Dalteparin: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
{{ | |authorTag={{SS}} | ||
{{ | |genericName=Dalteparin | ||
|aOrAn=a | |||
|drugClass=low molecular weight heparin | |||
|indicationType=prophylaxis | |||
|indication=ischemic complications in [[unstable angina]] and non-q-wave [[myocardial infarction]], [[deep vein thrombosis]], extended treatment of symptomatic [[venous thromboembolism]] in patients with cancer | |||
|adverseReactions=[[hematoma]] and irritation symptom | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">WARNING: SPINAL/EPIDURAL HEMATOMA</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;"> | |||
*Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: | |||
:* Use of indwelling epidural catheters | |||
:* Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants. | |||
:* A history of traumatic or repeated epidural or spinal punctures | |||
:* A history of spinal deformity or spinal surgery | |||
:* Optimal timing between the administration of Dalteparin and neuraxial procedures is not known. | |||
:* Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. | |||
:* Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis.</span></i> | |||
|fdaLIADAdult=<H4>Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction</H4> | |||
* Dosing information | |||
''' | :* Recommended dosage: ''' 120 IU/kg IV SC q12h''', but not more than '''10,000 IU''', | ||
::* Concurrent with: '''aspirin 75 to 165 mg PO qd‘’‘. Treatment should be continued until the patient is clinically stabilized. | |||
:::* Concurrent aspirin therapy is recommended except when contraindicated. | |||
::* The usual duration: '''5 to 8 days'''. | |||
*Table 1 lists the volume of Dalteparin, based on the 9.5 mL multiple-dose vial (10,000 IU/mL), to be administered for a range of patient weights. | |||
[[File: Dalteparin_administration_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<h4>Prophylaxis of Venous Thromboembolism Following Hip Replacement Surgery</h4> | |||
* Dosing information | |||
''' | :* Usual duration: '''5 to 10 days''' after surgery; up to '''14 days''' of treatment with Dalteparin have been well tolerated in clinical trials. | ||
''' | |||
:* Table 2 presents the dosing options for patients undergoing hip replacement surgery. | |||
[[File: Dalteparin_administration_02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<H4>Abdominal Surgery</H4> | |||
[[ | * Dosing information | ||
:* In patients undergoing abdominal surgery with a risk of thromboembolic complications | |||
::* Recommended dose: '''2500 IU IV SC qd''', starting 1 to 2 hours prior to surgery and repeated once daily postoperatively. | |||
:::* Usual duration:''' 5 to 10 days'''. | |||
* In patients undergoing abdominal surgery associated with a high risk of thromboembolic complications | |||
:* Recommended dose: '''5000 IU IV SC’‘’ the evening before surgery, then once daily postoperatively. | |||
::* Usual duration: '''5 to 10 days'''. | |||
::* Alternatively, in patients with malignancy, '''2500 IU IV SC''' 1 to 2 hours before surgery followed by '''2500 IU IV SC''' 12 hours later, and then ’‘’5000 IU IV SC qd‘’‘ postoperatively. | |||
::* Usual duration: ''' 5 to 10 days'''. | |||
<H4>Medical Patients During Acute Illness</H4> | |||
* Dosing information | |||
:* Recommended dosage: '''5000 IU IV SC qd‘’‘. | |||
::* Usual duration: '''12 to 14 days'''. | |||
<H4>Extended Treatment of Symptomatic Venous Thromboembolism in Patients with Cancer</H4> | |||
* Dosing information | |||
:* Recommended dosage: | |||
::* For the first 30 days: ‘’‘200 IU/kg total body weight IV SC qd’‘’. | |||
::* The total daily dose should not exceed ’‘’18,000 IU‘’‘. | |||
::* Table 3 lists the dose of Dalteparin to be administered once daily during the first month for a range of patient weights. | |||
'''Month 1''' | |||
[[File: Dalteparin_administration_03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
'''Months 2 to 6''' | |||
*Administer Dalteparin at a dose of approximately ’‘’150 IU/kg‘’‘, subcutaneously once daily during Months 2 through 6. The total daily dose should not exceed ’‘’18,000 IU‘’‘. Table 4 lists the dose of Dalteparin to be administered once daily for a range of patient weights during months 2-6. | |||
[[File: Dalteparin_administration_04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Safety and efficacy beyond six months have not been evaluated in patients with cancer and acute symptomatic [[VTE]]. | |||
<h4>Dose Reductions for Thrombocytopenia in Patients with Cancer and Acute Symptomatic VTE</h4> | |||
* Dosing information | |||
:* In patients receiving Dalteparin who experience platelet counts between 50,000 and 100,000/mm3, reduce the daily dose of Dalteparin by '''2,500 IU''' until the platelet count recovers to ≥ 100,000/mm3. In patients receiving Dalteparin who experience [[platelet count]]s < 50,000/mm3, discontinue Dalteparin until the platelet count recovers above 50,000/mm3. | |||
<H4>Dose Reductions for Renal Insufficiency in Extended Treatment of Acute Symptomatic Venous Thromboembolism in Patients with Cancer</H4> | |||
* Dosing information | |||
:* In patients with severely impaired renal function (CrCl < 30 mL/min), monitor anti-Xa levels to determine the appropriate Dalteparin dose. | |||
:* Target anti-Xa range is '''0.5-1.5 IU/mL'''. When monitoring anti-Xa in these patients, perform sampling 4-6 hrs after Dalteparin dosing and only after the patient has received 3-4 doses. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Dalteparin in pediatric patients. | |||
|offLabelAdultNoGuideSupport=<H4>Disseminated intravascular coagulation</H4> | |||
* Dosing information | |||
:* '''150 anti-Xa units/kilogram/day'''<ref name="pmid1962902">{{cite journal| author=Oguma Y, Sakuragawa N, Maki M, Hasegawa H, Nakagawa M| title=Treatment of disseminated intravascular coagulation with low molecular weight heparin. Research Group of FR-860 on DIC in Japan. | journal=Semin Thromb Hemost | year= 1990 | volume= 16 Suppl | issue= | pages= 34-40 | pmid=1962902 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1962902 }} </ref> | |||
<h4>Adjunct treatment of Percutaneous coronary intervention</h4> | |||
* Dosing information | |||
:* '''60 IU/kg IV'''<ref name="pmid11231430">{{cite journal| author=Kereiakes DJ, Kleiman NS, Fry E, Mwawasi G, Lengerich R, Maresh K et al.| title=Dalteparin in combination with abciximab during percutaneous coronary intervention. | journal=Am Heart J | year= 2001 | volume= 141 | issue= 3 | pages= 348-52 | pmid=11231430 | doi=10.1067/mhj.2001.113217 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11231430 }} </ref> | |||
<H4>Static Ulcer</H4> | |||
* Dosing information | |||
:* Not applicable <ref name="pmid11190899">{{cite journal| author=Bick RL, Scott RG| title=Stasis ulcers refractory to therapy--accelerated healing by treatment with clopidogrel +/- dalteparin: a preliminary report. | journal=Clin Appl Thromb Hemost | year= 2001 | volume= 7 | issue= 1 | pages= 21-4 | pmid=11190899 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11190899 }} </ref> | |||
<H4>Prophylaxis of Arterial thromboembolism</H4> | |||
* Dosing information | |||
:* '''100 IU/kg IV SC bid '''<ref name="pmid15226166">{{cite journal| author=Douketis JD, Johnson JA, Turpie AG| title=Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. | journal=Arch Intern Med | year= 2004 | volume= 164 | issue= 12 | pages= 1319-26 | pmid=15226166 | doi=10.1001/archinte.164.12.1319 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15226166 }} </ref> | |||
|fdaLIADPed=Safety and effectiveness in pediatric patients have not been established. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Dalteparin in pediatric patients. | |||
|offLabelPedNoGuideSupport=<H4>Prophylaxis of Arterial thromboembolism</H4> | |||
* Dosing information | |||
:* Not applicable 10650853 | |||
|contraindications=* Active major bleeding | |||
* History of [[heparin induced thrombocytopenia]] or [[heparin induced thrombocytopenia]] with [[thrombosis]]. | |||
* [[Hypersensitivity]] to dalteparin sodium (e.g., [[pruritis]], [[rash]], anaphylactic reactions) | |||
* In patients undergoing Epidural/Neuraxial anesthesia, do not administer Dalteparin; | |||
* As a treatment for [[unstable angina]] and non_q wave [[MI]] | |||
* For prolonged [[VTE]] prophylaxis. | |||
* [[Hypersensitivity]] to [[heparin]] or pork products | |||
|warnings=====Increased Risk of hemorrhage==== | |||
*Spinal or epidural [[hemorrhage]] and subsequent [[hematomas]] can occur with the associated use of [[low molecular weight heparins]] or heparinoids and neuraxial (spinal/epidural) anesthesia or [[spinal puncture]]. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting [[hemostasis]] such as [[NSAIDs]], with traumatic or repeated epidural or [[spinal puncture]], or in patients with a history of spinal surgery or [[spinal deformity]]. | |||
*To reduce the potential risk of bleeding associated with the concurrent use of dalteparin sodium and epidural or [[spinal anesthesia]]/analgesia or [[spinal puncture]], consider the pharmacokinetic profile of dalteparin. | |||
*Placement or removal of an epidural catheter or [[lumbar puncture]] is best performed when the anticoagulant effect of dalteparin is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known. No additional [[hemostasis]]-altering medications should be administered due to the additive effects. | |||
*Patients on preoperative Dalteparin thromboprophylaxis can be assumed to have altered coagulation. The first postoperative Dalteparin [[thrombophylaxis]] dose (2500 IU) should be administered 6 to 8 hrs postoperatively. The second postoperative dose (2500 or 5000 IU) should occur no sooner than 24 hrs after the first dose. Placement or removal of a catheter should be delayed for at least 12 hours after administration of lower doses (2500 IU or 5000 IU once daily) of Dalteparin, and at least 24 hours after the administration of higher doses (200 IU/kg once daily, 120 IU/kg twice daily) of Dalteparin. Anti-Xa levels are still detectable at these time points, and these delays are not a guarantee that neuraxial [[hematoma]] will be avoided. | |||
*Although a specific recommendation for timing of a subsequent Dalteparin dose after catheter removal cannot be made, consider delaying this next dose for at least four hours, based on a benefit-risk assessment considering both the risk for [[thrombosis]] and the risk for bleeding in the context of the procedure and patient risk factors. For patients with creatinine clearance <30mL/minute, additional considerations are necessary because elimination of Dalteparin may be more prolonged; consider doubling the timing of removal of a catheter, at least 24 hours for the lower prescribed dose of Dalteparin (2500 IU or 5000 IU once daily) and at least 48 hours for the higher dose (200 IU/kg once daily, 120 IU/kg twice daily). | |||
*Should the physician decide to administer [[anticoagulation]] in the context of epidural or [[spinal anesthesia]]/analgesia or [[lumbar puncture]], frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. *Instruct patients to report immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal [[hematoma]] are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae. | |||
*Use Dalteparin with extreme caution in patients who have an increased risk of [[hemorrhage]], such as those with severe uncontrolled [[hypertension]], [[bacterial endocarditis]], congenital or acquired bleeding disorders, active ulceration and angiodysplastic gastrointestinal disease, [[hemorrhagic stroke]], or shortly after brain, spinal or ophthalmological surgery. Dalteparin may enhance the risk of bleeding in patients with [[thrombocytopenia]] or platelet defects; severe liver or [[renal insufficiency]], hypertensive or [[diabetic retinopathy]], and recent [[gastrointestinal bleeding]]. Bleeding can occur at any site during therapy with Dalteparin. | |||
====Thrombocytopenia==== | |||
*[[Heparin-induced thrombocytopenia]] can occur with the administration of Dalteparin. The incidence of this complication is unknown at present. In clinical practice, cases of [[thrombocytopenia]] with [[thrombosis]], [[amputation]] and death have been observed. Closely monitor [[thrombocytopenia]] of any degree. | |||
*In Dalteparin clinical trials supporting non-cancer indications, platelet counts of < 50,000/mm3 occurred in < 1% of patients. | |||
*In the clinical trial of patients with cancer and acute symptomatic venous [[thromboembolism]] treated for up to 6 months in the Dalteparin treatment arm, platelet counts of < 100,000/mm3 occurred in 13.6% of patients, including 6.5% who also had platelet counts less than 50,000/mm3. In the same clinical trial, [[thrombocytopenia]] was reported as an adverse event in 10.9% of patients in the Dalteparin arm and 8.1% of patients in the OAC arm. Dalteparin dose was decreased or interrupted in patients whose platelet counts fell below 100,000/mm3. | |||
====Benzyl Alcohol==== | |||
*Each multiple-dose vial of Dalteparin contains benzyl alcohol as a preservative. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants. Because benzyl alcohol may cross the placenta, use caution when administering Dalteparin preserved with benzyl alcohol to pregnant women. If [[anticoagulation]] with Dalteparin is needed during pregnancy, use preservative-free formulations, where possible. | |||
====Laboratory Tests==== | |||
*Periodic routine complete blood counts, including platelet count, blood chemistry, and stool occult blood tests are recommended during the course of treatment with Dalteparin. When administered at recommended prophylaxis doses, routine coagulation tests such as [[Prothrombin Time]] (PT) and [[Activated Partial Thromboplastin Time]] (APTT) are relatively insensitive measures of Dalteparin activity and, therefore, unsuitable for monitoring the anticoagulant effect of Dalteparin. Anti-Factor Xa may be used to monitor the anticoagulant effect of Dalteparin, such as in patients with severe [[renal impairment]] or if abnormal coagulation parameters or bleeding occurs during Dalteparin therapy. | |||
|clinicalTrials=*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not accurately reflect the rates observed in practice. | |||
====Hemorrhage==== | |||
*The incidence of hemorrhagic complications during treatment with Dalteparin Injection has been low. The most commonly reported side effect is [[hematoma]] at the injection site. The risk for bleeding varies with the indication and may increase with higher doses. | |||
====Unstable Angina and Non-Q-Wave Myocardial Infarction==== | |||
*Table 5 summarizes major bleeding reactions that occurred with Dalteparin, [[heparin]], and placebo in clinical trials of [[unstable angina]] and non-Q-wave [[myocardial infarction]]. | |||
[[File:Dalteparin_advese_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Hip Replacement Surgery==== | |||
*Table 6 summarizes: 1) all major bleeding reactions and, 2) other bleeding reactions possibly or probably related to treatment with Dalteparin (preoperative dosing regimen), [[warfarin]] sodium, or [[heparin]] in two hip replacement surgery clinical trials. | |||
[[File:Dalteparin_advese_02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Six of the patients treated with Dalteparin experienced seven major bleeding reactions. Two of the reactions were wound hematoma (one requiring reoperation), three were bleeding from the operative site, one was intraoperative bleeding due to vessel damage, and one was [[gastrointestinal bleeding]]. None of the patients experienced retroperitoneal or [[intracranial hemorrhage]] or died of bleeding complications. | |||
*In the third [[hip replacement surgery]] clinical trial, the incidence of major bleeding reactions was similar in all three treatment groups: 3.6% (18/496) for patients who started Dalteparin before surgery; 2.5% (12/487) for patients who started Dalteparin after surgery; and 3.1% (15/489) for patients treated with [[warfarin]] sodium. | |||
====Abdominal Surgery==== | |||
*Table 7 summarizes bleeding reactions that occurred in clinical trials which studied Dalteparin 2500 and 5000 IU administered once daily to abdominal surgery patients. | |||
[[File:Dalteparin_advese_03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*In a trial comparing Dalteparin 5000 IU once daily to Dalteparin 2500 IU once daily in patients undergoing surgery for [[malignancy]], the incidence of bleeding reactions was 4.6% and 3.6%, respectively (n.s.). In a trial comparing Dalteparin 5000 IU once daily to [[heparin]] 5000 U twice daily, in the malignancy subgroup the incidence of bleeding reactions was 3.2% and 2.7%, respectively for Dalteparin and [[Heparin]] (n.s.). | |||
====Medical Patients with Severely Restricted Mobility During Acute Illness==== | |||
*Table 8 summarizes major bleeding reactions that occurred in a clinical trial of medical patients with severely restricted mobility during acute illness. | |||
[[File:Dalteparin_advese_04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Three of the major bleeding reactions that occurred by Day 21 were fatal, all due to gastrointestinal hemorrhage (two patients in the group treated with Dalteparin and one in the group receiving placebo). | |||
====Patients with Cancer and Acute Symptomatic Venous Thromboembolism==== | |||
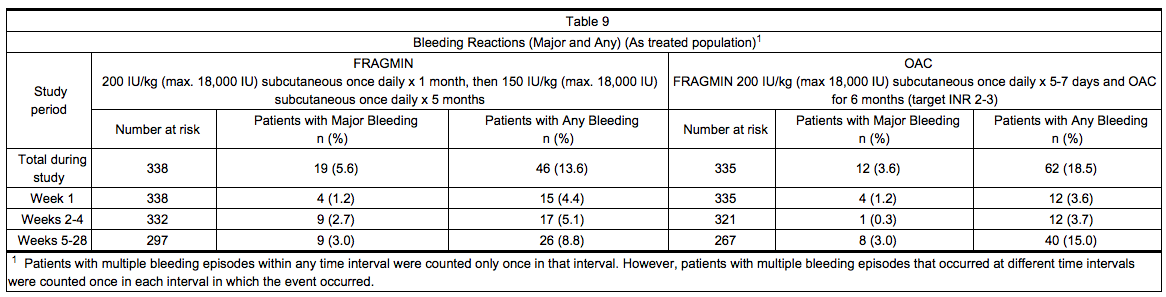
*Table 9 summarizes the number of patients with bleeding reactions that occurred in the clinical trial of patients with cancer and acute symptomatic [[venous thromboembolism]]. A bleeding event was considered major if it: 1) was accompanied by a decrease in [[hemoglobin]] of ≥ 2 g/dL in connection with clinical symptoms; 2) occurred at a critical site (intraocular, spinal/epidural, intracranial, retroperitoneal, or pericardial bleeding); 3) required transfusion of ≥ 2 units of blood products; or 4) led to death. Minor bleeding was classified as clinically overt bleeding that did not meet criteria for major bleeding. | |||
*At the end of the six-month study, a total of 46 (13.6%) patients in the Dalteparin arm and 62 (18.5%) patients in the OAC arm experienced any bleeding event. One bleeding event ([[hemoptysis]] in a patient in the Dalteparin arm at Day 71) was fatal. | |||
[[File:Dalteparin_advese_05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Elevations of Serum Transaminases==== | |||
*In Dalteparin clinical trials supporting non-cancer indications, where hepatic transaminases were measured, asymptomatic increases in transaminase levels (SGOT/AST and SGPT/ALT) greater than three times the upper limit of normal of the laboratory reference range were seen in 4.7% and 4.2%, respectively, of patients during treatment with Dalteparin. | |||
*In the Dalteparin clinical trial of patients with [[cancer]] and acute symptomatic [[venous thromboembolism]] treated with Dalteparin for up to 6 months, asymptomatic increases in transaminase levels, AST and ALT, greater than three times the upper limit of normal of the laboratory reference range were reported in 8.9% and 9.5% of patients, respectively. The frequencies of Grades 3 and 4 increases in AST and ALT, as classified by the National Cancer Institute, Common Toxicity Criteria (NCI-CTC) Scoring System, were 3% and 3.8%, respectively. Grades 2, 3 & 4 combined have been reported in 12% and 14% of patients, respectively. | |||
====Other==== | |||
*'''Allergic Reactions''': Allergic reactions (i.e., [[pruritus]], [[rash]], [[fever]], injection site reaction, [[bullous eruption]]) have occurred. Cases of anaphylactoid reactions have been reported. | |||
*'''Local Reactions''': Pain at the injection site, the only non-bleeding event determined to be possibly or probably related to treatment with Dalteparin and reported at a rate of at least 2% in the group treated with Dalteparin, was reported in 4.5% of patients treated with Dalteparin 5000 IU once daily vs 11.8% of patients treated with [[heparin]] 5000 U twice daily in the abdominal surgery trials. In the hip replacement trials, pain at injection site was reported in 12% of patients treated with Dalteparin 5000 IU once daily vs 13% of patients treated with [[heparin]] 5000 U three times a day. | |||
|postmarketing=*The following adverse reactions have been identified during postapproval use of Dalteparin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
*Since first international market introduction in 1985, there have been more than 15 reports of epidural or [[spinal hematoma]] formation with concurrent use of dalteparin sodium and spinal/epidural [[anesthesia]] or [[spinal puncture]]. The majority of patients had postoperative indwelling epidural catheters placed for [[analgesia]] or received additional drugs affecting [[hemostasis]]. In some cases the [[hematoma]] resulted in long-term or permanent paralysis (partial or complete). | |||
*[[Skin necrosis]] has occurred. There have been cases of [[alopecia]] reported that improved on drug discontinuation. | |||
|drugInteractions=*Use Dalteparin with care in patients receiving oral [[anticoagulants]], platelet inhibitors, and thrombolytic agents because of increased risk of bleeding. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=*There are no adequate and well-controlled studies of Dalteparin use in pregnant women. In reproductive and developmental toxicity studies, pregnant rats and rabbits received dalteparin sodium at intravenous doses up to 2400 IU/kg (14,160 IU/m2) (rats) and 4800 IU/kg (40,800 IU/m2) (rabbits). These exposures were 2 to 4 times (rats) and 4 times (rabbits) the human dose of 100 IU/kg dalteparin based on the body surface area. No evidence of impaired fertility or harm to the fetuses occurred in these studies. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
*Cases of "Gasping Syndrome" have occurred in premature infants when large amounts of benzyl alcohol have been administered (99-404 mg/kg/day). The 9.5 mL and the 3.8 mL multiple-dose vials of Dalteparin contain 14 mg/mL of benzyl alcohol. | |||
|useInNursing=*Based on limited published data dalteparin is minimally excreted in human milk. One study of 15 lactating women receiving prophylactic doses of dalteparin, in the immediate postpartum period, detected small amounts of anti-Xa activity (range < 0.005 to 0.037 IU/ml) in breast milk that were equivalent to a milk/plasma ratio of < 0.025-0.224. Oral absorption of LMWH is extremely low, but the clinical implications, if any, of this small amount of anticoagulant activity on a nursing infant are unknown. Caution should be exercised when Dalteparin is administered to a nursing woman. | |||
|useInPed=*Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri=*Of the total number of patients in clinical studies of Dalteparin, 5516 patients were 65 years of age or older and 2237 were 75 or older. No overall differences in effectiveness were observed between these subjects and younger subjects. Some studies suggest that the risk of bleeding increases with age. Postmarketing surveillance and literature reports have not revealed additional differences in the safety of Dalteparin between elderly and younger patients. Give careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) in geriatric patients, particularly in those with low body weight (< 45 kg) and those predisposed to decreased renal function. | |||
|administration=*Subcutaneous injection technique: Patients should be sitting or lying down and Dalteparin administered by deep subcutaneous injection. Dalteparin may be injected in a U-shape area around the navel, the upper outer side of the thigh or the upper outer quadrangle of the buttock. The injection site should be varied daily. When the area around the navel or the thigh is used, using the thumb and forefinger, you must lift up a fold of skin while giving the injection. The entire length of the needle should be inserted at a 45 to 90 degree angle. | |||
*Inspect Dalteparin prefilled syringes and vials visually for particulate matter and discoloration prior to administration | |||
*After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. Discard any unused solution after 2 weeks. | |||
[[File:Dalteparin_administration_05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*<u>Fixed dose syringes</u>: To ensure delivery of the full dose, do not expel the air bubble from the prefilled syringe before injection. Hold the syringe assembly by the open sides of the device. Remove the needle shield. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange <u>'''until the entire dose has been given'''</u>. The needle guard will '''not''' be activated unless the '''entire''' dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers. | |||
*<u>Graduated syringes</u>: Hold the syringe assembly by the open sides of the device. Remove the needle shield. With the needle pointing up, prepare the syringe by expelling the air bubble and then continuing to push the plunger to the desired dose or volume, discarding the extra solution in an appropriate manner. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange <u>'''until the entire dose remaining in the syringe has been given'''</u>. The needle guard will '''not''' be activated unless the '''entire''' dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers. | |||
|monitoring=FDA Package Insert for Dalteparin contains no information regarding drug monitoring. | |||
|IVCompat=There is limited information about the IV Compatibility. | |||
|overdose=*An excessive dosage of Dalteparin Injection may lead to [[hemorrhagic complications]]. These may generally be stopped by slow intravenous injection of protamine sulfate (1% solution), at a dose of 1 mg protamine for every 100 anti-Xa IU of Dalteparin given. If the APTT measured 2 to 4 hours after the first infusion remains prolonged, a second infusion of 0.5 mg [[protamine]] sulfate per 100 anti-Xa IU of Dalteparin may be administered. Even with these additional doses of [[protamine]], the APTT may remain more prolonged than would usually be found following administration of [[unfractionated heparin]]. In all cases, the anti-Factor Xa activity is never completely neutralized (maximum about 60 to 75%). | |||
*Take particular care to avoid overdosage with [[protamine]] sulfate. Administration of [[protamine]] sulfate can cause severe [[hypotensive]] and [[anaphylactoid reactions]]. Because fatal reactions, often resembling [[anaphylaxis]], have been reported with [[protamine]] sulfate, give [[protamine]] sulfate only when resuscitation techniques and treatment for [[anaphylactic shock]] are readily available. For additional information, consult the labeling of Protamine Sulfate Injection, USP, products. | |||
|drugBox={{Drugbox2 | |||
| IUPAC_name = | |||
| image = | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|dalteparin-sodium}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number = 9041-08-1 | |||
| ATC_prefix = B01 | |||
| ATC_suffix = AB04 | |||
| PubChem = | |||
| DrugBank = DB06779 | |||
| KEGG = D03353 | |||
| ChemSpiderID = NA | |||
<!--Chemical data--> | |||
| chemical_formula = | |||
| molecular_weight = | |||
}} | |||
|mechAction=*Dalteparin is a [[low molecular weight heparin]] with antithrombotic properties. It acts by enhancing the inhibition of Factor Xa and thrombin by antithrombin. In humans, dalteparin potentiates preferentially the inhibition of coagulation Factor Xa, while only slightly affecting the activated partial thromboplastin time (APTT). | |||
|structure=*Dalteparin Injection (dalteparin sodium injection) is a sterile, low molecular weight heparin. It is available in single-dose, prefilled syringes preassembled with a needle guard device, and multiple-dose vials. With reference to the W.H.O. First International Low Molecular Weight Heparin Reference Standard, each syringe contains either 2500, 5000, 7500, 10,000, 12,500, 15,000 or 18,000 anti-Factor Xa international units (IU), equivalent to 16, 32, 48, 64, 80, 96 or 115.2 mg dalteparin sodium, respectively. Each multiple-dose vial contains either 10,000 or 25,000 anti-Factor Xa IU per 1 mL (equivalent to 64 or 160 mg dalteparin sodium, respectively), for a total of 95,000 anti-Factor Xa IU per vial. | |||
*Each prefilled syringe also contains Water for Injection and sodium chloride, when required, to maintain physiologic ionic strength. The prefilled syringes are preservative-free. Each multiple-dose vial also contains Water for Injection and 14 mg of benzyl alcohol per mL as a preservative. The pH of both formulations is 5.0 to 7.5. | |||
*Dalteparin sodium is produced through controlled nitrous acid depolymerization of sodium heparin from porcine intestinal mucosa followed by a chromatographic purification process. It is composed of strongly acidic sulfated polysaccharide chains (oligosaccharide, containing 2,5-anhydro-D-mannitol residues as end groups) with an average molecular weight of 5000 and about 90% of the material within the range 2000-9000. The molecular weight distribution is: | |||
:*< 3000 daltons 3.0-15% | |||
:*3000 to 8000 daltons 65.0-78.0% | |||
:*> 8000 daltons 14.0-26.0% | |||
[[File:Dalteparin_structure_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|PD=*Doses of Dalteparin Injection of up to 10,000 anti-Factor Xa IU administered subcutaneously as a single dose or two 5000 IU doses 12 hours apart to healthy subjects did not produce a significant change in platelet aggregation, [[fibrinolysis]], or global clotting tests such as [[prothrombin time]] (PT), [[thrombin time]] (TT) or APTT. Subcutaneous administration of doses of 5000 IU twice daily of Dalteparin for seven consecutive days to patients undergoing abdominal surgery did not markedly affect APTT, Platelet Factor 4 (PF4), or lipoprotein lipase. | |||
|PK=*Mean peak levels of plasma anti-Factor Xa activity following single subcutaneous doses of 2500, 5000 and 10,000 IU were 0.19 ± 0.04, 0.41 ± 0.07 and 0.82 ± 0.10 IU/mL, respectively, and were attained in about 4 hours in most subjects. Absolute bioavailability in healthy volunteers, measured as the anti-Factor Xa activity, was 87 ± 6%. Increasing the dose from 2500 to 10,000 IU resulted in an overall increase in anti-Factor Xa AUC that was greater than proportional by about one-third. | |||
*Peak anti-Factor Xa activity increased more or less linearly with dose over the same dose range. There appeared to be no appreciable accumulation of anti-Factor Xa activity with twice-daily dosing of 100 IU/kg subcutaneously for up to 7 days. | |||
*The volume of distribution for dalteparin anti-Factor Xa activity was 40 to 60 mL/kg. The mean plasma clearances of dalteparin anti-Factor Xa activity in normal volunteers following single intravenous bolus doses of 30 and 120 anti-Factor Xa IU/kg were 24.6 ± 5.4 and 15.6 ± 2.4 mL/hr/kg, respectively. The corresponding mean disposition half-lives were 1.47 ± 0.3 and 2.5 ± 0.3 hours. | |||
*Following intravenous doses of 40 and 60 IU/kg, mean terminal half-lives were 2.1 ± 0.3 and 2.3 ± 0.4 hours, respectively. Longer apparent terminal half-lives (3 to 5 hours) are observed following subcutaneous dosing, possibly due to delayed absorption. In patients with chronic renal insufficiency requiring hemodialysis, the mean terminal half-life of anti-Factor Xa activity following a single intravenous dose of 5000 IU Dalteparin was 5.7 ± 2.0 hours, i.e. considerably longer than values observed in healthy volunteers, therefore, greater accumulation can be expected in these patients. | |||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |||
*Dalteparin sodium has not been tested for its carcinogenic potential in long-term animal studies. It was not mutagenic in the in vitro Ames Test, mouse lymphoma cell forward mutation test and human lymphocyte chromosomal aberration test and in the in vivo mouse micronucleus test. Dalteparin sodium at subcutaneous doses up to 1200 IU/kg (7080 IU/m2) did not affect the fertility or reproductive performance of male and female rats. | |||
|clinicalStudies=====Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction==== | |||
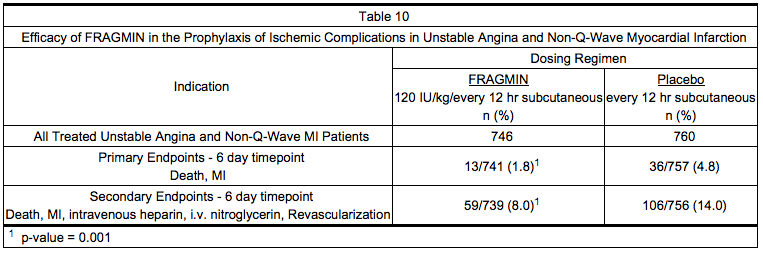
*In a double-blind, randomized, placebo-controlled clinical trial, patients who recently experienced [[unstable angina]] with EKG changes or non-Q-wave [[myocardial infarction]] (MI) were randomized to Dalteparin Injection 120 IU/kg or placebo every 12 hours subcutaneously. In this trial, unstable angina was defined to include only [[angina]] with EKG changes. All patients, except when contraindicated, were treated concurrently with [[aspirin]] (75 mg once daily) and beta blockers. Treatment was initiated within 72 hours of the event (the majority of patients received treatment within 24 hours) and continued for 5 to 8 days. A total of 1506 patients were enrolled and treated; 746 received Dalteparin and 760 received placebo. The mean age of the study population was 68 years (range 40 to 90 years) and the majority of patients were white (99.7%) and male (63.9%). The combined incidence of the endpoint of death or [[myocardial infarction]] was lower for Dalteparin compared with placebo at 6 days after initiation of therapy. These results were observed in an analysis of all-randomized and all-treated patients. The combined incidence of death, MI, need for intravenous [[heparin]] or intravenous [[nitroglycerin]], and revascularization was also lower for Dalteparin than for placebo (see Table 10). | |||
[[File:Dalteparin_clinical studies_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*In a second randomized, controlled trial designed to evaluate long-term treatment with Dalteparin (days 6 to 45), data were also collected comparing 1-week (5 to 8 days) treatment of Dalteparin 120 IU/kg every 12 hours subcutaneously with [[heparin]] at an APTT-adjusted dosage. All patients, except when contraindicated, were treated concurrently with aspirin (100 to 165 mg per day). Of the 1499 patients enrolled, 1482 patients were treated; 751 received Dalteparin and 731 received [[heparin]]. The mean age of the study population was 64 years (range 25 to 92 years) and the majority of patients were white (96.0%) and male (64.2%). The incidence of the combined endpoint of death, [[myocardial infarction]], or recurrent angina during this 1-week treatment period (5 to 8 days) was 9.3% for Dalteparin and 7.6% for heparin (p=0.323). | |||
====Prophylaxis of Deep Vein Thrombosis in Patients Following Hip Replacement Surgery==== | |||
*In an open-label randomized study, Dalteparin 5000 IU administered once daily subcutaneously was compared with warfarin sodium, administered orally, in patients undergoing hip replacement surgery. Treatment with Dalteparin was initiated with a 2500 IU dose subcutaneously within 2 hours before surgery, followed by a 2500 IU dose subcutaneously the evening of the day of surgery. Then, a dosing regimen of Dalteparin 5000 IU subcutaneously once daily was initiated on the first postoperative day. The first dose of [[warfarin]] sodium was given the evening before surgery, then continued daily at a dose adjusted for [[INR]] 2 to 3. Treatment in both groups was then continued for 5 to 9 days postoperatively. Of the 580 patients enrolled, 553 were treated and 550 underwent surgery. Of those who underwent surgery, 271 received Dalteparin and 279 received [[warfarin]] sodium. The mean age of the study population was 63 years (range 20 to 92 years) and the majority of patients were white (91.1%) and female (52.9%). The incidence of [[deep vein thrombosis]] ([[DVT]]), as determined by evaluable venography, was significantly lower for the group treated with Dalteparin compared with patients treated with [[warfarin]] sodium (see Table 11). | |||
|howSupplied=*After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. | |||
[[File:Dalteparin_how supplied_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|storage=*Store at controlled room temperature 20° to 25°C (68° to 77°F). | |||
|fdaPatientInfo=*If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant [[NSAIDs]], platelet inhibitors, or other [[anticoagulants]], inform the patients to watch for signs and symptoms of spinal or [[epidural hematoma]], such as tingling, numbness (especially in the lower limbs) and muscular weakness. If any of these symptoms occur the patient should contact his or her physician immediately. | |||
*Additionally, the use of [[aspirin]] and other [[NSAIDs]] may enhance the risk of [[hemorrhage]]. Discontinue their use prior to dalteparin therapy whenever possible; if co-administration is essential, the patient's clinical and laboratory status should be closely monitored. | |||
*Inform patients: | |||
[[ | :* of the instructions for injecting Dalteparin if their therapy is to continue after discharge from the hospitals. | ||
:* it may take them longer than usual to stop [[bleeding]]. | |||
:* they may bruise and/or bleed more easily when they are treated with Dalteparin. | |||
:* they should report any unusual [[bleeding]], bruising, or signs of [[thrombocytopenia]] (such as a rash of dark red spots under the skin) to their physician. | |||
:* to tell their physicians and dentists they are taking Dalteparin and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is take. | |||
:* to tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as [[aspirin]] or other [[NSAIDs]]. | |||
{{ | *Dalteparin is a registered trademark of Pfizer Health AB and is licensed to Eisai Inc. | ||
|alcohol=Alcohol-Dalteparin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=Fragmin | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_label_01.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin15.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin16.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin17.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin18.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin19.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin20.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin21.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin22.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_01.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_02.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_03.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_04.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_05.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_06.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_07.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_08.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_09.png | |||
}} | |||
{{LabelImage | |||
|fileName=Dalteparin_panel_10.png | |||
}} | |||
[[Category:Drug]] | |||
[[Category:Cardiovascular Drugs]] | |||
[[Category:Anticoagulants]] | |||
Latest revision as of 18:07, 19 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dalteparin is a low molecular weight heparin that is FDA approved for the prophylaxis of ischemic complications in unstable angina and non-q-wave myocardial infarction, deep vein thrombosis, extended treatment of symptomatic venous thromboembolism in patients with cancer. Common adverse reactions include hematoma and irritation symptom.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction
- Dosing information
- Recommended dosage: 120 IU/kg IV SC q12h, but not more than 10,000 IU,
- Concurrent with: aspirin 75 to 165 mg PO qd‘’‘. Treatment should be continued until the patient is clinically stabilized.
- Concurrent aspirin therapy is recommended except when contraindicated.
- The usual duration: 5 to 8 days.
- Table 1 lists the volume of Dalteparin, based on the 9.5 mL multiple-dose vial (10,000 IU/mL), to be administered for a range of patient weights.
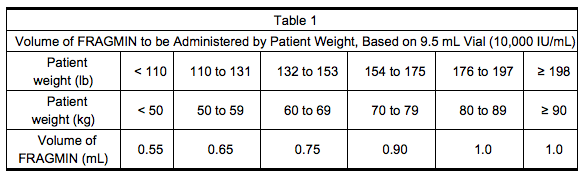
Prophylaxis of Venous Thromboembolism Following Hip Replacement Surgery
- Dosing information
- Usual duration: 5 to 10 days after surgery; up to 14 days of treatment with Dalteparin have been well tolerated in clinical trials.
- Table 2 presents the dosing options for patients undergoing hip replacement surgery.
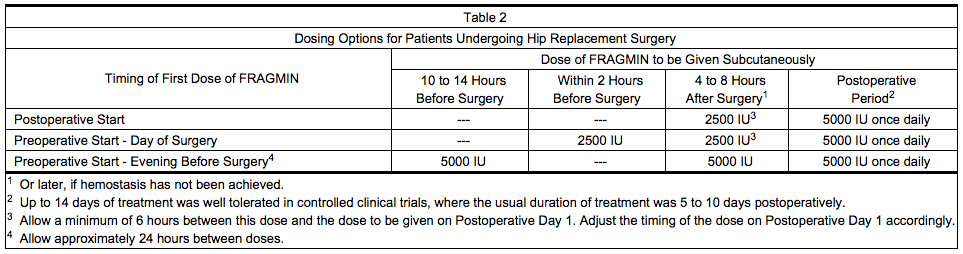
Abdominal Surgery
- Dosing information
- In patients undergoing abdominal surgery with a risk of thromboembolic complications
- Recommended dose: 2500 IU IV SC qd, starting 1 to 2 hours prior to surgery and repeated once daily postoperatively.
- Usual duration: 5 to 10 days.
- In patients undergoing abdominal surgery associated with a high risk of thromboembolic complications
- Recommended dose: 5000 IU IV SC’‘’ the evening before surgery, then once daily postoperatively.
- Usual duration: 5 to 10 days.
- Alternatively, in patients with malignancy, 2500 IU IV SC 1 to 2 hours before surgery followed by 2500 IU IV SC 12 hours later, and then ’‘’5000 IU IV SC qd‘’‘ postoperatively.
- Usual duration: 5 to 10 days.
Medical Patients During Acute Illness
- Dosing information
- Recommended dosage: 5000 IU IV SC qd‘’‘.
- Usual duration: 12 to 14 days.
Extended Treatment of Symptomatic Venous Thromboembolism in Patients with Cancer
- Dosing information
- Recommended dosage:
- For the first 30 days: ‘’‘200 IU/kg total body weight IV SC qd’‘’.
- The total daily dose should not exceed ’‘’18,000 IU‘’‘.
- Table 3 lists the dose of Dalteparin to be administered once daily during the first month for a range of patient weights.
Month 1
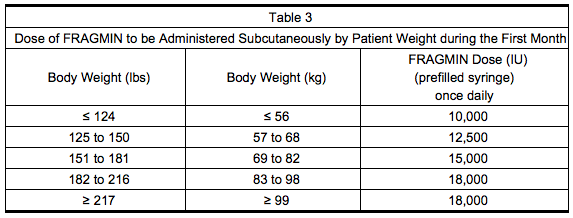
Months 2 to 6
- Administer Dalteparin at a dose of approximately ’‘’150 IU/kg‘’‘, subcutaneously once daily during Months 2 through 6. The total daily dose should not exceed ’‘’18,000 IU‘’‘. Table 4 lists the dose of Dalteparin to be administered once daily for a range of patient weights during months 2-6.
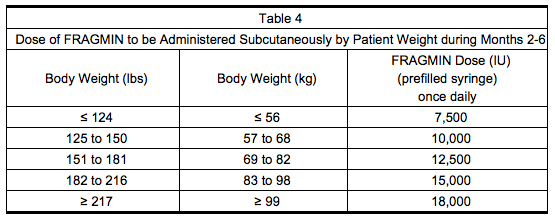
- Safety and efficacy beyond six months have not been evaluated in patients with cancer and acute symptomatic VTE.
Dose Reductions for Thrombocytopenia in Patients with Cancer and Acute Symptomatic VTE
- Dosing information
- In patients receiving Dalteparin who experience platelet counts between 50,000 and 100,000/mm3, reduce the daily dose of Dalteparin by 2,500 IU until the platelet count recovers to ≥ 100,000/mm3. In patients receiving Dalteparin who experience platelet counts < 50,000/mm3, discontinue Dalteparin until the platelet count recovers above 50,000/mm3.
Dose Reductions for Renal Insufficiency in Extended Treatment of Acute Symptomatic Venous Thromboembolism in Patients with Cancer
- Dosing information
- In patients with severely impaired renal function (CrCl < 30 mL/min), monitor anti-Xa levels to determine the appropriate Dalteparin dose.
- Target anti-Xa range is 0.5-1.5 IU/mL. When monitoring anti-Xa in these patients, perform sampling 4-6 hrs after Dalteparin dosing and only after the patient has received 3-4 doses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dalteparin in pediatric patients.
Non–Guideline-Supported Use
Disseminated intravascular coagulation
- Dosing information
- 150 anti-Xa units/kilogram/day[1]
Adjunct treatment of Percutaneous coronary intervention
- Dosing information
- 60 IU/kg IV[2]
Static Ulcer
- Dosing information
- Not applicable [3]
Prophylaxis of Arterial thromboembolism
- Dosing information
- 100 IU/kg IV SC bid [4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dalteparin in pediatric patients.
Non–Guideline-Supported Use
Prophylaxis of Arterial thromboembolism
- Dosing information
- Not applicable 10650853
Contraindications
- Active major bleeding
- History of heparin induced thrombocytopenia or heparin induced thrombocytopenia with thrombosis.
- Hypersensitivity to dalteparin sodium (e.g., pruritis, rash, anaphylactic reactions)
- In patients undergoing Epidural/Neuraxial anesthesia, do not administer Dalteparin;
- As a treatment for unstable angina and non_q wave MI
- For prolonged VTE prophylaxis.
- Hypersensitivity to heparin or pork products
Warnings
Increased Risk of hemorrhage
- Spinal or epidural hemorrhage and subsequent hematomas can occur with the associated use of low molecular weight heparins or heparinoids and neuraxial (spinal/epidural) anesthesia or spinal puncture. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity.
- To reduce the potential risk of bleeding associated with the concurrent use of dalteparin sodium and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dalteparin.
- Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of dalteparin is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known. No additional hemostasis-altering medications should be administered due to the additive effects.
- Patients on preoperative Dalteparin thromboprophylaxis can be assumed to have altered coagulation. The first postoperative Dalteparin thrombophylaxis dose (2500 IU) should be administered 6 to 8 hrs postoperatively. The second postoperative dose (2500 or 5000 IU) should occur no sooner than 24 hrs after the first dose. Placement or removal of a catheter should be delayed for at least 12 hours after administration of lower doses (2500 IU or 5000 IU once daily) of Dalteparin, and at least 24 hours after the administration of higher doses (200 IU/kg once daily, 120 IU/kg twice daily) of Dalteparin. Anti-Xa levels are still detectable at these time points, and these delays are not a guarantee that neuraxial hematoma will be avoided.
- Although a specific recommendation for timing of a subsequent Dalteparin dose after catheter removal cannot be made, consider delaying this next dose for at least four hours, based on a benefit-risk assessment considering both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors. For patients with creatinine clearance <30mL/minute, additional considerations are necessary because elimination of Dalteparin may be more prolonged; consider doubling the timing of removal of a catheter, at least 24 hours for the lower prescribed dose of Dalteparin (2500 IU or 5000 IU once daily) and at least 48 hours for the higher dose (200 IU/kg once daily, 120 IU/kg twice daily).
- Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. *Instruct patients to report immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
- Use Dalteparin with extreme caution in patients who have an increased risk of hemorrhage, such as those with severe uncontrolled hypertension, bacterial endocarditis, congenital or acquired bleeding disorders, active ulceration and angiodysplastic gastrointestinal disease, hemorrhagic stroke, or shortly after brain, spinal or ophthalmological surgery. Dalteparin may enhance the risk of bleeding in patients with thrombocytopenia or platelet defects; severe liver or renal insufficiency, hypertensive or diabetic retinopathy, and recent gastrointestinal bleeding. Bleeding can occur at any site during therapy with Dalteparin.
Thrombocytopenia
- Heparin-induced thrombocytopenia can occur with the administration of Dalteparin. The incidence of this complication is unknown at present. In clinical practice, cases of thrombocytopenia with thrombosis, amputation and death have been observed. Closely monitor thrombocytopenia of any degree.
- In Dalteparin clinical trials supporting non-cancer indications, platelet counts of < 50,000/mm3 occurred in < 1% of patients.
- In the clinical trial of patients with cancer and acute symptomatic venous thromboembolism treated for up to 6 months in the Dalteparin treatment arm, platelet counts of < 100,000/mm3 occurred in 13.6% of patients, including 6.5% who also had platelet counts less than 50,000/mm3. In the same clinical trial, thrombocytopenia was reported as an adverse event in 10.9% of patients in the Dalteparin arm and 8.1% of patients in the OAC arm. Dalteparin dose was decreased or interrupted in patients whose platelet counts fell below 100,000/mm3.
Benzyl Alcohol
- Each multiple-dose vial of Dalteparin contains benzyl alcohol as a preservative. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants. Because benzyl alcohol may cross the placenta, use caution when administering Dalteparin preserved with benzyl alcohol to pregnant women. If anticoagulation with Dalteparin is needed during pregnancy, use preservative-free formulations, where possible.
Laboratory Tests
- Periodic routine complete blood counts, including platelet count, blood chemistry, and stool occult blood tests are recommended during the course of treatment with Dalteparin. When administered at recommended prophylaxis doses, routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are relatively insensitive measures of Dalteparin activity and, therefore, unsuitable for monitoring the anticoagulant effect of Dalteparin. Anti-Factor Xa may be used to monitor the anticoagulant effect of Dalteparin, such as in patients with severe renal impairment or if abnormal coagulation parameters or bleeding occurs during Dalteparin therapy.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not accurately reflect the rates observed in practice.
Hemorrhage
- The incidence of hemorrhagic complications during treatment with Dalteparin Injection has been low. The most commonly reported side effect is hematoma at the injection site. The risk for bleeding varies with the indication and may increase with higher doses.
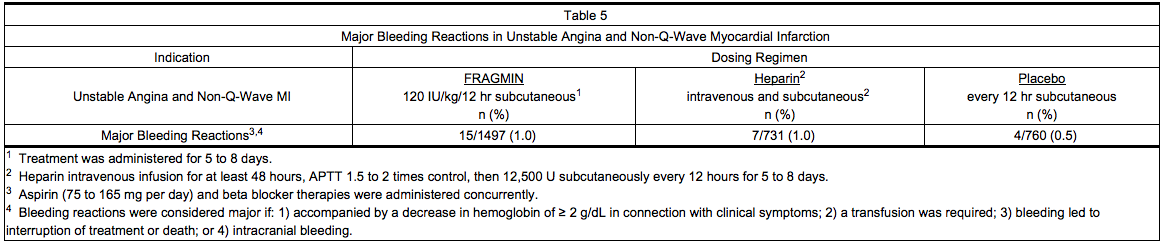
Unstable Angina and Non-Q-Wave Myocardial Infarction
- Table 5 summarizes major bleeding reactions that occurred with Dalteparin, heparin, and placebo in clinical trials of unstable angina and non-Q-wave myocardial infarction.
Hip Replacement Surgery
- Table 6 summarizes: 1) all major bleeding reactions and, 2) other bleeding reactions possibly or probably related to treatment with Dalteparin (preoperative dosing regimen), warfarin sodium, or heparin in two hip replacement surgery clinical trials.
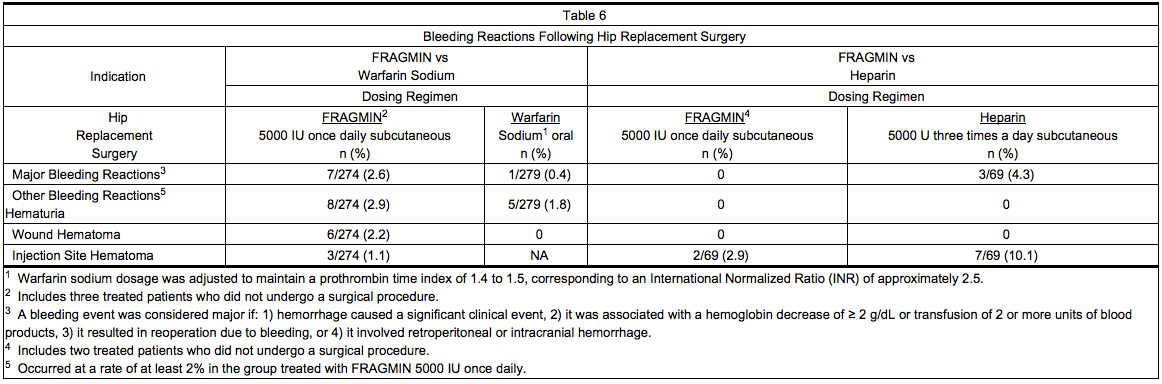
- Six of the patients treated with Dalteparin experienced seven major bleeding reactions. Two of the reactions were wound hematoma (one requiring reoperation), three were bleeding from the operative site, one was intraoperative bleeding due to vessel damage, and one was gastrointestinal bleeding. None of the patients experienced retroperitoneal or intracranial hemorrhage or died of bleeding complications.
- In the third hip replacement surgery clinical trial, the incidence of major bleeding reactions was similar in all three treatment groups: 3.6% (18/496) for patients who started Dalteparin before surgery; 2.5% (12/487) for patients who started Dalteparin after surgery; and 3.1% (15/489) for patients treated with warfarin sodium.
Abdominal Surgery
- Table 7 summarizes bleeding reactions that occurred in clinical trials which studied Dalteparin 2500 and 5000 IU administered once daily to abdominal surgery patients.
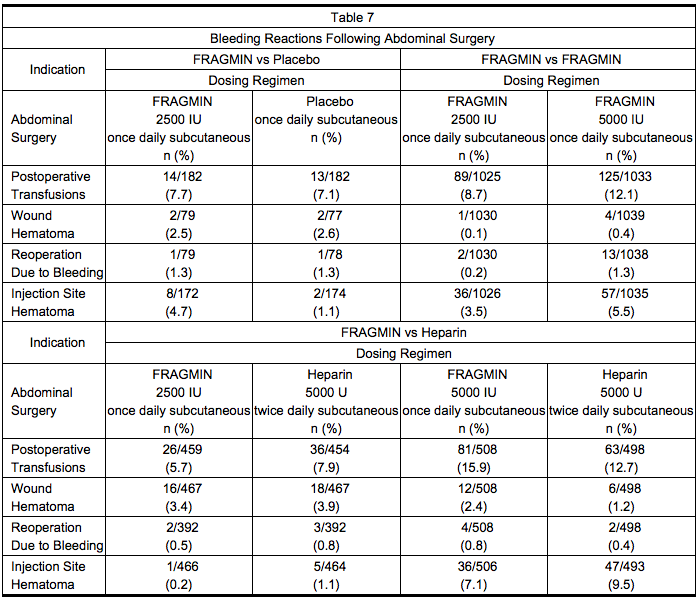
- In a trial comparing Dalteparin 5000 IU once daily to Dalteparin 2500 IU once daily in patients undergoing surgery for malignancy, the incidence of bleeding reactions was 4.6% and 3.6%, respectively (n.s.). In a trial comparing Dalteparin 5000 IU once daily to heparin 5000 U twice daily, in the malignancy subgroup the incidence of bleeding reactions was 3.2% and 2.7%, respectively for Dalteparin and Heparin (n.s.).
Medical Patients with Severely Restricted Mobility During Acute Illness
- Table 8 summarizes major bleeding reactions that occurred in a clinical trial of medical patients with severely restricted mobility during acute illness.
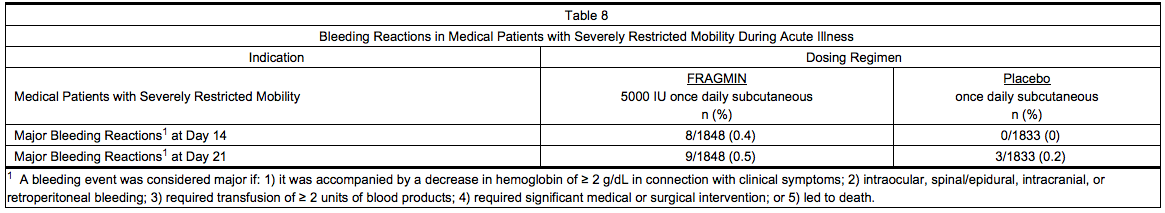
- Three of the major bleeding reactions that occurred by Day 21 were fatal, all due to gastrointestinal hemorrhage (two patients in the group treated with Dalteparin and one in the group receiving placebo).
Patients with Cancer and Acute Symptomatic Venous Thromboembolism
- Table 9 summarizes the number of patients with bleeding reactions that occurred in the clinical trial of patients with cancer and acute symptomatic venous thromboembolism. A bleeding event was considered major if it: 1) was accompanied by a decrease in hemoglobin of ≥ 2 g/dL in connection with clinical symptoms; 2) occurred at a critical site (intraocular, spinal/epidural, intracranial, retroperitoneal, or pericardial bleeding); 3) required transfusion of ≥ 2 units of blood products; or 4) led to death. Minor bleeding was classified as clinically overt bleeding that did not meet criteria for major bleeding.
- At the end of the six-month study, a total of 46 (13.6%) patients in the Dalteparin arm and 62 (18.5%) patients in the OAC arm experienced any bleeding event. One bleeding event (hemoptysis in a patient in the Dalteparin arm at Day 71) was fatal.
Elevations of Serum Transaminases
- In Dalteparin clinical trials supporting non-cancer indications, where hepatic transaminases were measured, asymptomatic increases in transaminase levels (SGOT/AST and SGPT/ALT) greater than three times the upper limit of normal of the laboratory reference range were seen in 4.7% and 4.2%, respectively, of patients during treatment with Dalteparin.
- In the Dalteparin clinical trial of patients with cancer and acute symptomatic venous thromboembolism treated with Dalteparin for up to 6 months, asymptomatic increases in transaminase levels, AST and ALT, greater than three times the upper limit of normal of the laboratory reference range were reported in 8.9% and 9.5% of patients, respectively. The frequencies of Grades 3 and 4 increases in AST and ALT, as classified by the National Cancer Institute, Common Toxicity Criteria (NCI-CTC) Scoring System, were 3% and 3.8%, respectively. Grades 2, 3 & 4 combined have been reported in 12% and 14% of patients, respectively.
Other
- Allergic Reactions: Allergic reactions (i.e., pruritus, rash, fever, injection site reaction, bullous eruption) have occurred. Cases of anaphylactoid reactions have been reported.
- Local Reactions: Pain at the injection site, the only non-bleeding event determined to be possibly or probably related to treatment with Dalteparin and reported at a rate of at least 2% in the group treated with Dalteparin, was reported in 4.5% of patients treated with Dalteparin 5000 IU once daily vs 11.8% of patients treated with heparin 5000 U twice daily in the abdominal surgery trials. In the hip replacement trials, pain at injection site was reported in 12% of patients treated with Dalteparin 5000 IU once daily vs 13% of patients treated with heparin 5000 U three times a day.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of Dalteparin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Since first international market introduction in 1985, there have been more than 15 reports of epidural or spinal hematoma formation with concurrent use of dalteparin sodium and spinal/epidural anesthesia or spinal puncture. The majority of patients had postoperative indwelling epidural catheters placed for analgesia or received additional drugs affecting hemostasis. In some cases the hematoma resulted in long-term or permanent paralysis (partial or complete).
- Skin necrosis has occurred. There have been cases of alopecia reported that improved on drug discontinuation.
Drug Interactions
- Use Dalteparin with care in patients receiving oral anticoagulants, platelet inhibitors, and thrombolytic agents because of increased risk of bleeding.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of Dalteparin use in pregnant women. In reproductive and developmental toxicity studies, pregnant rats and rabbits received dalteparin sodium at intravenous doses up to 2400 IU/kg (14,160 IU/m2) (rats) and 4800 IU/kg (40,800 IU/m2) (rabbits). These exposures were 2 to 4 times (rats) and 4 times (rabbits) the human dose of 100 IU/kg dalteparin based on the body surface area. No evidence of impaired fertility or harm to the fetuses occurred in these studies. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Cases of "Gasping Syndrome" have occurred in premature infants when large amounts of benzyl alcohol have been administered (99-404 mg/kg/day). The 9.5 mL and the 3.8 mL multiple-dose vials of Dalteparin contain 14 mg/mL of benzyl alcohol.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dalteparin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dalteparin during labor and delivery.
Nursing Mothers
- Based on limited published data dalteparin is minimally excreted in human milk. One study of 15 lactating women receiving prophylactic doses of dalteparin, in the immediate postpartum period, detected small amounts of anti-Xa activity (range < 0.005 to 0.037 IU/ml) in breast milk that were equivalent to a milk/plasma ratio of < 0.025-0.224. Oral absorption of LMWH is extremely low, but the clinical implications, if any, of this small amount of anticoagulant activity on a nursing infant are unknown. Caution should be exercised when Dalteparin is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Of the total number of patients in clinical studies of Dalteparin, 5516 patients were 65 years of age or older and 2237 were 75 or older. No overall differences in effectiveness were observed between these subjects and younger subjects. Some studies suggest that the risk of bleeding increases with age. Postmarketing surveillance and literature reports have not revealed additional differences in the safety of Dalteparin between elderly and younger patients. Give careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) in geriatric patients, particularly in those with low body weight (< 45 kg) and those predisposed to decreased renal function.
Gender
There is no FDA guidance on the use of Dalteparin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dalteparin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dalteparin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dalteparin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dalteparin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dalteparin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subcutaneous injection technique: Patients should be sitting or lying down and Dalteparin administered by deep subcutaneous injection. Dalteparin may be injected in a U-shape area around the navel, the upper outer side of the thigh or the upper outer quadrangle of the buttock. The injection site should be varied daily. When the area around the navel or the thigh is used, using the thumb and forefinger, you must lift up a fold of skin while giving the injection. The entire length of the needle should be inserted at a 45 to 90 degree angle.
- Inspect Dalteparin prefilled syringes and vials visually for particulate matter and discoloration prior to administration
- After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. Discard any unused solution after 2 weeks.
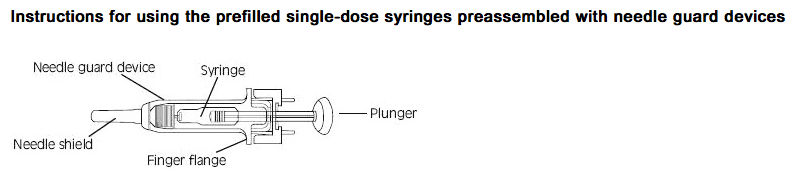
- Fixed dose syringes: To ensure delivery of the full dose, do not expel the air bubble from the prefilled syringe before injection. Hold the syringe assembly by the open sides of the device. Remove the needle shield. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
- Graduated syringes: Hold the syringe assembly by the open sides of the device. Remove the needle shield. With the needle pointing up, prepare the syringe by expelling the air bubble and then continuing to push the plunger to the desired dose or volume, discarding the extra solution in an appropriate manner. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose remaining in the syringe has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
Monitoring
FDA Package Insert for Dalteparin contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
- An excessive dosage of Dalteparin Injection may lead to hemorrhagic complications. These may generally be stopped by slow intravenous injection of protamine sulfate (1% solution), at a dose of 1 mg protamine for every 100 anti-Xa IU of Dalteparin given. If the APTT measured 2 to 4 hours after the first infusion remains prolonged, a second infusion of 0.5 mg protamine sulfate per 100 anti-Xa IU of Dalteparin may be administered. Even with these additional doses of protamine, the APTT may remain more prolonged than would usually be found following administration of unfractionated heparin. In all cases, the anti-Factor Xa activity is never completely neutralized (maximum about 60 to 75%).
- Take particular care to avoid overdosage with protamine sulfate. Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions, often resembling anaphylaxis, have been reported with protamine sulfate, give protamine sulfate only when resuscitation techniques and treatment for anaphylactic shock are readily available. For additional information, consult the labeling of Protamine Sulfate Injection, USP, products.
Pharmacology
Dalteparin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Dalteparin is a low molecular weight heparin with antithrombotic properties. It acts by enhancing the inhibition of Factor Xa and thrombin by antithrombin. In humans, dalteparin potentiates preferentially the inhibition of coagulation Factor Xa, while only slightly affecting the activated partial thromboplastin time (APTT).
Structure
- Dalteparin Injection (dalteparin sodium injection) is a sterile, low molecular weight heparin. It is available in single-dose, prefilled syringes preassembled with a needle guard device, and multiple-dose vials. With reference to the W.H.O. First International Low Molecular Weight Heparin Reference Standard, each syringe contains either 2500, 5000, 7500, 10,000, 12,500, 15,000 or 18,000 anti-Factor Xa international units (IU), equivalent to 16, 32, 48, 64, 80, 96 or 115.2 mg dalteparin sodium, respectively. Each multiple-dose vial contains either 10,000 or 25,000 anti-Factor Xa IU per 1 mL (equivalent to 64 or 160 mg dalteparin sodium, respectively), for a total of 95,000 anti-Factor Xa IU per vial.
- Each prefilled syringe also contains Water for Injection and sodium chloride, when required, to maintain physiologic ionic strength. The prefilled syringes are preservative-free. Each multiple-dose vial also contains Water for Injection and 14 mg of benzyl alcohol per mL as a preservative. The pH of both formulations is 5.0 to 7.5.
- Dalteparin sodium is produced through controlled nitrous acid depolymerization of sodium heparin from porcine intestinal mucosa followed by a chromatographic purification process. It is composed of strongly acidic sulfated polysaccharide chains (oligosaccharide, containing 2,5-anhydro-D-mannitol residues as end groups) with an average molecular weight of 5000 and about 90% of the material within the range 2000-9000. The molecular weight distribution is:
- < 3000 daltons 3.0-15%
- 3000 to 8000 daltons 65.0-78.0%
- > 8000 daltons 14.0-26.0%
Pharmacodynamics
- Doses of Dalteparin Injection of up to 10,000 anti-Factor Xa IU administered subcutaneously as a single dose or two 5000 IU doses 12 hours apart to healthy subjects did not produce a significant change in platelet aggregation, fibrinolysis, or global clotting tests such as prothrombin time (PT), thrombin time (TT) or APTT. Subcutaneous administration of doses of 5000 IU twice daily of Dalteparin for seven consecutive days to patients undergoing abdominal surgery did not markedly affect APTT, Platelet Factor 4 (PF4), or lipoprotein lipase.
Pharmacokinetics
- Mean peak levels of plasma anti-Factor Xa activity following single subcutaneous doses of 2500, 5000 and 10,000 IU were 0.19 ± 0.04, 0.41 ± 0.07 and 0.82 ± 0.10 IU/mL, respectively, and were attained in about 4 hours in most subjects. Absolute bioavailability in healthy volunteers, measured as the anti-Factor Xa activity, was 87 ± 6%. Increasing the dose from 2500 to 10,000 IU resulted in an overall increase in anti-Factor Xa AUC that was greater than proportional by about one-third.
- Peak anti-Factor Xa activity increased more or less linearly with dose over the same dose range. There appeared to be no appreciable accumulation of anti-Factor Xa activity with twice-daily dosing of 100 IU/kg subcutaneously for up to 7 days.
- The volume of distribution for dalteparin anti-Factor Xa activity was 40 to 60 mL/kg. The mean plasma clearances of dalteparin anti-Factor Xa activity in normal volunteers following single intravenous bolus doses of 30 and 120 anti-Factor Xa IU/kg were 24.6 ± 5.4 and 15.6 ± 2.4 mL/hr/kg, respectively. The corresponding mean disposition half-lives were 1.47 ± 0.3 and 2.5 ± 0.3 hours.
- Following intravenous doses of 40 and 60 IU/kg, mean terminal half-lives were 2.1 ± 0.3 and 2.3 ± 0.4 hours, respectively. Longer apparent terminal half-lives (3 to 5 hours) are observed following subcutaneous dosing, possibly due to delayed absorption. In patients with chronic renal insufficiency requiring hemodialysis, the mean terminal half-life of anti-Factor Xa activity following a single intravenous dose of 5000 IU Dalteparin was 5.7 ± 2.0 hours, i.e. considerably longer than values observed in healthy volunteers, therefore, greater accumulation can be expected in these patients.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Dalteparin sodium has not been tested for its carcinogenic potential in long-term animal studies. It was not mutagenic in the in vitro Ames Test, mouse lymphoma cell forward mutation test and human lymphocyte chromosomal aberration test and in the in vivo mouse micronucleus test. Dalteparin sodium at subcutaneous doses up to 1200 IU/kg (7080 IU/m2) did not affect the fertility or reproductive performance of male and female rats.
Clinical Studies
Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction
- In a double-blind, randomized, placebo-controlled clinical trial, patients who recently experienced unstable angina with EKG changes or non-Q-wave myocardial infarction (MI) were randomized to Dalteparin Injection 120 IU/kg or placebo every 12 hours subcutaneously. In this trial, unstable angina was defined to include only angina with EKG changes. All patients, except when contraindicated, were treated concurrently with aspirin (75 mg once daily) and beta blockers. Treatment was initiated within 72 hours of the event (the majority of patients received treatment within 24 hours) and continued for 5 to 8 days. A total of 1506 patients were enrolled and treated; 746 received Dalteparin and 760 received placebo. The mean age of the study population was 68 years (range 40 to 90 years) and the majority of patients were white (99.7%) and male (63.9%). The combined incidence of the endpoint of death or myocardial infarction was lower for Dalteparin compared with placebo at 6 days after initiation of therapy. These results were observed in an analysis of all-randomized and all-treated patients. The combined incidence of death, MI, need for intravenous heparin or intravenous nitroglycerin, and revascularization was also lower for Dalteparin than for placebo (see Table 10).
- In a second randomized, controlled trial designed to evaluate long-term treatment with Dalteparin (days 6 to 45), data were also collected comparing 1-week (5 to 8 days) treatment of Dalteparin 120 IU/kg every 12 hours subcutaneously with heparin at an APTT-adjusted dosage. All patients, except when contraindicated, were treated concurrently with aspirin (100 to 165 mg per day). Of the 1499 patients enrolled, 1482 patients were treated; 751 received Dalteparin and 731 received heparin. The mean age of the study population was 64 years (range 25 to 92 years) and the majority of patients were white (96.0%) and male (64.2%). The incidence of the combined endpoint of death, myocardial infarction, or recurrent angina during this 1-week treatment period (5 to 8 days) was 9.3% for Dalteparin and 7.6% for heparin (p=0.323).
Prophylaxis of Deep Vein Thrombosis in Patients Following Hip Replacement Surgery
- In an open-label randomized study, Dalteparin 5000 IU administered once daily subcutaneously was compared with warfarin sodium, administered orally, in patients undergoing hip replacement surgery. Treatment with Dalteparin was initiated with a 2500 IU dose subcutaneously within 2 hours before surgery, followed by a 2500 IU dose subcutaneously the evening of the day of surgery. Then, a dosing regimen of Dalteparin 5000 IU subcutaneously once daily was initiated on the first postoperative day. The first dose of warfarin sodium was given the evening before surgery, then continued daily at a dose adjusted for INR 2 to 3. Treatment in both groups was then continued for 5 to 9 days postoperatively. Of the 580 patients enrolled, 553 were treated and 550 underwent surgery. Of those who underwent surgery, 271 received Dalteparin and 279 received warfarin sodium. The mean age of the study population was 63 years (range 20 to 92 years) and the majority of patients were white (91.1%) and female (52.9%). The incidence of deep vein thrombosis (DVT), as determined by evaluable venography, was significantly lower for the group treated with Dalteparin compared with patients treated with warfarin sodium (see Table 11).
How Supplied
- After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks.
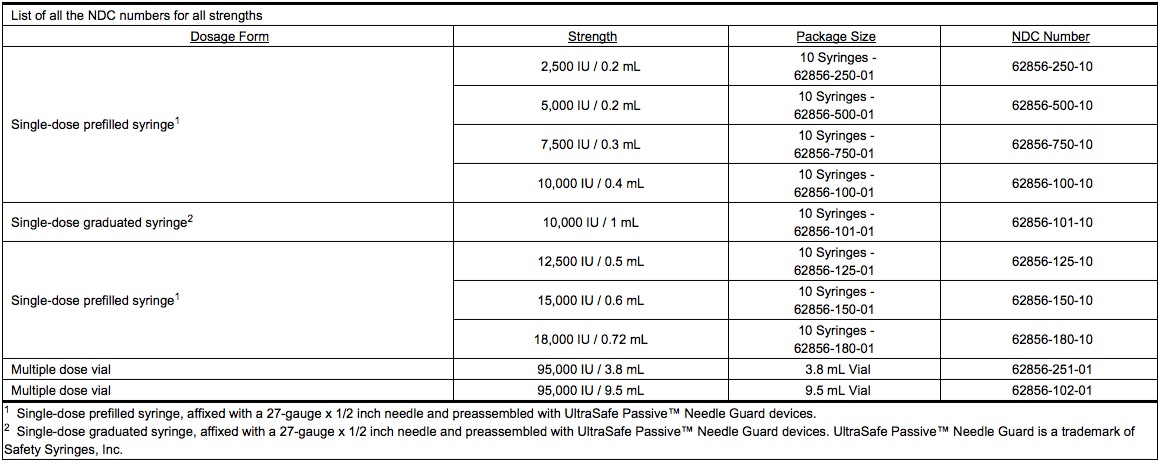
Storage
- Store at controlled room temperature 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Dalteparin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dalteparin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs, platelet inhibitors, or other anticoagulants, inform the patients to watch for signs and symptoms of spinal or epidural hematoma, such as tingling, numbness (especially in the lower limbs) and muscular weakness. If any of these symptoms occur the patient should contact his or her physician immediately.
- Additionally, the use of aspirin and other NSAIDs may enhance the risk of hemorrhage. Discontinue their use prior to dalteparin therapy whenever possible; if co-administration is essential, the patient's clinical and laboratory status should be closely monitored.
- Inform patients:
- of the instructions for injecting Dalteparin if their therapy is to continue after discharge from the hospitals.
- it may take them longer than usual to stop bleeding.
- they may bruise and/or bleed more easily when they are treated with Dalteparin.
- they should report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician.
- to tell their physicians and dentists they are taking Dalteparin and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is take.
- to tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAIDs.
- Dalteparin is a registered trademark of Pfizer Health AB and is licensed to Eisai Inc.
Precautions with Alcohol
Alcohol-Dalteparin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Fragmin
Look-Alike Drug Names
There is limited information regarding Dalteparin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Oguma Y, Sakuragawa N, Maki M, Hasegawa H, Nakagawa M (1990). "Treatment of disseminated intravascular coagulation with low molecular weight heparin. Research Group of FR-860 on DIC in Japan". Semin Thromb Hemost. 16 Suppl: 34–40. PMID 1962902.
- ↑ Kereiakes DJ, Kleiman NS, Fry E, Mwawasi G, Lengerich R, Maresh K; et al. (2001). "Dalteparin in combination with abciximab during percutaneous coronary intervention". Am Heart J. 141 (3): 348–52. doi:10.1067/mhj.2001.113217. PMID 11231430.
- ↑ Bick RL, Scott RG (2001). "Stasis ulcers refractory to therapy--accelerated healing by treatment with clopidogrel +/- dalteparin: a preliminary report". Clin Appl Thromb Hemost. 7 (1): 21–4. PMID 11190899.
- ↑ Douketis JD, Johnson JA, Turpie AG (2004). "Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen". Arch Intern Med. 164 (12): 1319–26. doi:10.1001/archinte.164.12.1319. PMID 15226166.
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_label_01.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin15.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin16.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin17.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin18.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin19.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin20.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin21.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin22.jpg
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_01.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_02.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_03.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_04.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_05.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_06.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_07.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_08.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_09.png
}}
{{#subobject:
|Label Page=Dalteparin |Label Name=Dalteparin_panel_10.png
}}