Chronic myelogenous leukemia pathophysiology
|
Chronic myelogenous leukemia Microchapters |
|
Differentiating Chronic myelogenous leukemia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Chronic myelogenous leukemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Chronic myelogenous leukemia pathophysiology |
|
Directions to Hospitals Treating Chronic myelogenous leukemia |
|
Risk calculators and risk factors for Chronic myelogenous leukemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
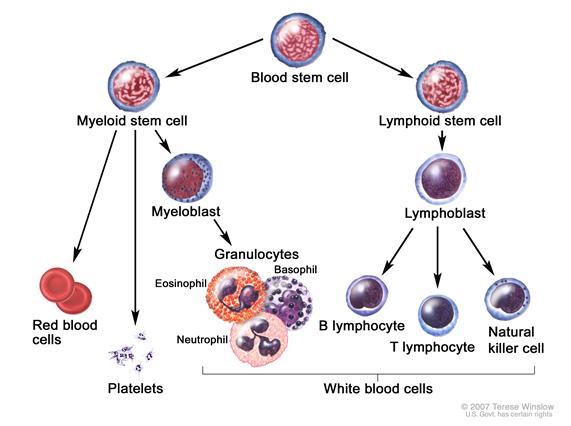
Pathophysiology
CML was the first malignancy to be linked to a clear genetic abnormality, the chromosomal translocation known as the Philadelphia chromosome. This chromosomal abnormality is so named because it was first discovered and described in 1960 by two scientists from Philadelphia, Pennsylvania: Peter Nowell of the University of Pennsylvania and David Hungerford of the Fox Chase Cancer Center. [1]
In this translocation, parts of two chromosomes (the 9th and 22nd by conventional karyotypic numbering) switch places. As a result, part of the BCR ("breakpoint cluster region") gene from chromosome 22 is fused with the ABL gene on chromosome 9. This abnormal "fusion" gene generates a protein of p210 or sometimes p185 weight (p is a weight measure of cellular proteins in kDa). Because abl carries a domain that can add phosphate groups to tyrosine residues (a tyrosine kinase), the bcr-abl fusion gene product is also a tyrosine kinase.[2]
The fused bcr-abl protein interacts with the interleukin 3beta(c) receptor subunit. The bcr-abl transcript is continuously active and does not require activation by other cellular messaging proteins. In turn, bcr-abl activates a cascade of proteins which control the cell cycle, speeding up cell division. Moreover, the bcr-abl protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities. The action of the bcr-abl protein is the pathophysiologic cause of chronic myelogenous leukemia. With improved understanding of the nature of the bcr-abl protein and its action as a tyrosine kinase, targeted therapies have been developed (the first of which was imatinib mesylate) which specifically inhibit the activity of the bcr-abl protein. These tyrosine kinase inhibitors can induce complete remissions in CML, confirming the central importance of bcr-abl as the cause of CML.[2]
References
- ↑ Nowell PC (2007). "Discovery of the Philadelphia chromosome: a personal perspective". Journal of Clinical Investigation. 117 (8): 2033–2035. PMID 17671636.
- ↑ 2.0 2.1 Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet (2007). "Chronic myeloid leukaemia". Lancet. 370 (9584): 342–50. PMID 17662883.