Congestive heart failure overview
| Resident Survival Guide |
| Congestive Heart Failure Microchapters |
|
Pathophysiology |
|---|
|
Differentiating Congestive heart failure from other Diseases |
|
Diagnosis |
|
Treatment |
|
Medical Therapy: |
|
Surgical Therapy: |
|
ACC/AHA Guideline Recommendations
|
|
Specific Groups: |
|
Congestive heart failure overview On the Web |
|
Directions to Hospitals Treating Congestive heart failure overview |
|
Risk calculators and risk factors for Congestive heart failure overview |
| Congestive Heart Failure Microchapters |
|
Pathophysiology |
|---|
|
Differentiating Congestive heart failure from other Diseases |
|
Diagnosis |
|
Treatment |
|
Medical Therapy: |
|
Surgical Therapy: |
|
ACC/AHA Guideline Recommendations
|
|
Specific Groups: |
|
Congestive heart failure overview On the Web |
|
Directions to Hospitals Treating Congestive heart failure overview |
|
Risk calculators and risk factors for Congestive heart failure overview |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Heart failure is a complex syndrome whereby there is inadequate output of the heart to meet the metabolic demands of the body. Abnormal function of different anatomic parts of the heart cause heart failure including the pericardium, the myocardium, the endocardium, the heart valves and the great vessels. Symptoms of heart failure are due to a lack of both forward blood flow to the body, and backward flow into the lungs. Heart failure is a clinical syndrome characterized by symptoms of dyspnea, edema and fatigue and signs such as rales on physical examination.
Classification
Several classification schemes are used to characterize heart failure based on:
- The pathophysiology of heart failure (systolic vs diastolic; left vs right; low output vs high output)
- The chronicity of heart failure (acute vs chronic)
- The severity of heart failure (the NY Heart Association Class)
- The stage of congestive heart failure (AHA Class A,B,C,D)
- The most recent classification according to the left ventricular ejection fraction; heart failure with reduced ejection fraction(HFrEF) vs heart failure with preserved ejection fraction (HFpEF)[1]
Pathophysiology
Heart failure can result from an abnormality of any one of the anatomical structures of the heart; the pericardium, the myocardium, the endocardium, the valvular structures or the great vessels. Heart failure was once thought to be secondary to a reduced left ventricular ejection fraction (heart failure with reduced EF). However, studies have shown that approximately 50% of patients who are diagnosed with heart failure have a normal/preserved ejection fraction (diastolic dysfunction) or (HF with preserved EF). Patients may be broadly classified as having heart failure with reduced left ventricular ejection fraction (systolic dysfunction) or normal/preserved ejection fraction (diastolic dysfunction). Systolic and diastolic dysfunction commonly occur in conjunction with each other. In most cases, systolic and diastolic dysfunction coexist, irrespective of EF.
Causes
Differential Diagnosis of Causes of Heart Failure Segregated by Left and Right Sided Heart Failure
Left Ventricular Failure
Most Common Causes:
Expanded List of Causes:
- Atrial fibrillation
- Alcoholism
- Anemia
- Angina
- Aortic regurgitation
- Aortic Stenosis
- Arteriovenous fistula
- Beriberi
- Cardiac aneurysm
- Cardiomyopathy
- Constrictive pericarditis
- Drugs, toxins
- Hypertension
- Hyperthyroidism
- Hypovolemia
- Hypoxia
- Mediastinal tumors
- Mitral Regurgitation
- Myocardial Infarction
- Paget's Disease
- Pancoast's Tumor
- Pericardial effusion
- Pericardial tamponade
- Perimyocarditis
- Protein deficiency
- Restrictive cardiomyopathy
- Rupture of the papillary muscles
- Sepsis
- Superior Vena Cava thrombosis
Right Ventricular Failure
Most Common Causes:
- Cardiomyopathy
- Cor pulmonale
- Diffuse myocarditis
- Left heart failure
Other Causes:
- After left ventricular failure
- After pulmonary resection
- Allergic alveolitis
- Bronchial asthma
- Chronic bronchitis
- Honeycomb lung
- Hyperglobulia
- Emphysema
- Mitral Stenosis
- Right ventricular myocardial infarction
- Pickwickian Syndrome
- Pleural fibrosis
- Pneumoconiosis
- Pulmonary fibrosis
- Pulmonic regurgitation
- Pulmonic stenosis
- Sarcoidosis
- Severe relapsing pulmonary emboli
- Silicosis
- Tachycardia
- Tricuspid insufficiency
Others
- Ascorbic acid deficiency
- Cardiac amyloidosis
- Carnitine deficiency
- Cervical vein stasis of non-cardiac genesis
- Congenital heart disease
- Cyanosis of non-cardiac genesis
- Diabetes Mellitus
- Dyspnea of non-cardiac genesis
- Edema of non-cardiac genesis
- Hemochromatosis
- Pleural effusion of non-cardiac genesis
- Pulmonary edema of non-cardiac genesis
- Thiamine deficiency
- Thyroid disease
Epidemiology and Demographics
Heart failure affects close to 5 million people in the United States of America and each year close to 500,000 new cases are diagnosed. Congestive heart failure is responsible for a significant portion of the healthcare budget, and more than 50% of patients seek re-admission within 6 months after treatment and the average duration of hospital stay is 6 days. In 2005 the prevalence among adults aged 20 and older in the United States was 5,300,000 (about 2,650,000 males, and 2,650,000 females).
Natural History, Complications, and Prognosis
Heart failure is associated with significantly reduced physical and mental health, resulting in a markedly decreased quality of life.[2][3] Congestive heart failure is also associated with a poor prognosis. With the exception of heart failure caused by reversible conditions, the condition usually worsens with time. Although some people survive many years, progressive disease is associated with an overall annual mortality rate of 10%.[4] In the Framingham experience, 80% of men and 70% of women with heart failure who were under 65 years of age had died within 8 years of the diagnosis.
Diagnosis
Symptoms
The classic symptoms of heart failure include dyspnea, fatigue, and fluid retention.
Other symptoms include:
- Ankle edema or swelling of the feet and legs
- Claudication or pain with walking
- Confusion and altered mentation
- Cool extremities or cold and clammy hands
- Cyanosis or bluish color to the skin
- Dizziness
- Dyspnea on ordinary exertion or greater shortness of breath with usual activities
- Fainting
- Fatigue
- Hemoptysis or frothy sputum
- Nocturia or urination during the night
- Nocturnal cough
- Orthopnea or sleeping on pillows
- Palpitations or extra heart beats
- Paroxysmal nocturnal dyspnea or awakening at night with shortness of breath
- Shortness of breath
- Syncope or passing out
- Weakness
- Wheezing or cardiac asthma
Physical Examination
General
- The patient's weight should be recorded to ascertain how far they are from their "dry" weight.
- Tachycardia
- Tachypnea (an increased rate of breathing) and an increased work of breathing
- Narrow pulse pressure (systolic blood pressure minus diastolic blood pressure is < 25 mm Hg)
Appearance
- The patient is often sitting upright and had labored breathing during an acute episode.
Skin
- The skin is cool and clammy consistent with hypoperfusion or cardiogenic shock
- Cyanosis is observed if severe hypoxemia is present
- Anasarca
Neck
- Jugular vein distention
- Central venous pressure > 16 cm H2O
Lungs
- Pleural effusion with dullness to percussion at the bases
- Rales
Abdomen
Heart
- Third heart sound (S3) and a gallop rhythm
- A displaced point of maximum impulse (PMI) consistent with an enlarged left ventrile
- If the right ventricular pressure is increased, a parasternal heave may be present, signifying the compensatory increase in contraction strength.
- A functional holosystolic murmur of mitral regurgitation may be heard if the heart dilates excessively
- Underlying valvular heart disease causes of congestive heart failure such as aortic stenosis,aortic regurgitation and mitral regurgitation may be auscultated.
Extremities
Neurologic
- Confusion and altered mentation
Signs that represent left sided failure include cool clammy skin, cyanosis, rales, a gallop rhythm, and a laterally displaced PMI. Signs that represent right sided failure include an elevated JVP, pedal edema, ascites, hepatomegaly, a parasternal heave and hepatojugular reflux. Commonly signs of both left and right sided failure are present.
Laboratory Studies
BNP
BNP levels may be useful in the initial establishment of the diagnosis of heart failure in the patient with dyspnea of unclear etiology. In a meta-analysis, BNP was superior N-terminal pro-BNP (NTproBNP) and was associated with a sensitivity of 85% and specificity of 84% in the diagnosis of heart failure in the primary care setting.[5] Once the diagnosis of heart failure is made, subsequent laboratory studies should be directed toward the identification of an underlying cause of heart failure.
Renal Function
Renal function should be assessed as a rough guide to the patient's intravascular volume status and renal perfusion. A urinalysis is helpful in the assessment of the patient's volume status. Electrolyte assessment and the correction of electrolyte disturbances such as hypokalemia, hyperkalemia and hypomagnesemia is critical in those patients treated with diuretics. Hyponatremia (due to poor stimulation of the baroreceptors and appropriate ADH release and free water retention) is associated with a poor prognosis.
Electrocardiogram
Low QRS Voltage:
- The EKG often shows low QRS voltage. There are two broad underlying causes of low QRS voltage in the patient with heart failure:
- Electrically inert myocardium due to a loss of viable myocardium
- Infiltration of the myocardium (myxedematous, Chagas disease)
- In alphabetical order the differential diagnosis of causes of low QRS voltage in the patient with heart failure includes:
Poor R wave progression:
- Poor R wave progression in the precordial leads may be secondary to a prior myocardial infarction but can also be observed in the absence of a prior myocardial infarction in the patient with heart failure.
Left bundle branch block (LBBB):
- Left bundle branch block (LBBB) can be observed in both ischemic and non-ischemic cases of heart failure.
Left ventricular hypertrophy:
- Changes of left ventricular hypertrophy consistent with a history of hypertension can be seen
Left atrial enlargement:
- Dilation of the left atrium can occur in congestive heart failure and the accompanying EKG abnormalities can be observed on the EKG.
Non-specific ST segment and T wave changes:
- T wave and ST segment inversions and flattening can be observed in heart failure.
Chest X Ray
- Since the left ventricle often dilates in the anteroposterior direction, the cardiac silhouette may appear deceptively normal.
- Chest x-ray findings include:
- Kerley B lines or thickening of the interlobular septa
- Peribronchial cuffing
- Thickening of the fissures
- Cephalization
- Increased vascular markings
- Interstitial edema
- Pleural effusions
Kerley B Lines
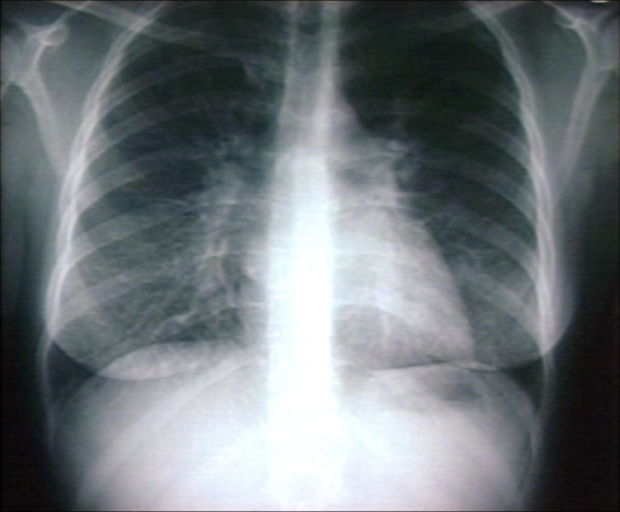
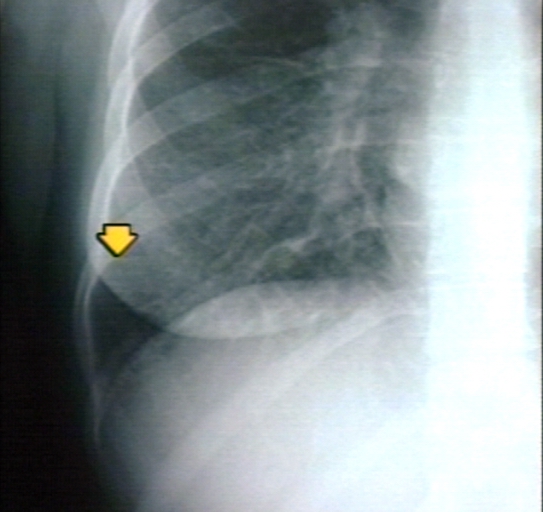
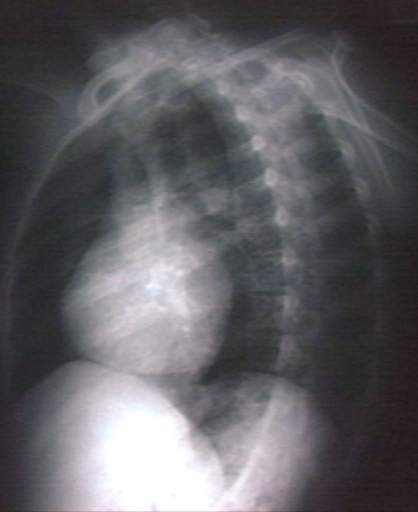
Kerley B lines are short parallel lines at the lung periphery. These lines represent distended interlobular septa, which are usually less than 1 cm in length and parallel to one another at right angles to the pleura. They are located peripherally in contact with the pleura, but are generally absent along fissural surfaces. They may be seen in any zone but are most frequently observed at the lung bases at the costophrenic angles on the PA radiograph, and in the substernal region on lateral radiographs.
-
Plain film: Mitral stenosis, Kerley B lines
-
Plain film: Mitral stenosis, Kerley B lines
-
Plain film: Mitral stenosis, Kerley B lines
Peribronchial Cuffing
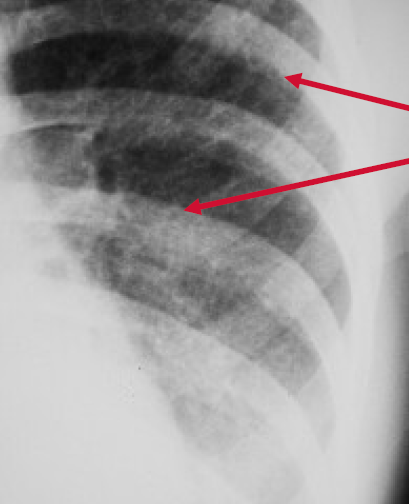
Peribronchial cuffing is an abnormality on a chest x-ray whereby the usually thin bronchial walls are thickened and take on a doughnut-like appearance.
Cephalization

Cephalization refers to the redistribution of blood into the upper lobe vessels. It has been hypothesized that once the hydrostatic pressure exceeds 10 mm Hg, then fluid begins to leak into the interstitium of the lung. This excess fluid initially compresses the lower lobe vessels, perhaps as a result of gravity. After this, the upper lobe vessels are recruited to distribute a greater volume of blood. In order to carry a greater volume of blood, the upper lobe vessels increase in size.
Echocardiography
Echocardiography can be used in the following ways:
- To evaluate left ventricular function and ejection fraction to distinguish systolic dysfunction with a low ejection fraction (<40%) from diastolic dysfunction with a preserved ejection fraction.
- To assess for the presence of regional wall motion abnormalities that would suggest an ischemic basis for the heart failure
- Detection and quantification of mitral regurgitation
- Detection and quantification oof aortic stenosis
- Measurement of pulmonary artery pressure
- Pericardial diseases such as cardiac tamponade can be rapidly diagnosed by echocardiography.
- Echocardiography may also aid in deciding what treatments will help the patient, such as medication, insertion of an implantable cardioverter-defibrillator or cardiac resynchronization therapy.
Treatment
General Measures in the Management of Heart Failure
- Treat the underlying cause of heart failure such as ischemic heart disease, hypertension, and valvular heart disease.
- Treat other non cardiac diseases that might contribute to the symptoms of heart failure such as diabetes and hyperthyroidism[8].
- Treat with a low salt diet[9]
- Follow the patient's weight to check for fluid overload
- Treat with vaccines for influenza and pneumococcus [10][11]
Diuresis: First Step in the Management of Heart Failure
The treatment of chronic heart failure often begins with the administration of diuretics, particularly if the patient has signs or symptoms of volume overload. While increased left ventricular volume increases contractility to a point, if the heart is filled beyond that point, its contractility diminishes (the patient "falls of the Staring curve"). Diuretics can reduce volume overload and reduce shortness of breath and edema. There are three kinds of diuretics, loop diuretics, thiazides and potassium-sparing diuretics. Diuretics rapidly improve the symptoms of heart failure (within hours to days). Diuretics reduce excess volume that accumulates with heart failure and decrease pulmonary edema that causes symptoms of dyspnea and orthopnea[12]. Lasix 20 to 40 mg PO daily is a conventional starting dose, but in some patients, torsemide may be a better choice due to its more predictable absorption. Once a day dosing of a given diuretic is preferred to twice a day dosing at a lower dose. A rise in BUN and Cr may reflect a reduction in renal perfusion, and further diuresis should only be undertaken with careful monitoring of renal function. The patient should weigh themselves each morning at the same time on the same scale, and the diuretic dosing should be adjusted to maintain a constant weight. Given the risk of hypokalemia or hyperkalemia, the blood level of electrolyes should be checked regularly.
ACE Inhibition and Angiotensin Receptor Blockade: Second Step in the Management of Heart Failure
After diuretics are started or at the same time they are started, an ACE inhibitor can be initiated [13]. This includes a large group of drugs, such as Enalapril (Vasotec/Renitec), Ramipril (Altace/Tritace/Ramace/Ramiwin), Quinapril (Accupril), Perindopril (Coversyl/Aceon), Lisinopril (Lisodur/Lopril/Novatec/Prinivil/Zestril) and Benazepril (Lotensin). They can improve symptoms and prognosis of heart failure in several ways including afterload reduction and favorable ventricular remodeling. Usual side effects include dry cough and angioedema. Patients with bilateral renal artery stenosis or severe renal impairment are not appropriate for angiotensin converting enzyme inhibitor (ACEI).
During or after the initiation of diuresis, one could start, for example, lisinopril 5 mg Q day. Every 1 - 2 weeks, the dose would be escalated to achieve a target dose of 15 to 20 mg Q day. An ACE inhibitor is initiated before a beta blocker because an ACE inhibitor achieves its hemodynamic effect more rapidly, and is less likely to cause a decline in hemodynamics. Although there is some data to suggest that aspirin blunts the hemodynamic effect of ACE inhibitors, there is no data to suggest that aspirin reduces the clinical efficacy of ACE inhibitors in heart failure patients. Aspirin should be administered to patients with ischemic heart disease, but not to patients without it.
If a patient cannot tolerate a an ACE inhibitor (develops a cough), then an Angiotensin II receptor blocker can be administered in its place. Angiotensin II receptor antagonists block the activation of angiotensin II AT1 receptors. Blockade of AT1 receptors directly causes vasodilation, reduces secretion of vasopressin, reduces production and secretion of aldosterone. Because angiotensin II receptor antagonists do not inhibit the breakdown of bradykinin or other kinins, and are thus only rarely associated with the persistent dry cough and/or angioedema that limit ACE inhibitor therapy. Commonly administered agents in the management of heart failure include Candesartan, Valsartan, Telmisartan, Losartan, Irbesartan, and Olmesartan. The effectiveness of switching to an ARB from and ACE inhibitor was demonstrated for candesartan in the CHARM Alternative trial [14].
In general, ARBs are as effective or slightly less effective than ACE inhibitors in the treatment of congestive heart failure.[15][16] It is a class 2a recommendation to substitute an ARB as an alternative to ACE inhibitors if the patient is already taking an ARB for another indication.[17]
The efficacy of adding an ARB to an ACE inhibitor was assessed in the CHARM Added trial[18]. While there was a reduction in the composite primary endpoint in the study, there was no reduction in mortality. Furthermore, the VALIANT trial demonstrated that an ARB should not be added to an ACE inhibitor in the post MI setting. These results for ARBs are in contrast to the results of the EMPHASIS HF trial showed that the addition of eplerenone (an aldosterone antagonist) to ACE inhibition improved clinical outcomes including mortality among patients with class II or III heart failure with a reduced LVEF.[19] Thus, based upon the mortality benefit observed in the EMPHASIS HF trial, an aldosterone antagonist rather than and ARB should be added to an ACE inhibitor in patients with NYHA class II heart failure and an LVEF < 30%, in the post-MI patient who has an LVEF < 40% who has heart failure symptoms or diabetes, and the patient with class III or IV heart failure who has an LVEF < 35%.
"Triple therapy", the combined use of an ACE inhibitor, an ARB and an aldosterone antagonist is a relative contraindication.
Beta blockers: Third Step in the Management of Heart Failure
Beta blockers reduce the heart rate which lowers the myocardial energy expenditure. They also prolong diastolic filling and lengthen the period of coronary perfusion. Beta blockers can also decrease the toxicity of catecholamines on the myocardium.
Once you have achieved a stable dose of a diuretic and an ACE inhibitor, then one of the three beta blockers that have been associated with improved survival (carvedilol, metoprolol succinate or bisoprolol) can be added and the dose titrated based upon the patient's tolerance. You should avoid beta-blockers with intrinsic sympathomimetic activity (pindolol or acebutolol). It should be noted that the 35% reduction in one year mortality observed in meta-analyses of beta-blockers in heart failure was when these drugs were added to ACE inhibitors[20]. There are no direct comparisons of the various beta-blockers, but some data does suggest that carvedilol may improve LVEF more than the others, but it may not be as well tolerated due to its vasodilatory properties. If the patient has been over diuresed, they may not tolerate the addition of a beta blocker.
- Relative contraindications to beta-blocker administration include the following:
- Asthma or bronchospasm
- Hypotension resulting in poor end organ perfusion or symptoms
- Bradycardia or heart block (first degree heart block with a PR interval > 0.24, second degree heart block, third degree heart block
- Peripheral arterial disease with limb ischemia at rest
- Moderate or greater peripheral edema
- Recent intravenous inotropic therapy
Given the potential for hemodynamic decompensation, the initiation of beta-blockers is best undertaken by an individual or center specializing in heart failure management. The patient should be aware of potential side effects, and should be aware that it may take one to three months for the beta-blockers to improve heart failure symptoms. Therapy is initiated with very low doses, and the dose of the beta-blocker should be doubled every two weeks until the target dose is achieved or symptoms prevent further dose escalation.
- Carvedilol: Initial dose 3.125 mg twice daily, target dose 25 to 50 mg twice daily
- Metoprolol succinate: Initial dose 12.5 mg daily, target dose 200 mg daily
- Bisoprolol: Initial dose 1.25 mg daily, target dose 5 to 10 mg daily
Weight gain or peripheral edema that is not responsive to diuresis may require a reduction in the dose of beta-blockers.
Aldosterone Antagonism: Fourth Step in the Management of Heart Failure
An aldosterone antagonist can be added to the regimen of 'select' patients. These selected patients include:
- Class II heart failure and a left ventricular ejection fraction (LVEF) < 30%
- Class III/IV heart failure and a LVEF <35%
- Post ST segment elevation MI and a LVEF < 40% who have either symptomatic heart failure or diabetes.
- The serum potassium must be under 5.0 meq/li and the glomerular filtration rate (GFR) should be > 30 cc per minute
A requirement for aldosterone antagonist is that the patient's renal function and potassium can be carefully monitored. Eplerenone has fewer endocrine side effects (1%) than spironolactone (10%), but is more costly. A reasonable strategy is to initiate therapy with spironolactone at a dose of 25 to 50 mg daily, and then switch to eplerenone at a dose of 25 to 50 mg daily if endocrine side effects develop.
Risk Factors for the Development of Hyperkalemia on an Aldosterone Antagonist
- Triple therapy with an ACE inhibitor and angiotensin II receptor blocker makes this combination a contraindication
- Higher doses of either an ACE inhibitor or an angiotensin receptor blocker (ARB)
- Hyperkalemia prior to initiation of spironolactone
- Comorbidities such as diabetes and chronic renal insufficiency
- Higher NYHA heart failure class
- Concomitant administration of beta blockers, nonsteroidal anti-inflammatory drugs (NSAIDs) or potassium supplements
- A daily dose of Spironolactone greater than 50 mg
The Combination of Hydralazine and a Nitrate: Fifth step in the Management of Heart Failure
The combination of hydralazine and a nitrate (particularly among black patients) can be added if the patient continues to have symptoms on a diuretic, ACE inhibitor (or ARB in the intolerant patient) and a beta blocker. The initial dose is isosorbide dinitrate 20 mg three times a day along with hydralazine 25 mg three times a day. The dose(s) can be increased every 2 to 4 weeks to a target dose of isosorbide dinitrate 40 mg three times a day and hydralazine 75 mg three times a day.
Digoxin: Sixth step in the Management of Heart Failure
Digitalis can strengthen the contractility of the heart and can also be useful to achieve rate control in patients with heart failure who also have atrial fibrillation. In the DIG trial, digoxin reduced the rate of re-hospitalization but did not improve mortality among all patients enrolled in the trial.[21] However, in a retrospective analysis, mortality was reduced in male patients who had digoxin levels between 0.5 and 0.8 ng/mL and was increased in male patients with digoxin levels > 1.2 ng/ml.[22] A similar trend was observed among women patients: there was a trend towards lower mortality at digoxin concentrations between 0.5 to 0.9 ng/ml, but significantly higher mortality at digoxin concentrations > 1.2 ng/ml.[23]
Digoxin should not be used as primary therapy for congestive heart failure. The administration of digoxin is reasonable in patients with NYHA class II-IV heart failure symptoms who have an LVEF of < 40% despite treatment with diuretics, angiotensin-converting enzyme inhibitors, beta blockers, and an aldosterone antagonist. Small doses of 0.125 mg per day of digoxin are often effective in maintaining a serum digoxin level between 0.5 and 0.8 ng/ml.
Cardiac Resynchronization Therapy
Cardiac Resynchronization Therapy (CRT) is recommended in congestive heart failure patients with:
- Symptoms: NYHA Class II-IV
- QRS: A prolonged QRS interval > 0.12
- LVEF: A LVEF < 30% to 35%
Percutaneous Coronary Intervention (PCI):
Coronary artery disease (CAD) and impaired blood flow to the heart is one of the main causes of heart failure. Relieving the blockages in the coronary arteries can improve overall heart function, which may improve or resolve heart failure symptoms. The procedure is usually performed in a cardiac catherization laboratory. A catheter, a very small tube with a tiny deflated balloon on the end, is inserted through an incision in the groin area and then guided over a floppy wire to the section of the diseased artery. The balloon is then inflated to prop open the artery. The balloon is deflated and withdrawn once the artery has been fully opened. A stent may be placed during the procedure to keep the blood vessel open. Clinical trials have demonstrated that percutaneous coronary intervention (PCI) is a very effective and safe procedure to dilate blocked vessels and can improve a patient's symptoms if ischemia or lack of blood flow is the problem.
Left Ventricular Assist Device (LVAD):
A left ventricular assist device (LVAD) is a mechanical pump-type device that can help maintain the pumping ability of a failing heart. One type of LVAD has tubing that pulls blood from the left ventricle into a pump. The pump then ejects blood into the aorta. LVADs are typically used for weeks to months as a "bridge" to more definitive therapy such as a heart transplant rather than as a final or "destination" therapy.
Heart Transplantation
A Heart transplant may be the only effective treatment option for patients with severe, progressive heart failure that can not be helped by medications, dietary and lifestyle changes. During a heart transplant procedure, the surgeons connect the patient to a heart-lung machine, which takes over the functions of the heart and lungs. Then the surgeons remove the diseased heart and replace it with the donor heart. Finally, the major blood vessels are reconnected and the new heart is ready to work. The outlook for people with heart transplants is good during the first few years after the transplant. Over 85 percent of patients are alive more than a year after their heart transplant.
References
- ↑ Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE; et al. (2013). "2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. doi:10.1016/j.jacc.2013.05.019. PMID 23747642.
- ↑ Juenger J, Schellberg D, Kraemer S; et al. (2002). "Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables". Heart. 87 (3): 235–41. doi:10.1136/heart.87.3.235. PMC 1767036. PMID 11847161. Unknown parameter
|month=ignored (help) - ↑ Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK (2002). "Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population". Eur. Heart J. 23 (23): 1867–76. doi:10.1053/euhj.2002.3255. PMID 12445536. Unknown parameter
|month=ignored (help) - ↑ Neubauer S (2007). "The failing heart — an engine out of fuel". N Engl J Med. 356 (11): 1140–51. doi:10.1056/NEJMra063052. PMID 17360992.
- ↑ Ewald B, Ewald D, Thakkinstian A, Attia J (2008). "Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction". Intern Med J. 38 (2): 101–13. doi:10.1111/j.1445-5994.2007.01454.x. PMID 18290826.
- ↑ 6.0 6.1 Madias JE (2008). "Low QRS voltage and its causes". J Electrocardiol. 41 (6): 498–500. doi:10.1016/j.jelectrocard.2008.06.021. PMID 18804788.
- ↑ Chinitz JS, Cooper JM, Verdino RJ (2008). "Electrocardiogram voltage discordance: interpretation of low QRS voltage only in the limb leads". J Electrocardiol. 41 (4): 281–6. doi:10.1016/j.jelectrocard.2007.12.001. PMID 18353352.
- ↑ DeGroot WJ, Leonard JJ (1970). "Hyperthyroidism as a high cardiac output state". Am Heart J. 79 (2): 265–75. PMID 4903771.
- ↑ Evangelista LS, Shinnick MA (2008). "What do we know about adherence and self-care?". J Cardiovasc Nurs. 23 (3): 250–7. doi:10.1097/01.JCN.0000317428.98844.4d. PMC 2880251. PMID 18437067.
- ↑ Martins Wde A, Ribeiro MD, Oliveira LB, Barros Lda S, Jorge AC, Santos CM; et al. (2011). "Influenza and pneumococcal vaccination in heart failure: a little applied recommendation". Arq Bras Cardiol. 96 (3): 240–5. PMID 21271169.
- ↑ Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM (2003). "Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both". The New England Journal of Medicine. 349 (20): 1893–906. doi:10.1056/NEJMoa032292. PMID 14610160. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Michael Felker G (2010). "Diuretic management in heart failure". Congest Heart Fail. 16 Suppl 1: S68–72. doi:10.1111/j.1751-7133.2010.00172.x. PMID 20653715.
- ↑ Shiokawa Y (1975). "Proceedings: Streptococcus surveys in Ryukyu Islands, Japan". Jpn Circ J. 39 (2): 168–71. PMID 1117548.
- ↑ Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K (2003). "Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial". Lancet. 362 (9386): 772–6. doi:10.1016/S0140-6736(03)14284-5. PMID 13678870. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Jong P, Demers C, McKelvie RS, Liu PP (2002). "Angiotensin receptor blockers in heart failure: meta-analysis of randomized controlled trials". Journal of the American College of Cardiology. 39 (3): 463–70. PMID 11823085. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B (2000). "Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II". Lancet. 355 (9215): 1582–7. PMID 10821361. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009). "2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation". Circulation. 119 (14): e391–479. doi:10.1161/CIRCULATIONAHA.109.192065. PMID 19324966. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA (2003). "Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial". Lancet. 362 (9386): 767–71. doi:10.1016/S0140-6736(03)14283-3. PMID 13678869. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B (2011). "Eplerenone in patients with systolic heart failure and mild symptoms". The New England Journal of Medicine. 364 (1): 11–21. doi:10.1056/NEJMoa1009492. PMID 21073363. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Brophy JM, Joseph L, Rouleau JL (2001). "Beta-blockers in congestive heart failure. A Bayesian meta-analysis". Annals of Internal Medicine. 134 (7): 550–60. PMID 11281737. Retrieved 2013-04-28. Unknown parameter
|month=ignored (help) - ↑ "The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group". The New England Journal of Medicine. 336 (8): 525–33. 1997. doi:10.1056/NEJM199702203360801. PMID 9036306. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM (2003). "Association of serum digoxin concentration and outcomes in patients with heart failure". JAMA : the Journal of the American Medical Association. 289 (7): 871–8. PMID 12588271. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help) - ↑ Adams KF, Patterson JH, Gattis WA, O'Connor CM, Lee CR, Schwartz TA, Gheorghiade M (2005). "Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis". Journal of the American College of Cardiology. 46 (3): 497–504. doi:10.1016/j.jacc.2005.02.091. PMID 16053964. Retrieved 2013-04-29. Unknown parameter
|month=ignored (help)