorrhages

Dot hemorrhages are dark red round spots of hemorrhage seen on fundoscopic exam. They are frequently observed in patients with diabetic retinopathy. Dot hemorrhages are due to either capillary or venular leak. The site of hemorrhage is deep within the retina.
Boat Hemorrhages

Boat hemorrhages are rectangular dark red spots of hemorrhage seen on fundoscopic exam. They are frequently observed in patients with diabetic retinopathy. Boat hemorrhages are due to either capillary or venular leak. The site of hemorrhage is at the interface between the retina and the vitreous humor. The contents that leak out are under such high-pressure that they break through the internal membrane of the retina.
Treatment
People with advanced retinopathy have a 90 percent chance to retain their vision when they get treatment before the retina is severely damaged. Besides of the tight diabetic management, there are three major treatments for diabetic retinopathy.
- Laser surgery
- Triamcinolone injection to the eye
- vitrectomy
Caution should be exercised in treatment with laser surgery since it causes a loss of retinal tissue. It is often more prudent to inject triamcinolone. In some patients it results in a marked increase of vision, especially if there is an edema of the macula. Avoiding tobacco use and correction of associated hypertension are important therapeutic measures in the management of diabetic retinopathy. [9]
Laser surgery
A type of laser surgery called panretinal photocoagulation, or PRP, is used to treat severe macular edema and PDR. The goal is to create 1 000 - 2 000 burns in the retina to reduce retina's oxygen demand, and hence the possibility of ischemia. In treating advanced diabetic retinopathy, the burns are used to destroy the abnormal blood vessels that form at the back of the eye. Rather than focus the light on a single spot, the eye care professional may make hundreds of small laser burns away from the center of the retina, a procedure called scatter laser treatment or panretinal photocoagulation.The treatment shrinks the abnormal blood vessels. Patients may lose some of their peripheral vision after this surgery, but the procedure saves the rest of the patient's sight. Laser surgery may also slightly reduce color and night vision.
Vitrectomy
A vitrectomy is performed when there is a lot of blood in the vitreous. It involves removing the cloudy vitreous and replacing it with a saline solution made up of salt and water. Because the vitreous is mostly water, there should be no change between the saline solution and the normal vitreous. Studies show that people who have a vitrectomy soon after a large hemorrhage are more likely to protect their vision than someone who waits to have the operation.
References
- ↑ Wolfensberger TJ, Hamilton AM (2001). "Diabetic retinopathy--an historical review". Semin Ophthalmol. 16 (1): 2–7. PMID 15487691.
- ↑ Understanding diabetic retinopathy by Pardianto G et al., in Mimbar Ilmiah Oftalmologi Indonesia.2005;2: 65-6.
- ↑ Frank RN (2004). "Diabetic retinopathy". N Engl J Med. 350 (1): 48–58. doi:10.1056/NEJMra021678. PMID 14702427.
- ↑ Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003). "Vascular endothelial growth factors and angiogenesis in eye disease". Prog Retin Eye Res. 22 (1): 1–29. PMID 12597922.
- ↑ Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C; et al. (2012). "Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology". Diabetologia. 55 (3): 845–54. doi:10.1007/s00125-011-2393-0. PMID 22143324.
- ↑ "NIHSeniorHealth: Diabetic Retinopathy - Causes and Risk Factors". Diabetic Retinopathy. NIHSenior Health. 2005.
- ↑ Hirai FE, Moss SE, Klein BE, Klein R (2008). "Relationship of glycemic control, exogenous insulin, and C-peptide levels to ischemic heart disease mortality over a 16-year period in people with older-onset diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR)". Diabetes Care. 31 (3): 493–7. doi:10.2337/dc07-1161. PMC 2773445. PMID 18025409.
- ↑ Paine SK, Sen A, Choudhuri S, Mondal LK, Chowdhury IH, Basu A; et al. (2012). "Association of tumor necrosis factor α, interleukin 6, and interleukin 10 promoter polymorphism with proliferative diabetic retinopathy in type 2 diabetic subjects". Retina. 32 (6): 1197–203. doi:10.1097/IAE.0b013e31822f55f3. PMID 22105495.
- ↑ "Diabetes Ocular complications". Chronic Complications of Diabetes. Armenian Medical Network. 2006. Text " Umesh Masharani, MB, BS, MRCP " ignored (help)
Related chapters
External links
- Diabetic Retinopathy Resource Guide from the National Eye Institute (NEI).
cs:Diabetická retinopatie de:Diabetische Retinopathie nl:Diabetische retinopathie fi:Diabeettinen retinopatia

