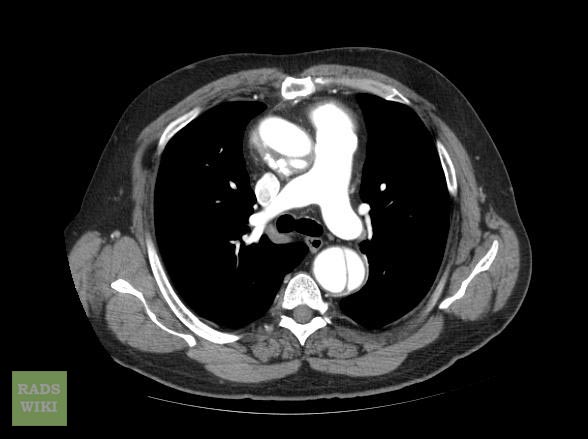
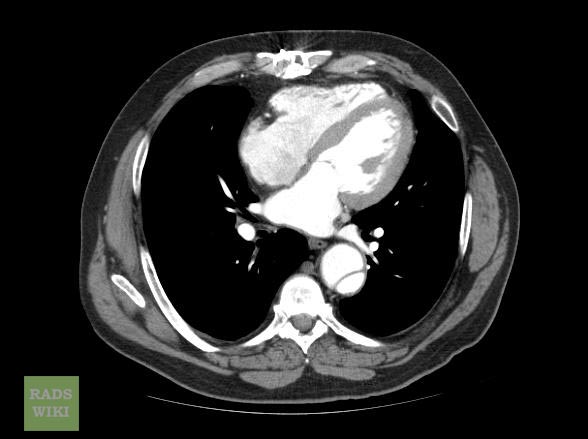
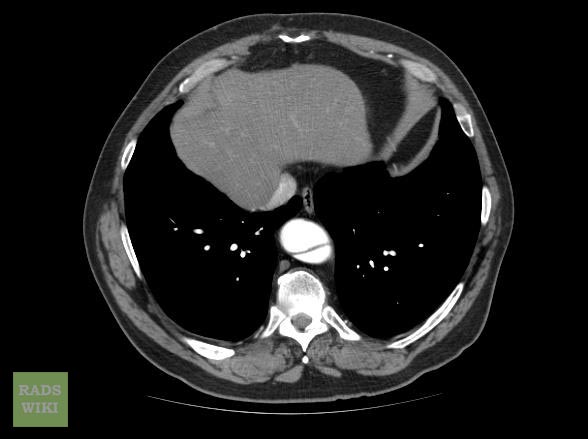
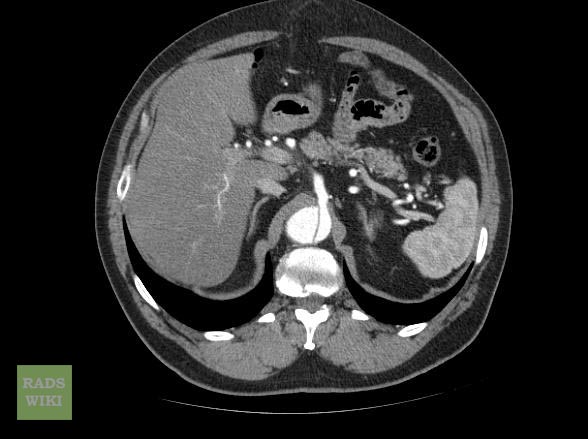
Aortic dissection CT
|
Aortic dissection Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Special Scenarios |
|
Case Studies |
|
|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Aarti Narayan, M.B.B.S [3]; Raviteja Guddeti, M.B.B.S. [4]
Overview
A CT scan can be used to diagnose aortic dissection if neither a TEE nor MRI is available in a timely fashion, or if there is a contraindication to their performance. An example would be after hours in an emergency room setting. If the results of the CT scan are non-diagnostic, then TEE or MRI should be performed to confirm the diagnosis.
Computed Tomography Angiography (CTA)
- Computed tomography angiography is a fast non-invasive test that will give an accurate three-dimensional view of the aorta. These images are produced by taking rapid thin cut slices of the chest and abdomen, and combining them in the computer to create cross-sectional slices.
- In order to delineate the aorta to the accuracy necessary to make the proper diagnosis, an iodinated contrast material is injected into a peripheral vein. Contrast is injected and the scan performed using a Bolus Tracking method. This is a type of scan timed to an injection, in order to capture the contrast as it enters the aorta.
- The scan will then follow the contrast as it flows though the vessel.
Advantages of CTA
- Readily available at most hospitals, even on an emergency basis.
- Easy identification of intraluminal thrombus and pericardial effusions.
- CT scanning with contrast has a reported sensitivity of 94% and a specificity of 87%. The accuracy of CT can be further improved with spiral CT and ultrafast CT (Electron beam CT).
Disadvantages of CTA
- The need for iodinated contrast material.
- Inability to see the intimal flap in more than 75 % of patients.
- Inability to diagnose the site of the intimal tear.
- Assessment of coronary arteries and aortic incompetence is difficult with a CT.
Differentiating a True Lumen from a False Lumen
- Beak sign: In the false lumen, there will be an acute angle between the dissection flap and the arterial wall.
- Aortic cobwebs: In the false lumen, there may be fibroelastic bands.
- Size: False lumen is usually larger than the true lumen.
- Diplaced intimal calcification: Usually faces the true lumen.
CT Examples
Type A Dissection
-
Aortic dissection Type Stanford A
-
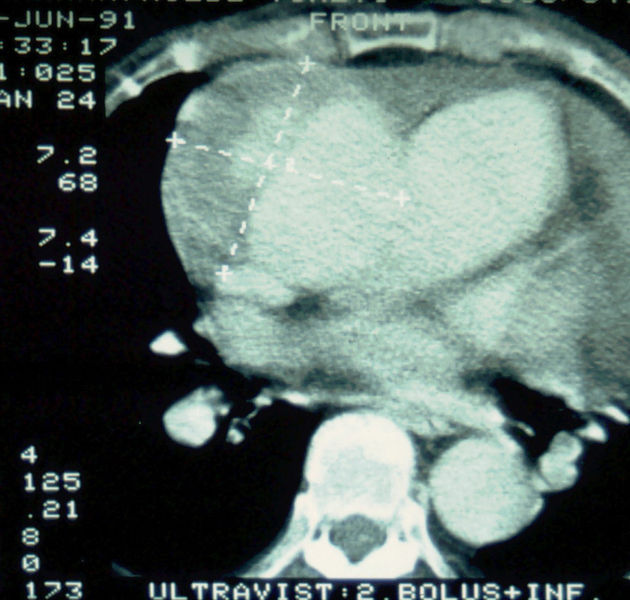
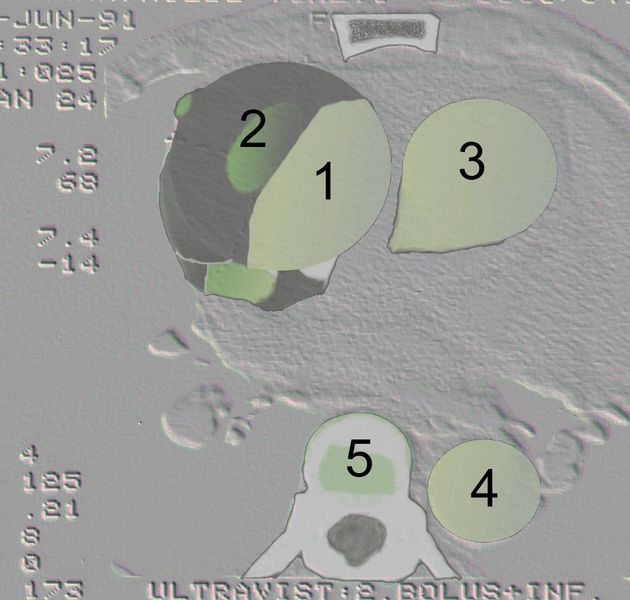
Aortic dissection Type Stanford A - '1 Aorta ascendens, true Lumen - 2 false Lumen - 3 Pulmonary artery - 4 Aorta descendens - 5 thoracic vertebra
Type B Dissection
2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease (DO NOT EDIT) [1]
Screening Tests (DO NOT EDIT)[1]
| Class I |
| "1. Urgent and definitive imaging of the aorta using transesophageal echocardiogram, computed tomographic imaging, or magnetic resonance imaging is recommended to identify or exclude thoracic aortic dissection in patients at high risk for the disease by initial screening. (Level of Evidence: B)" |
Determining the Presence and Measuring the Progression of Thoracic Aortic Disease (DO NOT EDIT)[1]
| Class I |
| "1. For measurements taken by computed tomographic imaging or magnetic resonance imaging, the external diameter should be measured perpendicular to the axis of blood flow. For aortic root measurements, the widest diameter, typically at the mid-sinus level, should be used. (Level of Evidence: C)" |
Takayasu Arteritis and Giant Cell Arteritis (DO NOT EDIT) [1]
| Class I |
| "1. The initial evaluation of Takayasu arteritis or giant cell arteritis should include thoracic aorta and branch vessel computed tomographic imaging or magnetic resonance imaging to investigate the possibility of aneurysm or occlusive disease in these vessels. (Level of Evidence: C)" |
Surveillance of Thoracic Aortic Disease or Previously Repaired Patients (DO NOT EDIT)[1]
| Class IIa |
| "1. Computed tomographic imaging or magnetic resonance imaging of the thoracic aorta is reasonable after a Type A or B aortic dissection or after prophylactic repair of the aortic root/ascending aorta. (Level of Evidence: C)" |
| "2. Computed tomographic imaging or magnetic resonance imaging of the aorta is reasonable at 1, 3, 6, and 12 months postdissection and, if stable, annually thereafter so that any threatening enlargement can be detected in a timely fashion. (Level of Evidence: C)" |
| "3. When following patients with imaging, utilization of the same modality at the same institution is reasonable, so that similar images of matching anatomic segments can be compared side by side. (Level of Evidence: C)" |
| "4. Surveillance imaging similar to classic aortic dissection is reasonable in patients with intramural hematoma. (Level of Evidence: C)" |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Hiratzka LF, Bakris GL, Beckman JA; et al. (2010). "2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine". Circulation. 121 (13): e266–369. doi:10.1161/CIR.0b013e3181d4739e. PMID 20233780. Unknown parameter
|month=ignored (help)