COVID-19-associated polyneuritis cranialis
|
COVID-19 Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
COVID-19-associated polyneuritis cranialis On the Web |
|
American Roentgen Ray Society Images of COVID-19-associated polyneuritis cranialis |
|
Risk calculators and risk factors for COVID-19-associated polyneuritis cranialis |
For COVID-19 frequently asked outpatient questions, click here
For COVID-19 frequently asked inpatient questions, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Javaria Anwer M.D.[2]
Synonyms and keywords: covid19 associated polyneuritis cranialis, SARS Cov2 associated polyneuritis cranialis, nCOV associated polyneuritis cranialis, coronavirus linked polyneuritis cranialis, covid linked polyneuritis cranialis, polyneuritis cranialis and coronavirus, polyneuritis cranialis and covid19, COVID-19 linked PNC.
Overview
Polyneuritis cranialis (PNC) literally means inflammation of the cranial nerves. It is a rare neurological disorder characterised by multiple cranial nerve palsies sparing the spinalcord. The novel coronavirus is also emerging as a neurotropic virus. The disease is a Guillain-Barré syndrome-Miller Fisher syndrome interface. The pathogenesis of polyneuritis cranials is characterized by demyelination of lower cranial nerves. COVID-19-associated polyneuritis cranials must be differentiated from other diseases that cause bulbar weakness, facial weakness, and ophthalmoparesis. The diagnosis of PNC is clinical and confirmed by NCS. Fixation nystagmus, bilateral abducens palsy, impaired visual acuity and gaze palsy abnormality and loss of deep tendon reflexes has been observed with no gait pathology. Treatment with acetaminophen caused complete recovery within 2 weeks. The disease itself is associated with COVID-19 infection as believed to be an immune response so prevention of the infection itself is the most promising primary prevention strategy at the moment.
Historical Perspective
- In 1937 French physicians Guillain G. et al. first described a postinfectious syndrome affecting the cranial nerves, associated with albuminocytological dissociation. The syndrome did not involve the limbs unlike Guillain-Barré syndrome and was called 'polyneuritis cranialis'.[1]
- The first COVID-19 outbreak news was first published by WHO on 5th January 2020.[2]
- Since mid-January 2020, right after the start of SARS-CoV2 outbreak neurological symptoms including the peripheral nervous system (PNS) symptoms have been reported in China (the first epicenter of the pandemic).[3]
- WHO declared the COVID-19 outbreak a pandemic on March 12, 2020.
- Polyneuritis cralialis associated with COVID-19 was first reported in a patient by Consuelo Gutiérrez-Ortiz et al. from Madrid, Spain on April 17th, 2020. The team reported both Miller Fisher syndrome (MFS) and polyneuritis cranialis in pattients with confirmed oropharyngeal RT PCR COVID-19 test.[4]
Classification
- There is no established system for the classification of COVID-19 associated polyneuritis cranialis.
- Basis of phenotypic appearance, the disease itself is a Guillain-Barré syndrome-Miller Fisher syndrome interface.[1]
Pathophysiology
- The exact pathogenesis of COVID-19-associated polyneuritis cranials is not fully understood.
- The pathogenesis of polyneuritis cranials is characterized by demyelination of lower cranial nerves.[5] Since polyneuritis cranials lies at the interface of GBS and Miller Fisher syndrome the pathogenesis involved in Miller Fisher syndrome can help understand the dynamics.
- Novel coronavirus is usually transmitted via respiratory droplets, direct contact with infected persons, or with contaminated objects and surfaces.[6]
- Neuropathogenic mechanism: The olfactory nerves are thought to be the primary site of direct viral inoculation in patients with neurological manifestations.[7] Following transmission, COVID-19's spike protein interacts with sialic acids linked to the patient's cell surface gangliosides to invade the neuron. The neurotropism of the novel human coronavirus is explained by the interaction between host cell proteases and Novel coronavirus's S protein spikes.[8]
- Immune mechanism:The presence of neurological symptoms in patients with severe COVID-19 disease and correlation of IL-6 with disease severity points towards the immune cause of neurological damage. novel human coronavirus being a neurotropic virus can induce a pro-inflammatory state in glial cells causing a rise in inflammatory factors such as interleukins as proved in vitro.[9][10]
- The absence of novel human coronavirus in the CSF in a patient reported, potentially clouds the possible passage through the blood-brain barrier or direct infection injury which have been included among the reasons for neurological manifestations.[10]
- The progression to polyneuritis cranials usually involves the nerve demyelination.
Causes
- COVID-19-associated polyneuritis cranialis (PNC) is caused after the infection with novel human coronavirus (a pan-betacoronavirus).
- PNC, in general, is caused by different viral or bacterial infections and in different disease states such as:
Differentiating COVID-19-associated polyneuritis cranialis from other Diseases
COVID-19-associated polyneuritis cranials must be differentiated from other diseases that cause bulbar weakness, facial weakness, and ophthalmoparesis. It may include close variants such as COVID-19-associated Guillain-Barre syndrome, COVID-19-associated Miller-Fischer syndrome (MFS)(differentiated by the absence of abnormal gait due to acute ataxic neuropathy and cervical-brachial weakness as in MFS) and commoner complications such as COVID-19-associated stroke. The table below illustrates possible differentials: [16][17][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][1][5]
| Diseases | History and Physical | Diagnostic tests | Other Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor Deficit | Sensory deficit | Cranial nerve Involvement | Autonomic dysfunction | Proximal/Distal/Generalized | Ascending/Descending/Systemic | Unilateral (UL)
or Bilateral (BL) or No Lateralization (NL) |
Onset | Lab or Imaging Findings | Specific test | ||
| Guillian-Barre syndrome | + | - | - | - | Generalized | Ascending | BL | Insidious | CSF: ↑Protein
↓Cells |
Clinical & Lumbar Puncture | Progressive ascending paralysis following infection, possible respiratory paralysis |
| Acute Flaccid Myelitis | + | + | + | - | Proximal > Distal | Ascending | UL/BL | Sudden | MRI (Longitudinal hyperintense lesions) | MRI and CSF PCR for viral etiology | Drooping eyelids
Difficulty swallowing Respiratory failure |
| Adult Botulism | + | - | + | + | Generalized | Descending | BL | Sudden | Toxin test | Blood, Wound, or Stool culture | Diplopia, Hyporeflexia, Hypotonia, possible respiratory paralysis |
| Infant Botulism | + | - | + | + | Generalized | Descending | BL | Sudden | Toxin test | Blood, Wound, or Stool culture | Flaccid paralysis (Floppy baby syndrome), possible respiratory paralysis |
| Eaton Lambert syndrome | + | - | + | + | Generalized | Systemic | BL | Intermittent | EMG, repetitive nerve stimulation test (RNS) | Voltage gated calcium channel (VGCC) antibody | Diplopia, ptosis, improves with movement (as the day progresses) |
| Myasthenia gravis | + | - | + | + | Generalized | Systemic | BL | Intermittent | EMG, Edrophonium test | Ach receptor antibody | Diplopia, ptosis, worsening with movement (as the day progresses) |
| Electrolyte disturbance | + | + | - | - | Generalized | Systemic | BL | Insidious | Electrolyte panel | ↓Ca++, ↓Mg++, ↓K+ | Possible arrhythmia |
| Organophosphate toxicity | + | + | - | + | Generalized | Ascending | BL | Sudden | Clinical diagnosis: physical exam & history | Clinical suspicion confirmed with RBC AchE activity | History of exposure to insecticide or living in farming environment. with : Diarrhea, Urination, Miosis, Bradycardia, Lacrimation, Emesis, Salivation, Sweating |
| Tick paralysis (Dermacentor tick) | + | - | - | - | Generalized | Ascending | BL | Insidious | Clinical diagnosis: physical exam & history | - | History of outdoor activity in Northeastern United States. The tick is often still latched to the patient at presentation (often in head and neck area) |
| Tetrodotoxin poisoning | + | - | + | + | Generalized | Systemic | BL | Sudden | Clinical diagnosis: physical exam & dietary history | - | History of consumption of puffer fish species. |
| Stroke | +/- | +/- | +/- | +/- | Generalized | Systemic | UL | Sudden | MRI +ve for ischemia or hemorrhage | MRI | Sudden unilateral motor and sensory deficit in a patient with a history of atherosclerotic risk factors (diabetes, hypertension, smoking) or atrial fibrillation. |
| Poliomyelitis | + | + | + | +/- | Proximal > Distal | Systemic | BL or UL | Sudden | PCR of CSF | Asymmetric paralysis following a flu-like syndrome. | |
| Transverse myelitis | + | + | + | + | Proximal > Distal | Systemic | BL or UL | Sudden | MRI & Lumbar puncture | MRI | History of chronic viral or autoimmune disease (e.g. HIV) |
| Neurosyphilis | + | + | - | +/- | Generalized | Systemic | BL | Insidious | MRI & Lumbar puncture | CSF VDRL-specifc
CSF FTA-Ab -sensitive |
History of unprotected sex or multiple sexual partners.
History of genital ulcer (chancre), diffuse maculopapular rash. |
| Muscular dystrophy | + | - | - | - | Proximal > Distal | Systemic | BL | Insidious | Genetic testing | Muscle biopsy | Progressive proximal lower limb weakness with calf pseudohypertrophy in early childhood. Gower sign positive. |
| Multiple sclerosis exacerbation | + | + | + | + | Generalized | Systemic | NL | Sudden | ↑CSF IgG levels
(monoclonal) |
Clinical assessment and MRI | Blurry vision, urinary incontinence, fatigue |
| Amyotrophic lateral sclerosis | + | - | - | - | Generalized | Systemic | BL | Insidious | Normal LP (to rule out DDx) | MRI & LP | Patient initially presents with upper motor neuron deficit (spasticity) followed by lower motor neuron deficit (flaccidity). |
| Inflammatory myopathy | + | - | - | - | Proximal > Distal | Systemic | UL or BL | Insidious | Elevated CK & Aldolase | Muscle biopsy | Progressive proximal muscle weakness in 3rd to 5th decade of life. With or without skin manifestations. |
- To view the differential diagnosis of COVID-19, click here
Epidemiology and Demographics
Till date (July 10th, 2020) a single case report of COVID-19 associated PNC ensures a very low incidence of this rare disease.[34]
Age
The median age at the diagnosis of PNC is 40 years. COVID-19 associated PNC was reported in a 39-year-old patient.[1][4]
Race
There is no racial predilection to COVID-19 associated with PNC.
Gender
The patient with COVID-19 associated PNC was a male. Data regarding gender distribution for PNC, in general, is not available.
Risk Factors
- In general more severe patients are likely to have neurologic symptoms.[3]
- There are no established risk factors for COVID-19-associated polyneuritis cranials (PNC).
Screening
- Currently, there are no recommended guidelines in place for the routine screening for COVID-19-associated polyneuritis cranials or coronavirus disease 2019 (COVID-19). Some countries use temperature monitoring as a screening tool. Certain companies have launched the Screening Tool but there are no formal guidelines. Click here for more information on COVID-19 screening. [35]
Natural History, Complications, and Prognosis
- About 80% patients with polyneuritis cranislis (PNC) present with preceding infection such as diarrhea or upper respiratory tract infection. In COVID-19 associated case, diarrhea and fever preceded the neurological symptoms. The disease develops within days. On average, 3-6 cranial nerves can be involved.[1]
- Prognosis of PNC is good and disease course is monophasic. Clinical improvement occurs within weeks or months. COVID-19 associated PNC case improved in 2 weeks.[5][4][1]
- No complications have been reported.[1]
Diagnosis
Diagnostic Study of Choice
The diagnosis of GBS and MFS is confirmed by Nerve conduction studies (NCS). A decreased amplitude shows nerve conduction pathology. Although other reports mention decreased nerve conduction in PNC patients, COVID-19 associated PNC report did not show NCS studies and consider that a limitation.
History and Symptoms
- The hallmark of polyneuritis cranialis (PNC) is bulbar weakness, facial weakness and ophthalmoparesis. Ocular symptoms occur in 73% and bulbar in 33% patients.[1]
- COVID-19 associated polyneuritis cranialis is preceded by COVID-19 infection symptoms such as diarrhea, fever which can be low-grade, and ageusia.
- Patient with polyneuritis cranialis may have the following symptoms as reported previously in literature:[5][14][1][1]
- Unpleasant sensations in the tongue and oral cavity (may last a few days)
- Dysphagia
- Asymmetrical facial weakness or diplegia
- Dysarthria
- Diplopia
- Headache
Physical Examination
- The presence of ophthalmoparesis with bulbar and facial weakness on physical examination is highly suggestive of polyneuritis cranialis (PNC). The disease is sometimes referred to as an oculopharyngeal variant of GBS and the early diagnosis essentially relies on physical exam findings.[36]
- According to the data from 15 polyneuritis cranialis cases asymmetric weakness with ocular signs (93% cases) such as ophthalmoplegia, ptosis, pupillary changes and bulbar signs such as dysarthria or dysphagia have been most commonly reported. facial palsy or numbness 73% cases has been reported.[1]
- The patient with OVID-19 associated polyneuritis cralialis has been describe to have following findings on physical exam:
- On Central nervous system exam:[4]
- Patient is well oriented to place, time, and person.
- Mental status examination is normal.
- Intracranial pressure is roughly estimated by fundoscopy has been reported normal.
- On Peripheral nervous system exam:
- Cranial nerve (CN)-1: Ageusia
- CN II: Visual acuity may be decreased such as in the COVID-19 associated polyneuritis cranialis patient had an acuity of 20/25 in both eyes
- CN III, IV, VI: Ophthalmoparesis, esotropia (abduction deficits), fixation nystagmus and Ptosis can also be present.[14]
- CN V: Facial sensory deficit was not reported but has been reported in other cases.[14]
- CN VII: SARS CoV2 associated PNC did not show facial palsy [14]
- CN VIII-XII: Normal.
- Deep tendon reflexes: All deep tendon reflexes are absent. Globally, brisk reflexes suggest an abnormality of the UMN or pyramidal tract, while decreased reflexes suggest abnormality in the anterior horn, LMN, peripheral nerve or motor end plate.
- Muscle strength (typically graded on the MRC scale I-V) was normal.
- Sensory system( fine touch, pain, temperature): Normal.
- Muscle tone was normal and no signs of rigidity were observed.
- Motor system exam: There was no ataxia or hypersomnolence.[4]
- Finger-to-nose test or heel-to-shin test did not show dysmetria or decomposition.[4]
Laboratory Findings
- A positive qualitative real-time oropharyngeal swab RT PCR COVID-19 test.[4]
- Cerebrospinal fluid (CSF) examination reveals:[4][37]
- Opening pressure is normal (normal range 8-15 mm Hg).
- WBC count was reported normal with all monocytes (normal range 0 - 5 WBCs all monocytes).
- CSF protein was a little high i.e, 62 mg/dl (normal range 15 to 60 mg/dl). CSF protein can be normal as in other cases of polyneuritis cranialis (PNC) due t other etiologies.[14][15] A high CSF protein and normal cell counts can be described as albuminocytologic dissociation and is seen in 67% PNC cases.[5][1]
- CSF glucose is normal (normal range 50-80 mg/dl).
- CSF cytology was normal.
- CSF cultures and serology were sterile and negative respectively.
- CSF RT PCR for COVID-19 was found negative in the patient.
- Anti-ganglioside GM-1 IgM and IgG antibody levels ( antiganglioside GQ1b and GD1b) should be checked.[1] The COVID-19 associated PNC patient reported could not get the planned laboratory tests done due to hospital saturation.
- CBC and differential, ESR, CRP, Basic Metabolic Panel, cardiac enzymes were all normal expect leukopenia was observed.[15]
Electrocardiogram
- There are no ECG findings associated with COVID-19-associated polyneuritis cranials (PNC).
- ECG shows significant findings in other manifestations or complications of COVID-19 infection such as COVID-19-associated myocardial injury, COVID-19-associated myocardial infarction, COVID-19-associated arrhythmia and conduction system disease, or COVID-19-associated pericarditis.
- The electrocardiogram findings on COVID-19 can be viewed by clicking here.
X-ray
- There are no x-ray findings associated with COVID-19-associated polyneuritis cranialis (PNC).[4]
- However, an x-ray may be helpful in the diagnosis of complications of COVID-19 such as COVID-19-associated pneumonia which is the most common finding associated with COVID-19 infection.
- The x-ray finidings on COVID-19 can be viewed by clicking here.
Echocardiography or Ultrasound
- There are no echocardiography/ultrasound findings associated with COVID-19-associated polyneuritis cranialis.
- However, echocardiography may be helpful in the diagnosis of cardiac complications of COVID-19 which include COVID-19-associated heart failure, or COVID-19-associated pericarditis. An abdominal ultrasound may be helpful in the case of COVID-19-associated abdominal pain.
- The echocardiographic findings on COVID-19 can be viewed by clicking here.
CT scan
- There are no CT scan findings associated with COVID-19-associated polyneuritis cranialis.[4]
- Chest CT scan may be helpful in suggesting other organ involvement in the COVID-19 which is a multi-organ disease.
- The CT scan findings in COVID-19 can be viewed by clicking here.
MRI
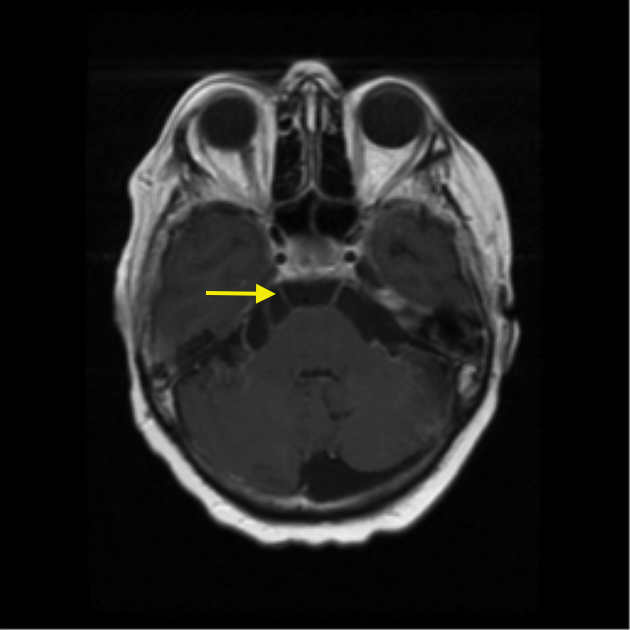
- There are no MRI findings reported in COVID-19-associated polyneuritis cranialis but the writer consider it a limitation to the study.[5]
- MRI in such neuropathies demonstrates nerve enhancement.[38][39] The MRI shown is not a case of COVID-19 related PNC but is to give an example of nerve enhancement.
- MRI may be helpful in suggesting other organ involvement in the COVID-19 which is a multi-organ disease.
- The MRI findings in COVID-19 can be viewed by clicking here.
Other Imaging Findings
There are no other imaging findings associated with COVID-19-associated polyneuritis cranialis.
Other Diagnostic Studies
There diagnostic studies associated with COVID-19-associated polyneuritis cranialis (PNC) that can help in the diagnosis include:
- Electromyography (EMG) in a patient with PNC will show neuropathic pattern helping differentiate neurological causes from primary muscle weakness. The case report on COVID-19 associated PNC considers not conducting [[EMG a limitation.
- Lyme (B.burgdorferi) IgG and IgM. (to rule out other commoner causes).
- TSH and T4 (rule out thyrotoxicosis especially in a patient with hyperthyroidism)[14]
Treatment
Medical Therapy
- The mainstay of therapy for COVID-19-associated polyneuritis cranialis is the administration of acetaminophen per oral (report does not mention the dose). It can be started after the neurological symptoms develop. The treatment can be continued on the outpatient basis depending upon the patient's condition, comorbidities, and complications. Acetaminophen works primarily as an analgesic, antipyretic and may work to ameliorate inflammation. It acts by inhibiting COX enzymes and eventually decreasing prostaglandin and prostacyclin production.[40]
- COVID-19 associated MFS patient treated with intravenous immunoglobulin 0.4 g/kg for 5 days caused complete resolution of neurological pathologies.[4] The patient with SARS CoV2 linked PNC was not administered immunoglobulins, cases of full recovery after intravenous immunoglobulin in same dosagehave been reported.[41][42] Randomised control trilas are required to consider a definitive treatment of the disease.
- COVID-19 medical therapy is as important as treating the associated polyneuritis cranialis.
- A few patients with COVID-19-associated polyneuritis cranialis may require physical therapy for residual muscle weakness.
Surgery
Surgical intervention is not recommended for the management of COVID-19-associated polyneuritis cranialis.
Primary Prevention
- The disease itself is associated with COVID-19 infection as believed to be an immune response so prevention of the infection itself is the most promising primary prevention strategy at the moment.
- There have been rigorous efforts in order to develop a vaccine for novel coronavirus and several vaccines are in the later phases of trials.[43]
- The only prevention for COVID-19 associated PNC is the prevention and early diagnosis of the coronavirus-19 infection itself. According to the CDC, the measures include:[44]
- Frequent handwashing with soap and water for at least 20 seconds or using a alcohol based hand sanitizer with at least 60% alcohol.
- Staying at least 6 feet (about 2 arms’ length) from other people who do not live with you.
- Covering your mouth and nose with a cloth face cover when around others and covering sneezes and coughs.
- Cleaning and disinfecting.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Wakerley, Benjamin R.; Yuki, Nobuhiro (2015). "Polyneuritis cranialis—subtype of Guillain–Barré syndrome?". Nature Reviews Neurology. 11 (11): 664–664. doi:10.1038/nrneurol.2015.115. ISSN 1759-4758.
- ↑ "WHO Timeline - COVID-19".
- ↑ 3.0 3.1 Mao, Ling; Wang, Mengdie; Chen, Shanghai; He, Quanwei; Chang, Jiang; Hong, Candong; Zhou, Yifan; Wang, David; Li, Yanan; Jin, Huijuan; Hu, Bo (2020). doi:10.1101/2020.02.22.20026500. Missing or empty
|title=(help) - ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 Gutiérrez-Ortiz, Consuelo; Méndez, Antonio; Rodrigo-Rey, Sara; San Pedro-Murillo, Eduardo; Bermejo-Guerrero, Laura; Gordo-Mañas, Ricardo; de Aragón-Gómez, Fernando; Benito-León, Julián (2020). "Miller Fisher Syndrome and polyneuritis cranialis in COVID-19". Neurology: 10.1212/WNL.0000000000009619. doi:10.1212/WNL.0000000000009619. ISSN 0028-3878.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Polo A, Manganotti P, Zanette G, De Grandis D (May 1992). "Polyneuritis cranialis: clinical and electrophysiological findings". J. Neurol. Neurosurg. Psychiatry. 55 (5): 398–400. doi:10.1136/jnnp.55.5.398. PMC 489084. PMID 1318358.
- ↑ "www.who.int" (PDF).
- ↑ Vavougios GD (July 2020). "Potentially irreversible olfactory and gustatory impairments in COVID-19: Indolent vs. fulminant SARS-CoV-2 neuroinfection". Brain Behav. Immun. 87: 107–108. doi:10.1016/j.bbi.2020.04.071. PMC 7185018 Check
|pmc=value (help). PMID 32353521 Check|pmid=value (help). - ↑ Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C (July 2020). "Nervous system involvement after infection with COVID-19 and other coronaviruses". Brain Behav. Immun. 87: 18–22. doi:10.1016/j.bbi.2020.03.031. PMC 7146689 Check
|pmc=value (help). PMID 32240762 Check|pmid=value (help). - ↑ Bohmwald, Karen; Gálvez, Nicolás M. S.; Ríos, Mariana; Kalergis, Alexis M. (2018). "Neurologic Alterations Due to Respiratory Virus Infections". Frontiers in Cellular Neuroscience. 12. doi:10.3389/fncel.2018.00386. ISSN 1662-5102.
- ↑ 10.0 10.1 Bohmwald K, Gálvez N, Ríos M, Kalergis AM (2018). "Neurologic Alterations Due to Respiratory Virus Infections". Front Cell Neurosci. 12: 386. doi:10.3389/fncel.2018.00386. PMC 6212673. PMID 30416428. Vancouver style error: initials (help)
- ↑ Schmutzhard E, Stanek G, Pohl P (November 1985). "Polyneuritis cranialis associated with Borrelia burgdorferi". J. Neurol. Neurosurg. Psychiatry. 48 (11): 1182–4. doi:10.1136/jnnp.48.11.1182. PMC 1028583. PMID 4078585.
- ↑ Yagnik, P M; Dhaduk, V (1986). "Polyneuritis cranialis in Lyme disease". Journal of Neurology, Neurosurgery & Psychiatry. 49 (8): 963–964. doi:10.1136/jnnp.49.8.963. ISSN 0022-3050.
- ↑ Nagel MA, Gilden D (August 2013). "Complications of varicella zoster virus reactivation". Curr Treat Options Neurol. 15 (4): 439–53. doi:10.1007/s11940-013-0246-5. PMC 3752706. PMID 23794213.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Kasundra GM, Bhargava AN, Bhushan B, Shubhakaran K, Sood I (2015). "Polyneuritis cranialis with generalized hyperreflexia as a presenting manifestation of thyrotoxicosis". Ann Indian Acad Neurol. 18 (2): 240–2. doi:10.4103/0972-2327.150625. PMC 4445207. PMID 26019429.
- ↑ 15.0 15.1 15.2 Torres, Alcy R; Salvador, Carla; Mora, Mauricio; Mirchandani, Sharam; Chavez, Wilson (2019). "Idiopathic Recurrent Polyneuritis Cranialis: A Rare Entity". Cureus. doi:10.7759/cureus.4488. ISSN 2168-8184.
- ↑ {{https://rarediseases.org/rare-diseases/miller-fisher-syndrome/}}
- ↑ 17.0 17.1 Kira R (February 2018). "[Acute Flaccid Myelitis]". Brain Nerve (in Japanese). 70 (2): 99–112. doi:10.11477/mf.1416200962. PMID 29433111.
- ↑ Hopkins SE (November 2017). "Acute Flaccid Myelitis: Etiologic Challenges, Diagnostic and Management Considerations". Curr Treat Options Neurol. 19 (12): 48. doi:10.1007/s11940-017-0480-3. PMID 29181601.
- ↑ Messacar K, Schreiner TL, Van Haren K, Yang M, Glaser CA, Tyler KL, Dominguez SR (September 2016). "Acute flaccid myelitis: A clinical review of US cases 2012-2015". Ann. Neurol. 80 (3): 326–38. doi:10.1002/ana.24730. PMC 5098271. PMID 27422805.
- ↑ Chong PF, Kira R, Mori H, Okumura A, Torisu H, Yasumoto S, Shimizu H, Fujimoto T, Hanaoka N, Kusunoki S, Takahashi T, Oishi K, Tanaka-Taya K (February 2018). "Clinical Features of Acute Flaccid Myelitis Temporally Associated With an Enterovirus D68 Outbreak: Results of a Nationwide Survey of Acute Flaccid Paralysis in Japan, August-December 2015". Clin. Infect. Dis. 66 (5): 653–664. doi:10.1093/cid/cix860. PMC 5850449. PMID 29028962.
- ↑ Messacar K, Asturias EJ, Hixon AM, Van Leer-Buter C, Niesters H, Tyler KL, Abzug MJ, Dominguez SR (August 2018). "Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality". Lancet Infect Dis. 18 (8): e239–e247. doi:10.1016/S1473-3099(18)30094-X. PMID 29482893. Vancouver style error: initials (help)
- ↑ Chen IJ, Hu SC, Hung KL, Lo CW (September 2018). "Acute flaccid myelitis associated with enterovirus D68 infection: A case report". Medicine (Baltimore). 97 (36): e11831. doi:10.1097/MD.0000000000011831. PMC 6133480. PMID 30200066.
- ↑ "Botulism | Botulism | CDC".
- ↑ McCroskey LM, Hatheway CL (May 1988). "Laboratory findings in four cases of adult botulism suggest colonization of the intestinal tract". J. Clin. Microbiol. 26 (5): 1052–4. PMC 266519. PMID 3290234.
- ↑ Lindström M, Korkeala H (April 2006). "Laboratory diagnostics of botulism". Clin. Microbiol. Rev. 19 (2): 298–314. doi:10.1128/CMR.19.2.298-314.2006. PMC 1471988. PMID 16614251.
- ↑ Brook I (2006). "Botulism: the challenge of diagnosis and treatment". Rev Neurol Dis. 3 (4): 182–9. PMID 17224901.
- ↑ Dimachkie MM, Barohn RJ (May 2013). "Guillain-Barré syndrome and variants". Neurol Clin. 31 (2): 491–510. doi:10.1016/j.ncl.2013.01.005. PMC 3939842. PMID 23642721.
- ↑ Walling AD, Dickson G (February 2013). "Guillain-Barré syndrome". Am Fam Physician. 87 (3): 191–7. PMID 23418763.
- ↑ Gilhus NE (2011). "Lambert-eaton myasthenic syndrome; pathogenesis, diagnosis, and therapy". Autoimmune Dis. 2011: 973808. doi:10.4061/2011/973808. PMC 3182560. PMID 21969911.
- ↑ Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA (May 2004). "Transverse Myelitis: pathogenesis, diagnosis and treatment". Front. Biosci. 9: 1483–99. PMID 14977560.
- ↑ Amato AA, Greenberg SA (December 2013). "Inflammatory myopathies". Continuum (Minneap Minn). 19 (6 Muscle Disease): 1615–33. doi:10.1212/01.CON.0000440662.26427.bd. PMID 24305450.
- ↑ Berger JR, Dean D (2014). "Neurosyphilis". Handb Clin Neurol. 121: 1461–72. doi:10.1016/B978-0-7020-4088-7.00098-5. PMID 24365430.
- ↑ Willison HJ, Jacobs BC, van Doorn PA (August 2016). "Guillain-Barré syndrome". Lancet. 388 (10045): 717–27. doi:10.1016/S0140-6736(16)00339-1. PMID 26948435.
- ↑ Román, Gustavo C.; Spencer, Peter S.; Reis, Jacques; Buguet, Alain; Faris, Mostafa El Alaoui; Katrak, Sarosh M.; Láinez, Miguel; Medina, Marco Tulio; Meshram, Chandrashekhar; Mizusawa, Hidehiro; Öztürk, Serefnur; Wasay, Mohammad (2020). "The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries". Journal of the Neurological Sciences. 414: 116884. doi:10.1016/j.jns.2020.116884. ISSN 0022-510X.
- ↑ Wakerley BR, Yuki N (September 2015). "Polyneuritis cranialis: oculopharyngeal subtype of Guillain-Barré syndrome". J. Neurol. 262 (9): 2001–12. doi:10.1007/s00415-015-7678-7. PMID 25712542.
- ↑ "Cerebral spinal fluid (CSF) collection: MedlinePlus Medical Encyclopedia".
- ↑ Lavi ES, Sklar EM (August 2001). "Enhancement of the eighth cranial nerve and labyrinth on MR imaging in sudden sensorineural hearing loss associated with human herpesvirus 1 infection: case report". AJNR Am J Neuroradiol. 22 (7): 1380–2. PMID 11498431.
- ↑ Aho TR, Wallace RC, Pitt AM, Sivakumar K (March 2004). "Charcot-Marie-Tooth disease: extensive cranial nerve involvement on CT and MR imaging". AJNR Am J Neuroradiol. 25 (3): 494–7. PMID 15037479.
- ↑ Capuano A, Scavone C, Racagni G, Scaglione F (July 2020). "NSAIDs in patients with viral infections, including Covid-19: Victims or perpetrators?". Pharmacol. Res. 157: 104849. doi:10.1016/j.phrs.2020.104849. PMC 7189871 Check
|pmc=value (help). PMID 32360482 Check|pmid=value (help). - ↑ Toro, Jaime; Millán, Carlos; Díaz, Camilo; Reyes, Saúl (2013). "Multiple Cranial Neuropathy (A Teaching Case)". Multiple Sclerosis and Related Disorders. 2 (4): 395–398. doi:10.1016/j.msard.2013.03.003. ISSN 2211-0348.
- ↑ Wiles CM, Brown P, Chapel H, Guerrini R, Hughes RA, Martin TD, McCrone P, Newsom-Davis J, Palace J, Rees JH, Rose MR, Scolding N, Webster AD (April 2002). "Intravenous immunoglobulin in neurological disease: a specialist review". J. Neurol. Neurosurg. Psychiatry. 72 (4): 440–8. doi:10.1136/jnnp.72.4.440. PMC 1737833. PMID 11909900.
- ↑ "NIH clinical trial of investigational vaccine for COVID-19 begins | National Institutes of Health (NIH)".
- ↑ "How to Protect Yourself & Others | CDC".