Amyloid beta: Difference between revisions
m (Disambiguating links to Impedance (link changed to Electrical resistance) using DisamAssist.) |
Matt Pijoan (talk | contribs) m (1 revision imported) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 13: | Line 13: | ||
| SCOP = 2lfm | | SCOP = 2lfm | ||
| TCDB = 1.C.50 | | TCDB = 1.C.50 | ||
| OPM family = | | OPM family = 304 | ||
| OPM protein = 2y3k | | OPM protein = 2y3k | ||
| CAZy = | | CAZy = | ||
| CDD = | | CDD = | ||
| Membranome superfamily= 45 | |||
}} | }} | ||
{{infobox protein | {{infobox protein | ||
| Line 38: | Line 39: | ||
| LocusSupplementaryData = | | LocusSupplementaryData = | ||
}} | }} | ||
'''Amyloid beta''' ('''Aβ''' or '''Abeta''') denotes [[peptide]]s of 36–43 [[amino acid]]s that are crucially involved in [[Alzheimer's disease]] as the main component of the [[amyloid plaque]]s found in the brains of Alzheimer patients.<ref name="pmid22813427">{{cite journal |vauthors=Hamley IW |title=The Amyloid Beta Peptide: A Chemist's Perspective. Role in Alzheimer's and Fibrillization |journal=Chemical Reviews |volume=112 |pages= | '''Amyloid beta''' ('''Aβ''' or '''Abeta''') denotes [[peptide]]s of 36–43 [[amino acid]]s that are crucially involved in [[Alzheimer's disease]] as the main component of the [[amyloid plaque]]s found in the brains of Alzheimer patients.<ref name="pmid22813427">{{cite journal | vauthors = Hamley IW | title = The Amyloid Beta Peptide: A Chemist's Perspective. Role in Alzheimer's and Fibrillization | journal = Chemical Reviews | volume = 112 | issue = 10 | pages = 5147–92 | date = October 2012 | pmid = 22813427 | doi = 10.1021/cr3000994 }}</ref> The peptides derive from the [[amyloid precursor protein]] (APP), which is cleaved by [[Beta-secretase 1|beta secretase]] and [[gamma secretase]] to yield Aβ. Aβ molecules can aggregate to form flexible soluble [[oligomer]]s which may exist in several forms. It is now believed that certain misfolded oligomers (known as "seeds") can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a [[prion]] infection. The oligomers are toxic to nerve cells.<ref>{{cite journal | vauthors = Haass C, Selkoe DJ | title = Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide | journal = Nature Reviews. Molecular Cell Biology | volume = 8 | issue = 2 | pages = 101–12 | date = February 2007 | pmid = 17245412 | doi = 10.1038/nrm2101 }}</ref> The other protein implicated in Alzheimer's disease, [[tau protein]], also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.<ref>{{cite journal | vauthors = Nussbaum JM, Seward ME, Bloom GS | title = Alzheimer disease: a tale of two prions | journal = Prion | volume = 7 | issue = 1 | pages = 14–9 | date = Jan–Feb 2013 | pmid = 22965142 | pmc = 3609044 | doi = 10.4161/pri.22118 }}</ref><ref>{{cite journal | vauthors = Pulawski W, Ghoshdastider U, Andrisano V, Filipek S | title = Ubiquitous amyloids | journal = Applied Biochemistry and Biotechnology | volume = 166 | issue = 7 | pages = 1626–43 | date = April 2012 | pmid = 22350870 | pmc = 3324686 | doi = 10.1007/s12010-012-9549-3 }}</ref> | ||
A recent study suggested that APP and its amyloid potential is of ancient origins, dating as far back as early [[deuterostomes]].<ref name="Sarkar">{{cite journal | vauthors = Tharp WG, Sarkar IN | title = Origins of amyloid-β | journal = BMC Genomics | volume = 14 | issue = 1 | pages = 290 | date = April 2013 | pmid = 23627794 | doi = 10.1186/1471-2164-14-290 | A recent study suggested that APP and its amyloid potential is of ancient origins, dating as far back as early [[deuterostomes]].<ref name="Sarkar">{{cite journal | vauthors = Tharp WG, Sarkar IN | title = Origins of amyloid-β | journal = BMC Genomics | volume = 14 | issue = 1 | pages = 290 | date = April 2013 | pmid = 23627794 | pmc = 3660159 | doi = 10.1186/1471-2164-14-290 }}</ref> | ||
== Normal function == | == Normal function == | ||
The normal function of Aβ is not well understood.<ref name="pmid19584429">{{cite journal | vauthors = Hiltunen M, van Groen T, Jolkkonen J | title = Functional roles of amyloid-beta protein precursor and amyloid-beta peptides: evidence from experimental studies | journal = Journal of Alzheimer's Disease | volume = 18 | issue = 2 | pages = 401–12 | year = 2009 | pmid = 19584429 | doi = 10.3233/JAD-2009-1154 }}</ref> Though some animal studies have shown that the absence of Aβ does not lead to any obvious loss of physiological function,<ref>{{cite journal | vauthors = Sadigh-Eteghad S, Talebi M, Farhoudi M, EJ Golzari S, Sabermarouf B, Mahmoudi J | title = Beta-amyloid exhibits antagonistic effects on alpha 7 nicotinic acetylcholine receptors in orchestrated manner|journal=Journal of Medical Hypotheses and Ideas| year =2014 | volume = 8 | pages = 48–52 | doi = 10.1016/j.jmhi.2014.01.001 }}</ref><ref name="pmid13678669">{{cite journal | vauthors = Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M | title = BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time | journal = Neurobiology of Disease | volume = 14 | issue = 1 | pages = 81–8 | date = | The normal function of Aβ is not well understood.<ref name="pmid19584429">{{cite journal | vauthors = Hiltunen M, van Groen T, Jolkkonen J | title = Functional roles of amyloid-beta protein precursor and amyloid-beta peptides: evidence from experimental studies | journal = Journal of Alzheimer's Disease | volume = 18 | issue = 2 | pages = 401–12 | year = 2009 | pmid = 19584429 | doi = 10.3233/JAD-2009-1154 }}</ref> Though some animal studies have shown that the absence of Aβ does not lead to any obvious loss of physiological function,<ref>{{cite journal | vauthors = Sadigh-Eteghad S, Talebi M, Farhoudi M, EJ Golzari S, Sabermarouf B, Mahmoudi J | title = Beta-amyloid exhibits antagonistic effects on alpha 7 nicotinic acetylcholine receptors in orchestrated manner|journal=Journal of Medical Hypotheses and Ideas| year =2014 | volume = 8 | pages = 48–52 | doi = 10.1016/j.jmhi.2014.01.001 }}</ref><ref name="pmid13678669">{{cite journal | vauthors = Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M | title = BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time | journal = Neurobiology of Disease | volume = 14 | issue = 1 | pages = 81–8 | date = October 2003 | pmid = 13678669 | doi = 10.1016/S0969-9961(03)00104-9 }}</ref> several potential activities have been discovered for Aβ, including activation of [[kinase]] [[enzyme]]s,<ref name="pmid15023353">{{cite journal | vauthors = Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK | title = Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential | journal = Biochimica et Biophysica Acta | volume = 1697 | issue = 1-2 | pages = 89–101 | date = March 2004 | pmid = 15023353 | doi = 10.1016/j.bbapap.2003.11.016 }}</ref><ref name="pmid19747481">{{cite journal | vauthors = Tabaton M, Zhu X, Perry G, Smith MA, Giliberto L | title = Signaling effect of amyloid-beta(42) on the processing of AβPP | journal = Experimental Neurology | volume = 221 | issue = 1 | pages = 18–25 | date = January 2010 | pmid = 19747481 | pmc = 2812589 | doi = 10.1016/j.expneurol.2009.09.002 }}</ref> protection against [[oxidative stress]],<ref name="pmid12077180">{{cite journal | vauthors = Zou K, Gong JS, Yanagisawa K, Michikawa M | title = A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage | journal = The Journal of Neuroscience | volume = 22 | issue = 12 | pages = 4833–41 | date = June 2002 | pmid = 12077180 | doi = }}</ref><ref name="pmid19320465">{{cite journal | vauthors = Baruch-Suchodolsky R, Fischer B | title = Aβ40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems | journal = Biochemistry | volume = 48 | issue = 20 | pages = 4354–70 | date = May 2009 | pmid = 19320465 | doi = 10.1021/bi802361k }}</ref> regulation of [[cholesterol]] transport,<ref name="pmid12206998">{{cite journal | vauthors = Yao ZX, Papadopoulos V | title = Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity | journal = FASEB Journal | volume = 16 | issue = 12 | pages = 1677–9 | date = October 2002 | pmid = 12206998 | doi = 10.1096/fj.02-0285fje }}</ref><ref name="pmid19401218">{{cite journal | vauthors = Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG | title = Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes | journal = Neuroscience | volume = 162 | issue = 2 | pages = 328–38 | date = August 2009 | pmid = 19401218 | pmc = 3083247 | doi = 10.1016/j.neuroscience.2009.04.049 }}</ref> functioning as a [[transcription factor]],<ref name="pmid21699964">{{cite journal | vauthors = Maloney B, Lahiri DK | title = The Alzheimer's amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif | journal = Gene | volume = 488 | issue = 1-2 | pages = 1–12 | date = November 2011 | pmid = 21699964 | pmc = 3381326 | doi = 10.1016/j.gene.2011.06.004 }}</ref><ref name="pmid21708232">{{cite journal | vauthors = Bailey JA, Maloney B, Ge YW, Lahiri DK | title = Functional activity of the novel Alzheimer's amyloid β-peptide interacting domain (AβID) in the APP and BACE1 promoter sequences and implications in activating apoptotic genes and in amyloidogenesis | journal = Gene | volume = 488 | issue = 1-2 | pages = 13–22 | date = November 2011 | pmid = 21708232 | pmc = 3372404 | doi = 10.1016/j.gene.2011.06.017 }}</ref> and anti-microbial activity (potentially associated with Aβ's pro-[[Inflammation|inflammatory]] activity).<ref name="pmid20209079">{{cite journal | vauthors = Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD | title = The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide | journal = PLOS One | volume = 5 | issue = 3 | pages = e9505 | date = March 2010 | pmid = 20209079 | pmc = 2831066 | doi = 10.1371/journal.pone.0009505 | editor1-last = Bush | bibcode = 2010PLoSO...5.9505S | editor1-first = Ashley I. }}</ref> | ||
The [[glymphatic system]] clears metabolic waste from the mammalian brain, and in particular beta amyloids.<ref name="pmid22896675">{{cite journal | vauthors = Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M | title = A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β | journal = Science Translational Medicine | volume = 4 | issue = 147 | pages = 147ra111 | date = | The [[glymphatic system]] clears metabolic waste from the mammalian brain, and in particular beta amyloids.<ref name="pmid22896675">{{cite journal | vauthors = Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M | title = A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β | journal = Science Translational Medicine | volume = 4 | issue = 147 | pages = 147ra111 | date = August 2012 | pmid = 22896675 | pmc = 3551275 | doi = 10.1126/scitranslmed.3003748 }}</ref> The rate of removal is significantly increased during sleep.<ref>{{cite journal | vauthors = Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M | title = Sleep drives metabolite clearance from the adult brain | journal = Science | volume = 342 | issue = 6156 | pages = 373–7 | date = October 2013 | pmid = 24136970 | pmc = 3880190 | doi = 10.1126/science.1241224 | bibcode = 2013Sci...342..373X }}</ref> However, the significance of the lymphatic system in Aβ clearance in Alzheimer's disease is unknown.<ref>{{cite journal | vauthors = Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ | title = Clearance systems in the brain-implications for Alzheimer disease | journal = Nature Reviews. Neurology | volume = 11 | issue = 8 | pages = 457–70 | date = August 2015 | pmid = 26195256 | pmc = 4694579 | doi = 10.1038/nrneurol.2015.119 }}</ref> | ||
== Disease associations == | == Disease associations == | ||
Aβ is the main component of [[amyloid]] plaques (extracellular deposits found in the [[brain]]s of patients with Alzheimer's disease).<ref>{{cite journal |vauthors=Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J |title=Amyloid-beta: a crucial factor in Alzheimer's disease |journal=Medical Principles and Practice |volume=24 |issue=1 |pages=1–10 |year=2014 |pmid=25471398 |doi=10.1159/000369101}}</ref> Similar plaques appear in some variants of [[Lewy body dementia]] and in [[inclusion body myositis]] (a muscle disease), while Aβ can also form the aggregates that coat cerebral blood vessels in [[cerebral amyloid angiopathy]]. The plaques are composed of a tangle of regularly ordered fibrillar aggregates called amyloid fibers,<ref>{{cite journal |vauthors=Parker MH, Reitz AB |title=Assembly of β-Amyloid Aggregates at the Molecular Level |journal=Chemtracts-Organic Chemistry |year=2000 |volume=13 |issue=1 |pages=51–56}}</ref> a [[protein folding|protein fold]] shared by other peptides such as the [[prion]]s associated with protein misfolding diseases. | Aβ is the main component of [[amyloid]] plaques (extracellular deposits found in the [[brain]]s of patients with Alzheimer's disease).<ref>{{cite journal | vauthors = Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J | title = Amyloid-beta: a crucial factor in Alzheimer's disease | journal = Medical Principles and Practice | volume = 24 | issue = 1 | pages = 1–10 | year = 2014 | pmid = 25471398 | doi = 10.1159/000369101 }}</ref> Similar plaques appear in some variants of [[Lewy body dementia]] and in [[inclusion body myositis]] (a muscle disease), while Aβ can also form the aggregates that coat cerebral blood vessels in [[cerebral amyloid angiopathy]]. The plaques are composed of a tangle of regularly ordered fibrillar aggregates called amyloid fibers,<ref>{{cite journal |vauthors=Parker MH, Reitz AB |title=Assembly of β-Amyloid Aggregates at the Molecular Level |journal=Chemtracts-Organic Chemistry |year=2000 |volume=13 |issue=1 |pages=51–56}}</ref> a [[protein folding|protein fold]] shared by other peptides such as the [[prion]]s associated with protein misfolding diseases. | ||
===Alzheimer's disease=== | ===Alzheimer's disease=== | ||
Recent research suggests that soluble oligomeric forms of the peptide may be causative agents in the development of Alzheimer's disease.<ref name="pmid18568035">{{cite journal |vauthors=Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ |title=Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory |journal=Nature Medicine |volume=14 |issue=8 |pages=837–42 |date= | Recent research suggests that soluble oligomeric forms of the peptide may be causative agents in the development of Alzheimer's disease.<ref name="pmid18568035">{{cite journal | vauthors = Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ | title = Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory | journal = Nature Medicine | volume = 14 | issue = 8 | pages = 837–42 | date = August 2008 | pmid = 18568035 | pmc = 2772133 | doi = 10.1038/nm1782 | laysummary = http://www.foxnews.com/story/0,2933,370002,00.html | laysource = Fox News }}</ref><ref name="pmid3292706">{{cite journal | vauthors = Prelli F, Castaño E, Glenner GG, Frangione B | title = Differences between vascular and plaque core amyloid in Alzheimer's disease | journal = Journal of Neurochemistry | volume = 51 | issue = 2 | pages = 648–51 | date = August 1988 | pmid = 3292706 | doi = 10.1111/j.1471-4159.1988.tb01087.x }}</ref> It is generally believed that Aβ oligomers are the most toxic.<ref name="pmid22837695">{{cite journal | vauthors = Zhao LN, Long H, Mu Y, Chew LY | title = The toxicity of amyloid β oligomers | journal = International Journal of Molecular Sciences | volume = 13 | issue = 6 | pages = 7303–27 | year = 2012 | pmid = 22837695 | pmc = 3397527 | doi = 10.3390/ijms13067303 }}</ref> The [[Ion channel hypothesis of Alzheimer's disease|ion channel hypothesis]] postulates that oligomers of soluble, non-fibrillar Aβ form membrane [[ion channel]]s allowing the unregulated [[calcium]] influx into neurons<ref>{{cite journal | vauthors = Arispe N, Rojas E, Pollard HB | title = Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 90 | issue = 2 | pages = 567–71 | date = January 1993 | pmid = 8380642 | pmc = 45704 | doi = 10.1073/pnas.90.2.567 | bibcode = 1993PNAS...90..567A }}</ref> that underlies disrupted calcium ion [[homeostasis]] and [[apoptosis]] seen in Alzheimer's disease.<ref>{{cite journal | vauthors = Abramov AY, Canevari L, Duchen MR | title = Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture | journal = Biochimica et Biophysica Acta | volume = 1742 | issue = 1-3 | pages = 81–7 | date = December 2004 | pmid = 15590058 | doi = 10.1016/j.bbamcr.2004.09.006 | series = 8th European Symposium on Calcium }}</ref><ref>{{cite journal | vauthors = Ekinci FJ, Linsley MD, Shea TB | title = Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation | journal = Brain Research. Molecular Brain Research | volume = 76 | issue = 2 | pages = 389–95 | date = March 2000 | pmid = 10762716 | doi = 10.1016/S0169-328X(00)00025-5 }}</ref> Computational studies have demonstrated that also Aβ peptides embedded into the membrane as monomers with predominant helical configuration, can oligomerize<ref>{{cite journal | vauthors = Pannuzzo M, Milardi D, Raudino A, Karttunen M, La Rosa C | title = Analytical model and multiscale simulations of Aβ peptide aggregation in lipid membranes: towards a unifying description of conformational transitions, oligomerization and membrane damage | journal = Physical Chemistry Chemical Physics | volume = 15 | issue = 23 | pages = 8940–51 | date = June 2013 | pmid = 23588697 | doi = 10.1039/c3cp44539a | bibcode = 2013PCCP...15.8940P }}</ref> and eventually form channels whose stability and conformation are sensitively correlated to the concomitant presence and arrangement of cholesterol.<ref>{{cite journal | vauthors = Pannuzzo M | title = On the physiological/pathological link between Aβ peptide, cholesterol, calcium ions and membrane deformation: A molecular dynamics study | journal = Biochimica et Biophysica Acta | volume = 1858 | issue = 6 | pages = 1380–9 | date = June 2016 | pmid = 27003127 | doi = 10.1016/j.bbamem.2016.03.018 }}</ref> A number of genetic, cell biology, biochemical and animal studies support the concept that Aβ plays a central role in the development of Alzheimer's disease pathology.<ref name="Ghiso_2002">{{cite journal | vauthors = Ghiso J, Frangione B | title = Amyloidosis and Alzheimer's disease | journal = Advanced Drug Delivery Reviews | volume = 54 | issue = 12 | pages = 1539–51 | date = December 2002 | pmid = 12453671 | doi = 10.1016/S0169-409X(02)00149-7 }}</ref><ref name="Selkoe_2001">{{cite journal | vauthors = Selkoe DJ | title = Clearing the brain's amyloid cobwebs | journal = Neuron | volume = 32 | issue = 2 | pages = 177–80 | date = October 2001 | pmid = 11683988 | doi = 10.1016/S0896-6273(01)00475-5 }}</ref> | ||
Brain Aβ is elevated in patients with sporadic Alzheimer's disease. Aβ is the main constituent of brain [[parenchymal]] and vascular amyloid; it contributes to cerebrovascular lesions and is neurotoxic.<ref name="Ghiso_2002"/><ref name="Selkoe_2001"/><ref name="pmid10196523">{{cite journal |vauthors=Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M |title=Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau |journal=Nature Neuroscience |volume=1 |issue=5 |pages=355–8 |date= | Brain Aβ is elevated in patients with sporadic Alzheimer's disease. Aβ is the main constituent of brain [[parenchymal]] and vascular amyloid; it contributes to cerebrovascular lesions and is neurotoxic.<ref name="Ghiso_2002"/><ref name="Selkoe_2001"/><ref name="pmid10196523">{{cite journal | vauthors = Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M | title = Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau | journal = Nature Neuroscience | volume = 1 | issue = 5 | pages = 355–8 | date = September 1998 | pmid = 10196523 | doi = 10.1038/1565 }}</ref><ref name="pmid9514588">{{cite journal | vauthors = Roses AD | title = Alzheimer diseases: a model of gene mutations and susceptibility polymorphisms for complex psychiatric diseases | journal = American Journal of Medical Genetics | volume = 81 | issue = 1 | pages = 49–57 | date = February 1998 | pmid = 9514588 | doi = 10.1002/(SICI)1096-8628(19980207)81:1<49::AID-AJMG10>3.0.CO;2-W }}</ref> It is unresolved how Aβ accumulates in the central nervous system and subsequently initiates the disease of cells. Some researchers have found that the Aβ oligomers induce some of the symptoms of Alzheimer's Disease by competing with insulin for binding sites on the insulin receptor, thus impairing glucose metabolism in the brain.<ref name="pmid12006603">{{cite journal | vauthors = Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R | title = Alzheimer's beta-amyloid peptides compete for insulin binding to the insulin receptor | journal = The Journal of Neuroscience | volume = 22 | issue = 10 | pages = RC221 | date = May 2002 | pmid = 12006603 | doi = 10.1523/JNEUROSCI.22-10-j0001.2002 | url = http://www.jneurosci.org/content/22/10/RC221.full.pdf }}</ref> Significant efforts have been focused on the mechanisms responsible for Aβ production, including the proteolytic enzymes gamma- and β-secretases which generate Aβ from its precursor protein, APP (amyloid precursor protein).<ref name="pmid10593990">{{cite journal | vauthors = Ray WJ, Yao M, Mumm J, Schroeter EH, Saftig P, Wolfe M, Selkoe DJ, Kopan R, Goate AM | title = Cell surface presenilin-1 participates in the gamma-secretase-like proteolysis of Notch | journal = The Journal of Biological Chemistry | volume = 274 | issue = 51 | pages = 36801–7 | date = December 1999 | pmid = 10593990 | doi = 10.1074/jbc.274.51.36801 }}</ref><ref name="pmid12453675">{{cite journal | vauthors = Roberts SB | title = Gamma-secretase inhibitors and Alzheimer's disease | journal = Advanced Drug Delivery Reviews | volume = 54 | issue = 12 | pages = 1579–88 | date = December 2002 | pmid = 12453675 | doi = 10.1016/S0169-409X(02)00155-2 }}</ref><ref name="pmid10531052">{{cite journal | vauthors = Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M | title = Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE | journal = Science | volume = 286 | issue = 5440 | pages = 735–41 | date = October 1999 | pmid = 10531052 | doi = 10.1126/science.286.5440.735 }}</ref><ref name="pmid12453676">{{cite journal | vauthors = Vassar R | title = Beta-secretase (BACE) as a drug target for Alzheimer's disease | journal = Advanced Drug Delivery Reviews | volume = 54 | issue = 12 | pages = 1589–602 | date = December 2002 | pmid = 12453676 | doi = 10.1016/S0169-409X(02)00157-6 }}</ref> Aβ circulates in plasma, cerebrospinal fluid (CSF) and brain interstitial fluid (ISF) mainly as soluble Aβ40<ref name="Ghiso_2002"/><ref>{{cite book |vauthors=Zlokovic BV, Frangione B |title=Transport-clearance hypothesis for Alzheimer's disease and potential therapeutic implications |year=2003 |publisher=Landes Bioscience |pages=114–122 |url=https://www.ncbi.nlm.nih.gov/books/NBK5975/}}</ref> Senile plaques contain both Aβ40 and Aβ42,<ref name="pmid3159021">{{cite journal | vauthors = Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K | title = Amyloid plaque core protein in Alzheimer disease and Down syndrome | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 82 | issue = 12 | pages = 4245–9 | date = June 1985 | pmid = 3159021 | pmc = 397973 | doi = 10.1073/pnas.82.12.4245 | bibcode = 1985PNAS...82.4245M }}</ref> while vascular amyloid is predominantly the shorter Aβ40. Several sequences of Aβ were found in both lesions.<ref name="pmid8943274">{{cite journal | vauthors = Castaño EM, Prelli F, Soto C, Beavis R, Matsubara E, Shoji M, Frangione B | title = The length of amyloid-beta in hereditary cerebral hemorrhage with amyloidosis, Dutch type. Implications for the role of amyloid-beta 1-42 in Alzheimer's disease | journal = The Journal of Biological Chemistry | volume = 271 | issue = 50 | pages = 32185–91 | date = December 1996 | pmid = 8943274 | doi = 10.1074/jbc.271.50.32185 }}</ref><ref name="pmid8248178">{{cite journal | vauthors = Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ | title = beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 90 | issue = 22 | pages = 10836–40 | date = November 1993 | pmid = 8248178 | pmc = 47873 | doi = 10.1073/pnas.90.22.10836 | bibcode = 1993PNAS...9010836R }}</ref><ref name="pmid7668828">{{cite journal | vauthors = Shinkai Y, Yoshimura M, Ito Y, Odaka A, Suzuki N, Yanagisawa K, Ihara Y | title = Amyloid beta-proteins 1-40 and 1-42(43) in the soluble fraction of extra- and intracranial blood vessels | journal = Annals of Neurology | volume = 38 | issue = 3 | pages = 421–8 | date = September 1995 | pmid = 7668828 | doi = 10.1002/ana.410380312 }}</ref> Generation of Aβ in the central nervous system may take place in the neuronal axonal membranes after APP-mediated axonal transport of β-secretase and presenilin-1.<ref name="pmid11740561">{{cite journal | vauthors = Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS | title = Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP | journal = Nature | volume = 414 | issue = 6864 | pages = 643–8 | date = December 2001 | pmid = 11740561 | doi = 10.1038/414643a }}</ref> | ||
Increases in either total Aβ levels or the relative concentration of both Aβ40 and Aβ42 (where the former is more concentrated in cerebrovascular plaques and the latter in [[neurite|neuritic]] plaques)<ref name="pmid10487842">{{cite journal| | Increases in either total Aβ levels or the relative concentration of both Aβ40 and Aβ42 (where the former is more concentrated in cerebrovascular plaques and the latter in [[neurite|neuritic]] plaques)<ref name="pmid10487842">{{cite journal | vauthors = Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J | title = Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease | journal = The American Journal of Pathology | volume = 155 | issue = 3 | pages = 853–62 | date = September 1999 | pmid = 10487842 | pmc = 1866907 | doi = 10.1016/S0002-9440(10)65184-X }}</ref> have been implicated in the [[etiology|pathogenesis]] of both familial and sporadic Alzheimer's disease. Due to its more hydrophobic nature, the Aβ42 is the most amyloidogenic form of the peptide. However the central sequence KLVFFAE is known to form amyloid on its own, and probably forms the core of the fibril.{{citation needed|date=October 2017}} One study further correlated Aβ42 levels in the brain not only with onset of Alzheimer's, but also reduced cerebrospinal fluid pressure, suggesting that a build-up or inability to clear Aβ42 fragments may play a role into the pathology.<ref>{{cite journal | vauthors = Schirinzi T, Di Lazzaro G, Sancesario GM, Colona VL, Scaricamazza E, Mercuri NB, Martorana A, Sancesario G | title = Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer's disease | journal = Journal of Neural Transmission | volume = 124 | issue = 12 | pages = 1621–1625 | date = December 2017 | pmid = 28866757 | doi = 10.1007/s00702-017-1786-8 }}</ref> | ||
The "[[amyloid hypothesis]]", that the plaques are responsible for the pathology of Alzheimer's disease, is accepted by the majority of researchers but is by no means conclusively established. An alternative hypothesis is that amyloid [[oligomer]]s rather than plaques are responsible for the disease.<ref name="pmid22837695"/><ref name="pmid12702875">{{cite journal |vauthors=Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG |title=Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis |journal=Science |volume=300 |issue=5618 |pages=486–9 |date= | The "[[amyloid hypothesis]]", that the plaques are responsible for the pathology of Alzheimer's disease, is accepted by the majority of researchers but is by no means conclusively established. An alternative hypothesis is that amyloid [[oligomer]]s rather than plaques are responsible for the disease.<ref name="pmid22837695"/><ref name="pmid12702875">{{cite journal | vauthors = Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG | title = Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis | journal = Science | volume = 300 | issue = 5618 | pages = 486–9 | date = April 2003 | pmid = 12702875 | doi = 10.1126/science.1079469 | bibcode = 2003Sci...300..486K }}</ref> Mice that are genetically engineered to express oligomers but not plaques (APP<sup>E693Q</sup>) develop the disease. Furthermore, mice that are in addition engineered to convert oligomers into plaques (APP<sup>E693Q</sup> X [[PSEN1|PS1]]ΔE9), are no more impaired than the oligomer only mice.<ref name="Gandy_2010">{{cite journal | vauthors = Gandy S, Simon AJ, Steele JW, Lublin AL, Lah JJ, Walker LC, Levey AI, Krafft GA, Levy E, Checler F, Glabe C, Bilker WB, Abel T, Schmeidler J, Ehrlich ME | title = Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers | journal = Annals of Neurology | volume = 68 | issue = 2 | pages = 220–30 | date = August 2010 | pmid = 20641005 | pmc = 3094694 | doi = 10.1002/ana.22052 | laysummary = http://www.dddmag.com/news-Alzheimers-Problems-Caused-By-Protein-Clumps-Not-Plaques-51410.aspx | laysource = Drug Discovery and Development }}</ref> Intra-cellular deposits of [[tau protein]] are also seen in the disease, and may also be implicated, as has aggregation of [[alpha synuclein]]. | ||
=== Cancer === | === Cancer === | ||
While Aβ has been implicated in [[cancer]] development, prompting studies on a variety of cancers to elucidate the nature of its possible effects, results are largely inconclusive. Aβ levels have been assessed in relation to a number of cancers, including [[Esophageal cancer|esophageal]], [[Colorectal cancer|colorectal]], [[Lung cancer|lung]], and [[Hepatic cancer|hepatic]], in response to observed reductions in risk for developing Alzheimer’s in survivors of these cancers. All cancers were shown to be associated positively with increased Aβ levels, particularly hepatic cancers.<ref>{{ | While Aβ has been implicated in [[cancer]] development, prompting studies on a variety of cancers to elucidate the nature of its possible effects, results are largely inconclusive. Aβ levels have been assessed in relation to a number of cancers, including [[Esophageal cancer|esophageal]], [[Colorectal cancer|colorectal]], [[Lung cancer|lung]], and [[Hepatic cancer|hepatic]], in response to observed reductions in risk for developing Alzheimer’s in survivors of these cancers. All cancers were shown to be associated positively with increased Aβ levels, particularly hepatic cancers.<ref>{{cite journal | vauthors = Jin WS, Bu XL, Liu YH, Shen LL, Zhuang ZQ, Jiao SS, Zhu C, Wang QH, Zhou HD, Zhang T, Wang YJ | title = Plasma Amyloid-Beta Levels in Patients with Different Types of Cancer | journal = Neurotoxicity Research | volume = 31 | issue = 2 | pages = 283–288 | date = February 2017 | pmid = 27913965 | doi = 10.1007/s12640-016-9682-9 }}</ref> This direction of association however has not yet been established. Studies focusing on human breast cancer cell lines have further demonstrated that these cancerous cells display an increased level of expression of amyloid precursor protein.<ref>{{cite journal | vauthors = Lim S, Yoo BK, Kim HS, Gilmore HL, Lee Y, Lee HP, Kim SJ, Letterio J, Lee HG | title = Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer | journal = BMC Cancer | volume = 14 | pages = 928 | date = December 2014 | pmid = 25491510 | doi = 10.1186/1471-2407-14-928 }}</ref> | ||
=== Down Syndrome === | === Down Syndrome === | ||
Adults with [[Down syndrome]] had accumulation of amyloid in association with evidence of Alzheimer’s disease, including declines in cognitive functioning, memory, fine motor movements, executive functioning, and visuospatial skills.<ref name=":0">{{ | Adults with [[Down syndrome]] had accumulation of amyloid in association with evidence of Alzheimer’s disease, including declines in cognitive functioning, memory, fine motor movements, executive functioning, and visuospatial skills.<ref name=":0">{{cite journal | vauthors = Hartley SL, Handen BL, Devenny D, Mihaila I, Hardison R, Lao PJ, Klunk WE, Bulova P, Johnson SC, Christian BT | title = Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome | journal = Neurobiology of Aging | volume = 58 | pages = 68–76 | date = October 2017 | pmid = 28715661 | doi = 10.1016/j.neurobiolaging.2017.05.019 }}</ref> | ||
== Formation == | == Formation == | ||
Aβ is formed after sequential [[peptidase|cleavage]] of the [[amyloid precursor protein]] (APP), a [[transmembrane protein|transmembrane]] [[glycoprotein]] of undetermined function. APP can be cleaved by the [[proteolysis|proteolytic]] enzymes [[alpha secretase|α-]], [[BACE|β-]] and [[gamma secretase|γ-secretase]]; Aβ protein is generated by successive action of the β and γ secretases. The γ secretase, which produces the [[C-terminus|C-terminal]] end of the Aβ peptide, cleaves within the transmembrane region of APP and can generate a number of isoforms of 30-51 [[amino acid]] residues in length.<ref name="pmid24225948">{{cite journal | vauthors = Olsson F, Schmidt S, Althoff V, Munter LM, Jin S, Rosqvist S, Lendahl U, Multhaup G, Lundkvist J | title = Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions | journal = The Journal of Biological Chemistry | volume = 289 | issue = 3 | pages = 1540–50 | date = | Aβ is formed after sequential [[peptidase|cleavage]] of the [[amyloid precursor protein]] (APP), a [[transmembrane protein|transmembrane]] [[glycoprotein]] of undetermined function. APP can be cleaved by the [[proteolysis|proteolytic]] enzymes [[alpha secretase|α-]], [[BACE|β-]] and [[gamma secretase|γ-secretase]]; Aβ protein is generated by successive action of the β and γ secretases. The γ secretase, which produces the [[C-terminus|C-terminal]] end of the Aβ peptide, cleaves within the transmembrane region of APP and can generate a number of isoforms of 30-51 [[amino acid]] residues in length.<ref name="pmid24225948">{{cite journal | vauthors = Olsson F, Schmidt S, Althoff V, Munter LM, Jin S, Rosqvist S, Lendahl U, Multhaup G, Lundkvist J | title = Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions | journal = The Journal of Biological Chemistry | volume = 289 | issue = 3 | pages = 1540–50 | date = January 2014 | pmid = 24225948 | pmc = 3894335 | doi = 10.1074/jbc.M113.498246 }}</ref> The most common isoforms are Aβ<sub>40</sub> and Aβ<sub>42</sub>; the longer form is typically produced by cleavage that occurs in the [[endoplasmic reticulum]], while the shorter form is produced by cleavage in the trans-[[Golgi apparatus|Golgi]] network.<ref name="pmid9288729">{{cite journal | vauthors = Hartmann T, Bieger SC, Brühl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K | title = Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides | journal = Nature Medicine | volume = 3 | issue = 9 | pages = 1016–20 | date = September 1997 | pmid = 9288729 | doi = 10.1038/nm0997-1016 }}</ref> The Aβ<sub>40</sub> form is the more common of the two, but Aβ<sub>42</sub> is the more fibrillogenic and is thus associated with disease states. Mutations in APP associated with early-onset Alzheimer's have been noted to increase the relative production of Aβ<sub>42</sub>, and thus one suggested avenue of Alzheimer's therapy involves modulating the activity of β and γ secretases to produce mainly Aβ<sub>40</sub>.<ref name="Yin">{{cite journal | vauthors = Yin YI, Bassit B, Zhu L, Yang X, Wang C, Li YM | title = {gamma}-Secretase Substrate Concentration Modulates the Abeta42/Aβ40 Ratio: IMPLICATIONS FOR ALZHEIMER DISEASE | journal = The Journal of Biological Chemistry | volume = 282 | issue = 32 | pages = 23639–44 | date = August 2007 | pmid = 17556361 | doi = 10.1074/jbc.M704601200 }}</ref> | ||
One major issue with this therapeutic approach are the consequences of interfering with enzymes like β and γ secretases, which have other functional roles besides within the amyloidogenic pathway. Exemplary of this are the results which clinical trials that approach the amyloid beta problem using γ secretase inhibitors have faced, including severe cognitive dysfunction and an elevated incidence of skin cancers.<ref>{{ | One major issue with this therapeutic approach are the consequences of interfering with enzymes like β and γ secretases, which have other functional roles besides within the amyloidogenic pathway. Exemplary of this are the results which clinical trials that approach the amyloid beta problem using γ secretase inhibitors have faced, including severe cognitive dysfunction and an elevated incidence of skin cancers.<ref>{{cite journal | vauthors = Kikuchi K, Kidana K, Tatebe T, Tomita T | title = Dysregulated Metabolism of the Amyloid-β Protein and Therapeutic Approaches in Alzheimer Disease | journal = Journal of Cellular Biochemistry | volume = 118 | issue = 12 | pages = 4183–4190 | date = December 2017 | pmid = 28488760 | doi = 10.1002/jcb.26129 }}</ref> | ||
Aβ is also destroyed by several amyloid-degrading enzymes including [[neprilysin]].<ref name="pmid22900228">{{cite journal | vauthors = Nalivaeva NN, Belyaev ND, Zhuravin IA, Turner AJ | title = The Alzheimer's amyloid-degrading peptidase, neprilysin: can we control it? | journal = International Journal of Alzheimer's Disease | volume = 2012 | issue = | pages = 383796 | year = 2012 | pmid = 22900228 | pmc = 3412116 | doi = 10.1155/2012/383796 }}</ref> | Aβ is also destroyed by several amyloid-degrading enzymes including [[neprilysin]].<ref name="pmid22900228">{{cite journal | vauthors = Nalivaeva NN, Belyaev ND, Zhuravin IA, Turner AJ | title = The Alzheimer's amyloid-degrading peptidase, neprilysin: can we control it? | journal = International Journal of Alzheimer's Disease | volume = 2012 | issue = | pages = 383796 | year = 2012 | pmid = 22900228 | pmc = 3412116 | doi = 10.1155/2012/383796 }}</ref> | ||
| Line 75: | Line 76: | ||
== Genetics == | == Genetics == | ||
Autosomal-dominant mutations in APP cause [[hereditary]] [[familial Alzheimer disease|early-onset Alzheimer's disease]] (a.k.a. familial AD). This form of AD accounts for no more than 10% of all cases, and the vast majority of AD is not accompanied by such mutations.<ref name="pmid18631956">{{cite journal | vauthors = | Autosomal-dominant mutations in APP cause [[hereditary]] [[familial Alzheimer disease|early-onset Alzheimer's disease]] (a.k.a. familial AD). This form of AD accounts for no more than 10% of all cases, and the vast majority of AD is not accompanied by such mutations.<ref name="pmid18631956">{{cite journal | vauthors = | title = 2008 Alzheimer's disease facts and figures | journal = Alzheimer's & Dementia | volume = 4 | issue = 2 | pages = 110–33 | date = March 2008 | pmid = 18631956 | doi = 10.1016/j.jalz.2008.02.005 }}</ref> However, familial Alzheimer disease is likely to result from altered [[Proteolysis|proteolytic]] processing. | ||
The gene for the amyloid precursor protein is located on [[chromosome 21 (human)|chromosome 21]], and accordingly people with [[Down syndrome]] have a very high incidence of Alzheimer's disease.<ref>{{cite journal | vauthors = Glenner GG, Wong CW | title = Alzheimer's disease and Down's syndrome: | The gene for the amyloid precursor protein is located on [[chromosome 21 (human)|chromosome 21]], and accordingly people with [[Down syndrome]] have a very high incidence of Alzheimer's disease.<ref>{{cite journal | vauthors = Glenner GG, Wong CW | title = Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein | journal = Biochemical and Biophysical Research Communications | volume = 122 | issue = 3 | pages = 1131–5 | date = August 1984 | pmid = 6236805 | doi = 10.1016/0006-291X(84)91209-9 }}</ref> | ||
== Structure and toxicity == | == Structure and toxicity == | ||
Amyloid beta is commonly thought to be [[intrinsically unstructured protein|intrinsically unstructured]], meaning that in solution it does not acquire a unique tertiary [[protein folding|fold]] but rather populates a set of structures. As such, it cannot be crystallized and most structural knowledge on amyloid beta comes from [[NMR]] and [[molecular dynamics]]. Early NMR-derived models of a 26-aminoacid polypeptide from amyloid beta (Aβ 10-35) show a collapsed [[random coil|coil]] structure devoid of significant [[secondary structure]] content.<ref name="zhang2000">{{cite journal | vauthors = Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, Lu Y, Felix AM, Maggio JE, Lee JP | title = The Alzheimer's peptide a beta adopts a collapsed coil structure in water | journal = Journal of Structural Biology | volume = 130 | issue = | Amyloid beta is commonly thought to be [[intrinsically unstructured protein|intrinsically unstructured]], meaning that in solution it does not acquire a unique tertiary [[protein folding|fold]] but rather populates a set of structures. As such, it cannot be crystallized and most structural knowledge on amyloid beta comes from [[NMR]] and [[molecular dynamics]]. Early NMR-derived models of a 26-aminoacid polypeptide from amyloid beta (Aβ 10-35) show a collapsed [[random coil|coil]] structure devoid of significant [[secondary structure]] content.<ref name="zhang2000">{{cite journal | vauthors = Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, Lu Y, Felix AM, Maggio JE, Lee JP | title = The Alzheimer's peptide a beta adopts a collapsed coil structure in water | journal = Journal of Structural Biology | volume = 130 | issue = 2-3 | pages = 130–41 | date = June 2000 | pmid = 10940221 | doi = 10.1006/jsbi.2000.4288 }}</ref> However, the most recent (2012) NMR structure of (Aβ 1-40) has significant secondary and tertiary structure.<ref name="Vivekanandan_2011">{{cite journal | vauthors = Vivekanandan S, Brender JR, Lee SY, Ramamoorthy A | title = A partially folded structure of amyloid-beta(1-40) in an aqueous environment | journal = Biochemical and Biophysical Research Communications | volume = 411 | issue = 2 | pages = 312–6 | date = July 2011 | pmid = 21726530 | pmc = 3148408 | doi = 10.1016/j.bbrc.2011.06.133 }}</ref> [[Replica exchange]] molecular dynamics studies suggested that amyloid beta can indeed populate multiple discrete structural states;<ref name="yang2008">{{cite journal | vauthors = Yang M, Teplow DB | title = Amyloid beta-protein monomer folding: free-energy surfaces reveal alloform-specific differences | journal = Journal of Molecular Biology | volume = 384 | issue = 2 | pages = 450–64 | date = December 2008 | pmid = 18835397 | pmc = 2673916 | doi = 10.1016/j.jmb.2008.09.039 }}</ref> more recent studies identified a multiplicity of discrete conformational clusters by statistical analysis.<ref name="sgourakis2010">{{cite journal | vauthors = Sgourakis NG, Merced-Serrano M, Boutsidis C, Drineas P, Du Z, Wang C, Garcia AE | title = Atomic-level characterization of the ensemble of the Aβ(1-42) monomer in water using unbiased molecular dynamics simulations and spectral algorithms | journal = Journal of Molecular Biology | volume = 405 | issue = 2 | pages = 570–83 | date = January 2011 | pmid = 21056574 | pmc = 3060569 | doi = 10.1016/j.jmb.2010.10.015 }}</ref> By NMR-guided simulations, amyloid beta 1-40 and amyloid beta 1-42 also seem to feature highly different conformational states,<ref name="sgourakis2007">{{cite journal | vauthors = Sgourakis NG, Yan Y, McCallum SA, Wang C, Garcia AE | title = The Alzheimer's peptides Aβ40 and 42 adopt distinct conformations in water: a combined MD / NMR study | journal = Journal of Molecular Biology | volume = 368 | issue = 5 | pages = 1448–57 | date = May 2007 | pmid = 17397862 | pmc = 1978067 | doi = 10.1016/j.jmb.2007.02.093 }}</ref> with the C-terminus of amyloid beta 1-42 being more structured than that of the 1-40 fragment. | ||
Low-temperature and low-salt conditions allowed to isolate pentameric disc-shaped oligomers devoid of beta structure.<ref name="ahmed2010">{{cite journal | vauthors = Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO | title = Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils | journal = Nature Structural & Molecular Biology | volume = 17 | issue = 5 | pages = 561–7 | date = May 2010 | pmid = 20383142 | pmc = 2922021 | doi = 10.1038/nsmb.1799 }}</ref> In contrast, soluble oligomers prepared in the presence of detergents seem to feature substantial beta sheet content with mixed parallel and antiparallel character, different from fibrils;<ref name="yu2009">{{cite journal | vauthors = Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, Miesbauer L, Solomon L, Bartley D, Walter K, Johnson RW, Hajduk PJ, Olejniczak ET | title = Structural characterization of a soluble amyloid beta-peptide oligomer | journal = Biochemistry | volume = 48 | issue = 9 | pages = 1870–7 | date = | Low-temperature and low-salt conditions allowed to isolate pentameric disc-shaped oligomers devoid of beta structure.<ref name="ahmed2010">{{cite journal | vauthors = Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO | title = Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils | journal = Nature Structural & Molecular Biology | volume = 17 | issue = 5 | pages = 561–7 | date = May 2010 | pmid = 20383142 | pmc = 2922021 | doi = 10.1038/nsmb.1799 }}</ref> In contrast, soluble oligomers prepared in the presence of detergents seem to feature substantial beta sheet content with mixed parallel and antiparallel character, different from fibrils;<ref name="yu2009">{{cite journal | vauthors = Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, Miesbauer L, Solomon L, Bartley D, Walter K, Johnson RW, Hajduk PJ, Olejniczak ET | title = Structural characterization of a soluble amyloid beta-peptide oligomer | journal = Biochemistry | volume = 48 | issue = 9 | pages = 1870–7 | date = March 2009 | pmid = 19216516 | doi = 10.1021/bi802046n }}</ref> computational studies suggest an antiparallel beta-turn-beta motif instead for membrane-embedded oligomers.<ref name="wales2010">{{cite journal | vauthors = Strodel B, Lee JW, Whittleston CS, Wales DJ | title = Transmembrane structures for Alzheimer's Aβ(1-42) oligomers | journal = Journal of the American Chemical Society | volume = 132 | issue = 38 | pages = 13300–12 | date = September 2010 | pmid = 20822103 | doi = 10.1021/ja103725c }}</ref> | ||
The suggested mechanisms by which amyloid beta may damage and cause neuronal death include the generation of [[reactive oxygen species]] during the process of its self-aggregation. When this occurs on the membrane of neurons in vitro, it causes [[lipid peroxidation]] and the generation of a toxic aldehyde called [[4-hydroxynonenal]] which, in turn, impairs the function of ion-motive ATPases, [[glucose transporters]] and [[glutamate transporters]]. As a result, amyloid beta promotes depolarization of the synaptic membrane, excessive calcium influx and mitochondrial impairment.<ref name="pmid15295589">{{cite journal | vauthors = Mattson MP | title = Pathways towards and away from Alzheimer's disease | journal = Nature | volume = 430 | issue = 7000 | pages = 631–9 | date = | The suggested mechanisms by which amyloid beta may damage and cause neuronal death include the generation of [[reactive oxygen species]] during the process of its self-aggregation. When this occurs on the membrane of neurons in vitro, it causes [[lipid peroxidation]] and the generation of a toxic aldehyde called [[4-hydroxynonenal]] which, in turn, impairs the function of ion-motive ATPases, [[glucose transporters]] and [[glutamate transporters]]. As a result, amyloid beta promotes depolarization of the synaptic membrane, excessive calcium influx and mitochondrial impairment.<ref name="pmid15295589">{{cite journal | vauthors = Mattson MP | title = Pathways towards and away from Alzheimer's disease | journal = Nature | volume = 430 | issue = 7000 | pages = 631–9 | date = August 2004 | pmid = 15295589 | pmc = 3091392 | doi = 10.1038/nature02621 | bibcode = 2004Natur.430..631M }}</ref> Aggregations of the amyloid-beta peptide disrupt membranes in vitro.<ref name="FlagmeierDe2017">{{cite journal | vauthors = Flagmeier P, De S, Wirthensohn DC, Lee SF, Vincke C, Muyldermans S, Knowles TP, Gandhi S, Dobson CM, Klenerman D | title = 2+ Influx into Lipid Vesicles Induced by Protein Aggregates | journal = Angewandte Chemie | volume = 56 | issue = 27 | pages = 7750–7754 | date = June 2017 | pmid = 28474754 | doi = 10.1002/anie.201700966 }}</ref> | ||
== Intervention strategies == | == Intervention strategies == | ||
{{details|Alzheimer's disease research}} <!-- large overlap --> | {{details|Alzheimer's disease research}} <!-- large overlap --> | ||
Researchers in Alzheimer's disease have identified several strategies as possible interventions against amyloid:<ref name="pmid15322526">{{cite journal | vauthors = Citron M | title = Strategies for disease modification in Alzheimer's disease | journal = Nature Reviews. Neuroscience | volume = 5 | issue = 9 | pages = 677–85 | date = | Researchers in Alzheimer's disease have identified several strategies as possible interventions against amyloid:<ref name="pmid15322526">{{cite journal | vauthors = Citron M | title = Strategies for disease modification in Alzheimer's disease | journal = Nature Reviews. Neuroscience | volume = 5 | issue = 9 | pages = 677–85 | date = September 2004 | pmid = 15322526 | doi = 10.1038/nrn1495 }}</ref> | ||
* [[BACE|β-Secretase]] [[enzyme inhibitor|inhibitor]]s. These work to block the first cleavage of APP inside of the cell, at the endoplasmic reticulum. | * [[BACE|β-Secretase]] [[enzyme inhibitor|inhibitor]]s. These work to block the first cleavage of APP inside of the cell, at the endoplasmic reticulum. | ||
| Line 97: | Line 98: | ||
β- and γ-secretase are responsible for the generation of Aβ from the release of the intracellular domain of APP, meaning that compounds that can partially inhibit the activity of either β- and γ-secretase are highly sought after. In order to initiate partial inhibition of β- and γ-secretase, a compound is needed that can block the large active site of aspartyl proteases while still being capable of bypassing the blood-brain barrier. To date, human testing has been avoided due to concern that it might interfere with signaling via Notch proteins and other cell surface receptors.{{citation needed|date=October 2017}} | β- and γ-secretase are responsible for the generation of Aβ from the release of the intracellular domain of APP, meaning that compounds that can partially inhibit the activity of either β- and γ-secretase are highly sought after. In order to initiate partial inhibition of β- and γ-secretase, a compound is needed that can block the large active site of aspartyl proteases while still being capable of bypassing the blood-brain barrier. To date, human testing has been avoided due to concern that it might interfere with signaling via Notch proteins and other cell surface receptors.{{citation needed|date=October 2017}} | ||
* [[Immunotherapy]]. This stimulates the host immune system to recognize and attack Aβ, or provide antibodies that either prevent plaque deposition or enhance clearance of plaques or Aβ oligomers. Oligomerization is a chemical process that converts individual molecules into a chain consisting of a finite number of molecules. Prevention of oligomerization of Aβ has been exemplified by active or passive Aβ immunization. In this process antibodies to Aβ are used to decrease cerebral plaque levels. This is accomplished by promoting microglial clearance and/or redistributing the peptide from the brain to systemic circulation. | * [[Immunotherapy]]. This stimulates the host immune system to recognize and attack Aβ, or provide antibodies that either prevent plaque deposition or enhance clearance of plaques or Aβ oligomers. Oligomerization is a chemical process that converts individual molecules into a chain consisting of a finite number of molecules. Prevention of oligomerization of Aβ has been exemplified by active or passive Aβ immunization. In this process antibodies to Aβ are used to decrease cerebral plaque levels. This is accomplished by promoting microglial clearance and/or redistributing the peptide from the brain to systemic circulation. Antibodies that target Aβ that currently in clinical trials included [[aducanumab]], [[bapineuzumab]], [[crenezumab]], [[gantenerumab]], [[gantenerumab]], and [[solanezumab]].<ref name="Cummings_2017">{{cite journal | vauthors = Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K | title = Alzheimer's disease drug development pipeline: 2017 | journal = Alzheimer's & Dementia | volume = 3 | issue = 3 | pages = 367–384 | date = September 2017 | pmid = 29067343 | pmc = 5651419 | doi = 10.1016/j.trci.2017.05.002 | department = review }}</ref><ref name="Schilling_2018">{{cite journal | vauthors = Schilling S, Rahfeld JU, Lues I, Lemere CA | title = Passive Aβ Immunotherapy: Current Achievements and Future Perspectives | journal = Molecules | volume = 23 | issue = 5 | date = May 2018 | pmid = 29751505 | doi = 10.3390/molecules23051068 | department = review }}</ref> Beta-amyloid vaccines that are currently in clinical trials include [[CAD106]] and [[UB-311]].<ref name="Cummings_2017" /> However literature reviews have raised questions as to immunotherapy's overall efficacy. One such study assessing ten anti-Ab42 antibodies showed minimal cognitive protection and results within each trial, as symptoms were too far progressed by the time of application to be useful. Further development is still required for application to presymptomatic patients to assess their effectiveness early into disease progression.<ref name="pmid28787714">{{cite journal | vauthors = Wang Y, Yan T, Lu H, Yin W, Lin B, Fan W, Zhang X, Fernandez-Funez P | title = Lessons from Anti-Amyloid-β Immunotherapies in Alzheimer Disease: Aiming at a Moving Target | journal = Neuro-Degenerative Diseases | volume = 17 | issue = 6 | pages = 242–250 | date = 2017 | pmid = 28787714 | doi = 10.1159/000478741 | department = review }}</ref> | ||
* Anti-aggregation agents<ref name="pmid12167652">{{cite journal | vauthors = Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ | title = New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer's disease | journal = The Journal of Biological Chemistry | volume = 277 | issue = 45 | pages = 42881–90 | date = | * Anti-aggregation agents<ref name="pmid12167652">{{cite journal | vauthors = Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ | title = New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer's disease | journal = The Journal of Biological Chemistry | volume = 277 | issue = 45 | pages = 42881–90 | date = November 2002 | pmid = 12167652 | doi = 10.1074/jbc.M206593200 }}</ref> such as [[apomorphine]], or [[carbenoxolone]]. The latter has commonly been used as a treatment for peptic ulcers, but also displays neuroprotective properties, shown to improve cognitive functions such as verbal fluency and memory consolidation. By binding with high affinity to Aβ42 fragments, primarily via hydrogen bonding, carbenoxolone captures the peptides before they can aggregate together, rendering them inert, as well as destabilizes those aggregates already formed, helping to clear them.<ref name="pmid28652220">{{cite journal | vauthors = Sharma S, Nehru B, Saini A | title = Inhibition of Alzheimer's amyloid-beta aggregation in-vitro by carbenoxolone: Insight into mechanism of action | journal = Neurochemistry International | volume = 108 | issue = | pages = 481–493 | date = September 2017 | pmid = 28652220 | doi = 10.1016/j.neuint.2017.06.011 | department = primary }}</ref> This is a common mechanism of action of anti-aggregation agents at large.<ref name="pmid12213471">{{cite journal | vauthors = Parker MH, Chen R, Conway KA, Lee DH, Luo C, Boyd RE, Nortey SO, Ross TM, Scott MK, Reitz AB | title = Synthesis of (-)-5,8-dihydroxy-3R-methyl-2R-(dipropylamino)-1,2,3,4-tetrahydronaphthalene: an inhibitor of beta-amyloid(1-42) aggregation | journal = Bioorganic & Medicinal Chemistry | volume = 10 | issue = 11 | pages = 3565–9 | date = November 2002 | pmid = 12213471 | doi = 10.1016/S0968-0896(02)00251-1 }}</ref> | ||
* Studies comparing synthetic to recombinant Aβ<sub>42</sub> in assays measuring rate of fibrillation, fibril homogeneity, and cellular toxicity showed that recombinant Aβ<sub>42</sub> had a faster fibrillation rate and greater toxicity than synthetic amyloid beta 1-42 peptide.<ref>{{cite journal | vauthors = Finder VH, Vodopivec I, Nitsch RM, Glockshuber R | title = The recombinant amyloid-beta peptide | * Studies comparing synthetic to recombinant Aβ<sub>42</sub> in assays measuring rate of fibrillation, fibril homogeneity, and cellular toxicity showed that recombinant Aβ<sub>42</sub> had a faster fibrillation rate and greater toxicity than synthetic amyloid beta 1-42 peptide.<ref>{{cite journal | vauthors = Finder VH, Vodopivec I, Nitsch RM, Glockshuber R | title = The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42 | journal = Journal of Molecular Biology | volume = 396 | issue = 1 | pages = 9–18 | date = February 2010 | pmid = 20026079 | doi = 10.1016/j.jmb.2009.12.016 }}</ref><ref>{{cite journal | vauthors = | title = State of aggregation | journal = Nature Neuroscience | volume = 14 | issue = 4 | pages = 399 | date = April 2011 | pmid = 21445061 | doi = 10.1038/nn0411-399 }}</ref> | ||
* Modulating cholesterol homeostasis has yielded results that show that chronic use of cholesterol-lowering drugs, such as the statins, is associated with a lower incidence of AD. In APP genetically modified mice, cholesterol-lowering drugs have been shown to reduce overall pathology. While the mechanism is poorly understood it appears that cholesterol-lowering drugs have a direct effect on APP processing.<ref name="pmid11592856">{{cite journal | vauthors = Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE | title = A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease | journal = Neurobiology of Disease | volume = 8 | issue = 5 | pages = 890–9 | date = | * Modulating cholesterol homeostasis has yielded results that show that chronic use of cholesterol-lowering drugs, such as the statins, is associated with a lower incidence of AD. In APP genetically modified mice, cholesterol-lowering drugs have been shown to reduce overall pathology. While the mechanism is poorly understood it appears that cholesterol-lowering drugs have a direct effect on APP processing.<ref name="pmid11592856">{{cite journal | vauthors = Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE | title = A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease | journal = Neurobiology of Disease | volume = 8 | issue = 5 | pages = 890–9 | date = October 2001 | pmid = 11592856 | doi = 10.1006/nbdi.2001.0422 }}</ref><ref name="Lee_2002">{{cite journal | vauthors = Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY | title = Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 99 | issue = 11 | pages = 7705–10 | date = May 2002 | pmid = 12032347 | pmc = 124328 | doi = 10.1073/pnas.092034699 | bibcode = 2002PNAS...99.7705L }}</ref> | ||
* [[Memantine]] is an Alzheimer's drug which has received widespread approval. It is a non-competitive N-methyl-D-aspartate ([[NMDA]]) channel blocker. By binding to the NMDA receptor with a higher affinity than Mg2+ ions, memantine is able to inhibit the prolonged influx of Ca2+ ions, particularly from extrasynaptic receptors, which forms the basis of neuronal excitotoxicity. It is an option for the management of patients with moderate to severe Alzheimer's Disease (modest effect). The study showed that 20 mg/day improved cognition, functional ability and behavioural symptoms in patient population.<ref name="pmid23158762">{{cite journal | vauthors = Schneider JS, Pioli EY, Jianzhong Y, Li Q, Bezard E | title = Effects of memantine and galantamine on cognitive performance in aged rhesus macaques | journal = Neurobiology of Aging | volume = 34 | issue = 4 | pages = 1126–32 | date = | * [[Memantine]] is an Alzheimer's drug which has received widespread approval. It is a non-competitive N-methyl-D-aspartate ([[NMDA]]) channel blocker. By binding to the NMDA receptor with a higher affinity than Mg2+ ions, memantine is able to inhibit the prolonged influx of Ca2+ ions, particularly from extrasynaptic receptors, which forms the basis of neuronal excitotoxicity. It is an option for the management of patients with moderate to severe Alzheimer's Disease (modest effect). The study showed that 20 mg/day improved cognition, functional ability and behavioural symptoms in patient population.<ref name="pmid23158762">{{cite journal | vauthors = Schneider JS, Pioli EY, Jianzhong Y, Li Q, Bezard E | title = Effects of memantine and galantamine on cognitive performance in aged rhesus macaques | journal = Neurobiology of Aging | volume = 34 | issue = 4 | pages = 1126–32 | date = April 2013 | pmid = 23158762 | doi = 10.1016/j.neurobiolaging.2012.10.020 }}</ref> | ||
== Measuring amyloid beta == | == Measuring amyloid beta == | ||
[[Image:Cerebral amyloid angiopathy -2b- amyloid beta - intermed mag - cropped.jpg|thumb|[[Micrograph]] showing amyloid beta (brown) in [[senile plaques]] of the [[cerebral cortex]] (upper left of image) and cerebral [[blood vessel]]s (right of image) with [[immunostain]]ing.]] | [[Image:Cerebral amyloid angiopathy -2b- amyloid beta - intermed mag - cropped.jpg|thumb|[[Micrograph]] showing amyloid beta (brown) in [[senile plaques]] of the [[cerebral cortex]] (upper left of image) and cerebral [[blood vessel]]s (right of image) with [[immunostain]]ing.]] | ||
Imaging compounds, notably [[Pittsburgh compound B]], (6-OH-BTA-1, a [[thioflavin]]), can selectively bind to amyloid beta in vitro and in vivo. This technique, combined with [[Positron emission tomography|PET]] imaging, is used to image areas of plaque deposits in Alzheimer's patients.<ref>{{cite journal| | Imaging compounds, notably [[Pittsburgh compound B]], (6-OH-BTA-1, a [[thioflavin]]), can selectively bind to amyloid beta in vitro and in vivo. This technique, combined with [[Positron emission tomography|PET]] imaging, is used to image areas of plaque deposits in Alzheimer's patients.<ref>{{cite journal | vauthors = Heurling K, Leuzy A, Zimmer ER, Lubberink M, Nordberg A | title = Imaging β-amyloid using [(18)F]flutemetamol positron emission tomography: from dosimetry to clinical diagnosis | journal = European Journal of Nuclear Medicine and Molecular Imaging | volume = 43 | issue = 2 | pages = 362–73 | date = February 2016 | pmid = 26440450 | doi = 10.1007/s00259-015-3208-1 }}</ref> | ||
===Post mortem or in tissue biopsies=== | ===Post mortem or in tissue biopsies=== | ||
Amyloid beta can be measured semiquantitatively with [[immunostain]]ing, which also allows one to determine location. Amyloid beta may be primarily vascular, as in [[cerebral amyloid angiopathy]], or in [[senile plaques]] in [[white matter]].<ref>{{cite journal| | Amyloid beta can be measured semiquantitatively with [[immunostain]]ing, which also allows one to determine location. Amyloid beta may be primarily vascular, as in [[cerebral amyloid angiopathy]], or in [[senile plaques]] in [[white matter]].<ref>{{cite journal | vauthors = Ito H, Shimada H, Shinotoh H, Takano H, Sasaki T, Nogami T, Suzuki M, Nagashima T, Takahata K, Seki C, Kodaka F, Eguchi Y, Fujiwara H, Kimura Y, Hirano S, Ikoma Y, Higuchi M, Kawamura K, Fukumura T, Böö ÉL, Farde L, Suhara T | title = Quantitative Analysis of Amyloid Deposition in Alzheimer Disease Using PET and the Radiotracer ¹¹C-AZD2184 | journal = Journal of Nuclear Medicine | volume = 55 | issue = 6 | pages = 932–8 | date = June 2014 | pmid = 24732152 | doi = 10.2967/jnumed.113.133793 }}</ref> | ||
One sensitive method is [[ELISA]] which is an immunosorbent assay which utilizes a pair of [[antibodies]] that recognize amyloid beta.<ref name="pmid22528111">{{cite journal | vauthors = Schmidt SD, Nixon RA, Mathews PM | title = Tissue processing prior to analysis of Alzheimer's disease associated proteins and metabolites, including Aβ | journal = Methods in Molecular Biology | volume = 849 | pages = 493–506 | year = 2012 | pmid = 22528111 | doi = 10.1007/978-1-61779-551-0_33 | isbn = 978-1-61779-550-3 | series = Methods in Molecular Biology }}</ref><ref name="pmid22528112">{{cite journal | vauthors = Schmidt SD, Mazzella MJ, Nixon RA, Mathews PM | title = Aβ measurement by enzyme-linked immunosorbent assay | journal = Methods in Molecular Biology | volume = 849 | pages = 507–27 | year = 2012 | pmid = 22528112 | doi = 10.1007/978-1-61779-551-0_34 | isbn = 978-1-61779-550-3 | series = Methods in Molecular Biology }}</ref> | One sensitive method is [[ELISA]] which is an immunosorbent assay which utilizes a pair of [[antibodies]] that recognize amyloid beta.<ref name="pmid22528111">{{cite journal | vauthors = Schmidt SD, Nixon RA, Mathews PM | title = Tissue processing prior to analysis of Alzheimer's disease associated proteins and metabolites, including Aβ | journal = Methods in Molecular Biology | volume = 849 | pages = 493–506 | year = 2012 | pmid = 22528111 | doi = 10.1007/978-1-61779-551-0_33 | isbn = 978-1-61779-550-3 | series = Methods in Molecular Biology }}</ref><ref name="pmid22528112">{{cite journal | vauthors = Schmidt SD, Mazzella MJ, Nixon RA, Mathews PM | title = Aβ measurement by enzyme-linked immunosorbent assay | journal = Methods in Molecular Biology | volume = 849 | pages = 507–27 | year = 2012 | pmid = 22528112 | doi = 10.1007/978-1-61779-551-0_34 | isbn = 978-1-61779-550-3 | series = Methods in Molecular Biology }}</ref> | ||
[[atomic force microscope|Atomic force microscopy]], which can visualize nanoscale molecular surfaces, can be used to determine the aggregation state of amyloid beta in vitro.<ref name="pmid12499373">{{cite journal | vauthors = Stine WB, Dahlgren KN, Krafft GA, LaDu MJ | title = In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis | journal = The Journal of Biological Chemistry | volume = 278 | issue = 13 | pages = 11612–22 | date = | [[atomic force microscope|Atomic force microscopy]], which can visualize nanoscale molecular surfaces, can be used to determine the aggregation state of amyloid beta in vitro.<ref name="pmid12499373">{{cite journal | vauthors = Stine WB, Dahlgren KN, Krafft GA, LaDu MJ | title = In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis | journal = The Journal of Biological Chemistry | volume = 278 | issue = 13 | pages = 11612–22 | date = March 2003 | pmid = 12499373 | doi = 10.1074/jbc.M210207200 }}</ref> | ||
[[Dual polarisation interferometry]] is an optical technique which can measure early stages of aggregation by measuring the molecular size and densities as the fibrils elongate.<ref name="pmid17171334">{{cite journal | vauthors = Gengler S, Gault VA, Harriott P, Hölscher C | title = Impairments of hippocampal synaptic plasticity induced by aggregated beta-amyloid (25-35) are dependent on stimulation-protocol and genetic background | journal = Experimental Brain Research | volume = 179 | issue = 4 | pages = 621–30 | date = | [[Dual polarisation interferometry]] is an optical technique which can measure early stages of aggregation by measuring the molecular size and densities as the fibrils elongate.<ref name="pmid17171334">{{cite journal | vauthors = Gengler S, Gault VA, Harriott P, Hölscher C | title = Impairments of hippocampal synaptic plasticity induced by aggregated beta-amyloid (25-35) are dependent on stimulation-protocol and genetic background | journal = Experimental Brain Research | volume = 179 | issue = 4 | pages = 621–30 | date = June 2007 | pmid = 17171334 | doi = 10.1007/s00221-006-0819-6 }}</ref><ref name="pmid18005258">{{cite journal | vauthors = Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA | title = Monitoring the prevention of amyloid fibril formation by alpha-crystallin. Temperature dependence and the nature of the aggregating species | journal = The FEBS Journal | volume = 274 | issue = 24 | pages = 6290–304 | date = December 2007 | pmid = 18005258 | doi = 10.1111/j.1742-4658.2007.06144.x }}</ref> These aggregate processes can also be studied on lipid bilayer constructs.<ref name="pmid19703409">{{cite journal | vauthors = Sanghera N, Swann MJ, Ronan G, Pinheiro TJ | title = Insight into early events in the aggregation of the prion protein on lipid membranes | journal = Biochimica et Biophysica Acta | volume = 1788 | issue = 10 | pages = 2245–51 | date = October 2009 | pmid = 19703409 | doi = 10.1016/j.bbamem.2009.08.005 }}</ref> | ||
=== Blood samples=== | === Blood samples=== | ||
New research has shown promise in testing whole blood samples for amyloid beta levels on the basis | New research has shown promise in testing whole blood samples for amyloid beta levels on the basis of [[electrical impedance]]. Interdigitated microelectrodes prepared with amyloid beta antibody measure differentiated impedance of flow in samples before and after antibody reactions to amyloid beta, comparing with normalization to account for regular variance between electrodes. When applied to control mice versus transgenic amyloid precursor protein/presenilin 1 mice (APP/PS1), strains could be differentiated via their differing amyloid beta levels.<ref>{{cite journal | vauthors = Yoo YK, Kim J, Kim G, Kim YS, Kim HY, Lee S, Cho WW, Kim S, Lee SM, Lee BC, Lee JH, Hwang KS | title = A highly sensitive plasma-based amyloid-β detection system through medium-changing and noise cancellation system for early diagnosis of the Alzheimer's disease | language = En | journal = Scientific Reports | volume = 7 | issue = 1 | pages = 8882 | date = August 2017 | pmid = 28827785 | doi = 10.1038/s41598-017-09370-3 }}</ref> | ||
== See also == | |||
*[[TPM21]] | |||
== References == | == References == | ||
{{reflist| | {{reflist|32em}} | ||
== External links == | == External links == | ||
Latest revision as of 09:08, 9 January 2019
| Amyloid beta peptide (beta-APP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| File:Abeta 2lfm.jpg A partially folded structure of amyloid beta(1 40) in an aqueous environment (pdb 2lfm)[1] | |||||||||
| Identifiers | |||||||||
| Symbol | APP | ||||||||
| Pfam | PF03494 | ||||||||
| InterPro | IPR013803 | ||||||||
| SCOP | 2lfm | ||||||||
| SUPERFAMILY | 2lfm | ||||||||
| TCDB | 1.C.50 | ||||||||
| OPM superfamily | 304 | ||||||||
| OPM protein | 2y3k | ||||||||
| Membranome | 45 | ||||||||
| |||||||||
| amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease) | |
|---|---|
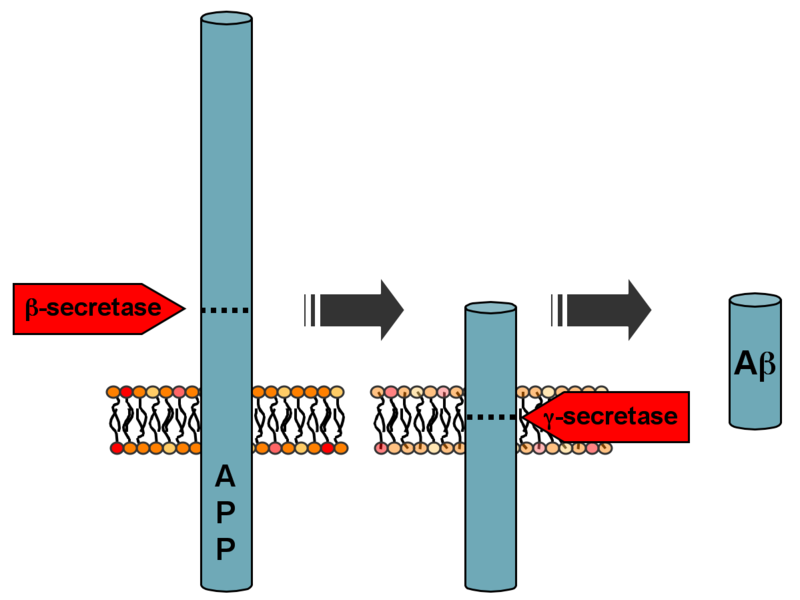 Processing of the amyloid precursor protein | |
| Identifiers | |
| Symbol | APP |
| Alt. symbols | AD1 |
| Entrez | 351 |
| HUGO | 620 |
| OMIM | 104760 |
| RefSeq | NM_000484 |
| UniProt | P05067 |
| Other data | |
| Locus | Chr. 21 q21.2 |
Amyloid beta (Aβ or Abeta) denotes peptides of 36–43 amino acids that are crucially involved in Alzheimer's disease as the main component of the amyloid plaques found in the brains of Alzheimer patients.[2] The peptides derive from the amyloid precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers (known as "seeds") can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells.[3] The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.[4][5]
A recent study suggested that APP and its amyloid potential is of ancient origins, dating as far back as early deuterostomes.[6]
Normal function
The normal function of Aβ is not well understood.[7] Though some animal studies have shown that the absence of Aβ does not lead to any obvious loss of physiological function,[8][9] several potential activities have been discovered for Aβ, including activation of kinase enzymes,[10][11] protection against oxidative stress,[12][13] regulation of cholesterol transport,[14][15] functioning as a transcription factor,[16][17] and anti-microbial activity (potentially associated with Aβ's pro-inflammatory activity).[18]
The glymphatic system clears metabolic waste from the mammalian brain, and in particular beta amyloids.[19] The rate of removal is significantly increased during sleep.[20] However, the significance of the lymphatic system in Aβ clearance in Alzheimer's disease is unknown.[21]
Disease associations
Aβ is the main component of amyloid plaques (extracellular deposits found in the brains of patients with Alzheimer's disease).[22] Similar plaques appear in some variants of Lewy body dementia and in inclusion body myositis (a muscle disease), while Aβ can also form the aggregates that coat cerebral blood vessels in cerebral amyloid angiopathy. The plaques are composed of a tangle of regularly ordered fibrillar aggregates called amyloid fibers,[23] a protein fold shared by other peptides such as the prions associated with protein misfolding diseases.
Alzheimer's disease
Recent research suggests that soluble oligomeric forms of the peptide may be causative agents in the development of Alzheimer's disease.[24][25] It is generally believed that Aβ oligomers are the most toxic.[26] The ion channel hypothesis postulates that oligomers of soluble, non-fibrillar Aβ form membrane ion channels allowing the unregulated calcium influx into neurons[27] that underlies disrupted calcium ion homeostasis and apoptosis seen in Alzheimer's disease.[28][29] Computational studies have demonstrated that also Aβ peptides embedded into the membrane as monomers with predominant helical configuration, can oligomerize[30] and eventually form channels whose stability and conformation are sensitively correlated to the concomitant presence and arrangement of cholesterol.[31] A number of genetic, cell biology, biochemical and animal studies support the concept that Aβ plays a central role in the development of Alzheimer's disease pathology.[32][33]
Brain Aβ is elevated in patients with sporadic Alzheimer's disease. Aβ is the main constituent of brain parenchymal and vascular amyloid; it contributes to cerebrovascular lesions and is neurotoxic.[32][33][34][35] It is unresolved how Aβ accumulates in the central nervous system and subsequently initiates the disease of cells. Some researchers have found that the Aβ oligomers induce some of the symptoms of Alzheimer's Disease by competing with insulin for binding sites on the insulin receptor, thus impairing glucose metabolism in the brain.[36] Significant efforts have been focused on the mechanisms responsible for Aβ production, including the proteolytic enzymes gamma- and β-secretases which generate Aβ from its precursor protein, APP (amyloid precursor protein).[37][38][39][40] Aβ circulates in plasma, cerebrospinal fluid (CSF) and brain interstitial fluid (ISF) mainly as soluble Aβ40[32][41] Senile plaques contain both Aβ40 and Aβ42,[42] while vascular amyloid is predominantly the shorter Aβ40. Several sequences of Aβ were found in both lesions.[43][44][45] Generation of Aβ in the central nervous system may take place in the neuronal axonal membranes after APP-mediated axonal transport of β-secretase and presenilin-1.[46]
Increases in either total Aβ levels or the relative concentration of both Aβ40 and Aβ42 (where the former is more concentrated in cerebrovascular plaques and the latter in neuritic plaques)[47] have been implicated in the pathogenesis of both familial and sporadic Alzheimer's disease. Due to its more hydrophobic nature, the Aβ42 is the most amyloidogenic form of the peptide. However the central sequence KLVFFAE is known to form amyloid on its own, and probably forms the core of the fibril.[citation needed] One study further correlated Aβ42 levels in the brain not only with onset of Alzheimer's, but also reduced cerebrospinal fluid pressure, suggesting that a build-up or inability to clear Aβ42 fragments may play a role into the pathology.[48]
The "amyloid hypothesis", that the plaques are responsible for the pathology of Alzheimer's disease, is accepted by the majority of researchers but is by no means conclusively established. An alternative hypothesis is that amyloid oligomers rather than plaques are responsible for the disease.[26][49] Mice that are genetically engineered to express oligomers but not plaques (APPE693Q) develop the disease. Furthermore, mice that are in addition engineered to convert oligomers into plaques (APPE693Q X PS1ΔE9), are no more impaired than the oligomer only mice.[50] Intra-cellular deposits of tau protein are also seen in the disease, and may also be implicated, as has aggregation of alpha synuclein.
Cancer
While Aβ has been implicated in cancer development, prompting studies on a variety of cancers to elucidate the nature of its possible effects, results are largely inconclusive. Aβ levels have been assessed in relation to a number of cancers, including esophageal, colorectal, lung, and hepatic, in response to observed reductions in risk for developing Alzheimer’s in survivors of these cancers. All cancers were shown to be associated positively with increased Aβ levels, particularly hepatic cancers.[51] This direction of association however has not yet been established. Studies focusing on human breast cancer cell lines have further demonstrated that these cancerous cells display an increased level of expression of amyloid precursor protein.[52]
Down Syndrome
Adults with Down syndrome had accumulation of amyloid in association with evidence of Alzheimer’s disease, including declines in cognitive functioning, memory, fine motor movements, executive functioning, and visuospatial skills.[53]
Formation
Aβ is formed after sequential cleavage of the amyloid precursor protein (APP), a transmembrane glycoprotein of undetermined function. APP can be cleaved by the proteolytic enzymes α-, β- and γ-secretase; Aβ protein is generated by successive action of the β and γ secretases. The γ secretase, which produces the C-terminal end of the Aβ peptide, cleaves within the transmembrane region of APP and can generate a number of isoforms of 30-51 amino acid residues in length.[54] The most common isoforms are Aβ40 and Aβ42; the longer form is typically produced by cleavage that occurs in the endoplasmic reticulum, while the shorter form is produced by cleavage in the trans-Golgi network.[55] The Aβ40 form is the more common of the two, but Aβ42 is the more fibrillogenic and is thus associated with disease states. Mutations in APP associated with early-onset Alzheimer's have been noted to increase the relative production of Aβ42, and thus one suggested avenue of Alzheimer's therapy involves modulating the activity of β and γ secretases to produce mainly Aβ40.[56]
One major issue with this therapeutic approach are the consequences of interfering with enzymes like β and γ secretases, which have other functional roles besides within the amyloidogenic pathway. Exemplary of this are the results which clinical trials that approach the amyloid beta problem using γ secretase inhibitors have faced, including severe cognitive dysfunction and an elevated incidence of skin cancers.[57]
Aβ is also destroyed by several amyloid-degrading enzymes including neprilysin.[58]
Genetics
Autosomal-dominant mutations in APP cause hereditary early-onset Alzheimer's disease (a.k.a. familial AD). This form of AD accounts for no more than 10% of all cases, and the vast majority of AD is not accompanied by such mutations.[59] However, familial Alzheimer disease is likely to result from altered proteolytic processing.
The gene for the amyloid precursor protein is located on chromosome 21, and accordingly people with Down syndrome have a very high incidence of Alzheimer's disease.[60]
Structure and toxicity
Amyloid beta is commonly thought to be intrinsically unstructured, meaning that in solution it does not acquire a unique tertiary fold but rather populates a set of structures. As such, it cannot be crystallized and most structural knowledge on amyloid beta comes from NMR and molecular dynamics. Early NMR-derived models of a 26-aminoacid polypeptide from amyloid beta (Aβ 10-35) show a collapsed coil structure devoid of significant secondary structure content.[61] However, the most recent (2012) NMR structure of (Aβ 1-40) has significant secondary and tertiary structure.[1] Replica exchange molecular dynamics studies suggested that amyloid beta can indeed populate multiple discrete structural states;[62] more recent studies identified a multiplicity of discrete conformational clusters by statistical analysis.[63] By NMR-guided simulations, amyloid beta 1-40 and amyloid beta 1-42 also seem to feature highly different conformational states,[64] with the C-terminus of amyloid beta 1-42 being more structured than that of the 1-40 fragment.
Low-temperature and low-salt conditions allowed to isolate pentameric disc-shaped oligomers devoid of beta structure.[65] In contrast, soluble oligomers prepared in the presence of detergents seem to feature substantial beta sheet content with mixed parallel and antiparallel character, different from fibrils;[66] computational studies suggest an antiparallel beta-turn-beta motif instead for membrane-embedded oligomers.[67]
The suggested mechanisms by which amyloid beta may damage and cause neuronal death include the generation of reactive oxygen species during the process of its self-aggregation. When this occurs on the membrane of neurons in vitro, it causes lipid peroxidation and the generation of a toxic aldehyde called 4-hydroxynonenal which, in turn, impairs the function of ion-motive ATPases, glucose transporters and glutamate transporters. As a result, amyloid beta promotes depolarization of the synaptic membrane, excessive calcium influx and mitochondrial impairment.[68] Aggregations of the amyloid-beta peptide disrupt membranes in vitro.[69]
Intervention strategies
Researchers in Alzheimer's disease have identified several strategies as possible interventions against amyloid:[70]
- β-Secretase inhibitors. These work to block the first cleavage of APP inside of the cell, at the endoplasmic reticulum.
- γ-Secretase inhibitors (e. g. semagacestat). These work to block the second cleavage of APP in the cell membrane and would then stop the subsequent formation of Aβ and its toxic fragments.
- Selective Aβ42 lowering agents (e. g. tarenflurbil). These modulate γ-secretase to reduce Aβ42 production in favor of other (shorter) Aβ versions.
β- and γ-secretase are responsible for the generation of Aβ from the release of the intracellular domain of APP, meaning that compounds that can partially inhibit the activity of either β- and γ-secretase are highly sought after. In order to initiate partial inhibition of β- and γ-secretase, a compound is needed that can block the large active site of aspartyl proteases while still being capable of bypassing the blood-brain barrier. To date, human testing has been avoided due to concern that it might interfere with signaling via Notch proteins and other cell surface receptors.[citation needed]
- Immunotherapy. This stimulates the host immune system to recognize and attack Aβ, or provide antibodies that either prevent plaque deposition or enhance clearance of plaques or Aβ oligomers. Oligomerization is a chemical process that converts individual molecules into a chain consisting of a finite number of molecules. Prevention of oligomerization of Aβ has been exemplified by active or passive Aβ immunization. In this process antibodies to Aβ are used to decrease cerebral plaque levels. This is accomplished by promoting microglial clearance and/or redistributing the peptide from the brain to systemic circulation. Antibodies that target Aβ that currently in clinical trials included aducanumab, bapineuzumab, crenezumab, gantenerumab, gantenerumab, and solanezumab.[71][72] Beta-amyloid vaccines that are currently in clinical trials include CAD106 and UB-311.[71] However literature reviews have raised questions as to immunotherapy's overall efficacy. One such study assessing ten anti-Ab42 antibodies showed minimal cognitive protection and results within each trial, as symptoms were too far progressed by the time of application to be useful. Further development is still required for application to presymptomatic patients to assess their effectiveness early into disease progression.[73]
- Anti-aggregation agents[74] such as apomorphine, or carbenoxolone. The latter has commonly been used as a treatment for peptic ulcers, but also displays neuroprotective properties, shown to improve cognitive functions such as verbal fluency and memory consolidation. By binding with high affinity to Aβ42 fragments, primarily via hydrogen bonding, carbenoxolone captures the peptides before they can aggregate together, rendering them inert, as well as destabilizes those aggregates already formed, helping to clear them.[75] This is a common mechanism of action of anti-aggregation agents at large.[76]
- Studies comparing synthetic to recombinant Aβ42 in assays measuring rate of fibrillation, fibril homogeneity, and cellular toxicity showed that recombinant Aβ42 had a faster fibrillation rate and greater toxicity than synthetic amyloid beta 1-42 peptide.[77][78]
- Modulating cholesterol homeostasis has yielded results that show that chronic use of cholesterol-lowering drugs, such as the statins, is associated with a lower incidence of AD. In APP genetically modified mice, cholesterol-lowering drugs have been shown to reduce overall pathology. While the mechanism is poorly understood it appears that cholesterol-lowering drugs have a direct effect on APP processing.[79][80]
- Memantine is an Alzheimer's drug which has received widespread approval. It is a non-competitive N-methyl-D-aspartate (NMDA) channel blocker. By binding to the NMDA receptor with a higher affinity than Mg2+ ions, memantine is able to inhibit the prolonged influx of Ca2+ ions, particularly from extrasynaptic receptors, which forms the basis of neuronal excitotoxicity. It is an option for the management of patients with moderate to severe Alzheimer's Disease (modest effect). The study showed that 20 mg/day improved cognition, functional ability and behavioural symptoms in patient population.[81]
Measuring amyloid beta
Imaging compounds, notably Pittsburgh compound B, (6-OH-BTA-1, a thioflavin), can selectively bind to amyloid beta in vitro and in vivo. This technique, combined with PET imaging, is used to image areas of plaque deposits in Alzheimer's patients.[82]
Post mortem or in tissue biopsies
Amyloid beta can be measured semiquantitatively with immunostaining, which also allows one to determine location. Amyloid beta may be primarily vascular, as in cerebral amyloid angiopathy, or in senile plaques in white matter.[83]
One sensitive method is ELISA which is an immunosorbent assay which utilizes a pair of antibodies that recognize amyloid beta.[84][85]
Atomic force microscopy, which can visualize nanoscale molecular surfaces, can be used to determine the aggregation state of amyloid beta in vitro.[86]
Dual polarisation interferometry is an optical technique which can measure early stages of aggregation by measuring the molecular size and densities as the fibrils elongate.[87][88] These aggregate processes can also be studied on lipid bilayer constructs.[89]
Blood samples
New research has shown promise in testing whole blood samples for amyloid beta levels on the basis of electrical impedance. Interdigitated microelectrodes prepared with amyloid beta antibody measure differentiated impedance of flow in samples before and after antibody reactions to amyloid beta, comparing with normalization to account for regular variance between electrodes. When applied to control mice versus transgenic amyloid precursor protein/presenilin 1 mice (APP/PS1), strains could be differentiated via their differing amyloid beta levels.[90]
See also
References
- ↑ 1.0 1.1 Vivekanandan S, Brender JR, Lee SY, Ramamoorthy A (July 2011). "A partially folded structure of amyloid-beta(1-40) in an aqueous environment". Biochemical and Biophysical Research Communications. 411 (2): 312–6. doi:10.1016/j.bbrc.2011.06.133. PMC 3148408. PMID 21726530.
- ↑ Hamley IW (October 2012). "The Amyloid Beta Peptide: A Chemist's Perspective. Role in Alzheimer's and Fibrillization". Chemical Reviews. 112 (10): 5147–92. doi:10.1021/cr3000994. PMID 22813427.
- ↑ Haass C, Selkoe DJ (February 2007). "Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide". Nature Reviews. Molecular Cell Biology. 8 (2): 101–12. doi:10.1038/nrm2101. PMID 17245412.
- ↑ Nussbaum JM, Seward ME, Bloom GS (Jan–Feb 2013). "Alzheimer disease: a tale of two prions". Prion. 7 (1): 14–9. doi:10.4161/pri.22118. PMC 3609044. PMID 22965142.
- ↑ Pulawski W, Ghoshdastider U, Andrisano V, Filipek S (April 2012). "Ubiquitous amyloids". Applied Biochemistry and Biotechnology. 166 (7): 1626–43. doi:10.1007/s12010-012-9549-3. PMC 3324686. PMID 22350870.
- ↑ Tharp WG, Sarkar IN (April 2013). "Origins of amyloid-β". BMC Genomics. 14 (1): 290. doi:10.1186/1471-2164-14-290. PMC 3660159. PMID 23627794.
- ↑ Hiltunen M, van Groen T, Jolkkonen J (2009). "Functional roles of amyloid-beta protein precursor and amyloid-beta peptides: evidence from experimental studies". Journal of Alzheimer's Disease. 18 (2): 401–12. doi:10.3233/JAD-2009-1154. PMID 19584429.
- ↑ Sadigh-Eteghad S, Talebi M, Farhoudi M, EJ Golzari S, Sabermarouf B, Mahmoudi J (2014). "Beta-amyloid exhibits antagonistic effects on alpha 7 nicotinic acetylcholine receptors in orchestrated manner". Journal of Medical Hypotheses and Ideas. 8: 48–52. doi:10.1016/j.jmhi.2014.01.001.
- ↑ Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M (October 2003). "BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time". Neurobiology of Disease. 14 (1): 81–8. doi:10.1016/S0969-9961(03)00104-9. PMID 13678669.
- ↑ Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK (March 2004). "Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential". Biochimica et Biophysica Acta. 1697 (1–2): 89–101. doi:10.1016/j.bbapap.2003.11.016. PMID 15023353.
- ↑ Tabaton M, Zhu X, Perry G, Smith MA, Giliberto L (January 2010). "Signaling effect of amyloid-beta(42) on the processing of AβPP". Experimental Neurology. 221 (1): 18–25. doi:10.1016/j.expneurol.2009.09.002. PMC 2812589. PMID 19747481.
- ↑ Zou K, Gong JS, Yanagisawa K, Michikawa M (June 2002). "A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage". The Journal of Neuroscience. 22 (12): 4833–41. PMID 12077180.
- ↑ Baruch-Suchodolsky R, Fischer B (May 2009). "Aβ40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems". Biochemistry. 48 (20): 4354–70. doi:10.1021/bi802361k. PMID 19320465.
- ↑ Yao ZX, Papadopoulos V (October 2002). "Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity". FASEB Journal. 16 (12): 1677–9. doi:10.1096/fj.02-0285fje. PMID 12206998.
- ↑ Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG (August 2009). "Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes". Neuroscience. 162 (2): 328–38. doi:10.1016/j.neuroscience.2009.04.049. PMC 3083247. PMID 19401218.
- ↑ Maloney B, Lahiri DK (November 2011). "The Alzheimer's amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif". Gene. 488 (1–2): 1–12. doi:10.1016/j.gene.2011.06.004. PMC 3381326. PMID 21699964.
- ↑ Bailey JA, Maloney B, Ge YW, Lahiri DK (November 2011). "Functional activity of the novel Alzheimer's amyloid β-peptide interacting domain (AβID) in the APP and BACE1 promoter sequences and implications in activating apoptotic genes and in amyloidogenesis". Gene. 488 (1–2): 13–22. doi:10.1016/j.gene.2011.06.017. PMC 3372404. PMID 21708232.
- ↑ Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD (March 2010). Bush AI, ed. "The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide". PLOS One. 5 (3): e9505. Bibcode:2010PLoSO...5.9505S. doi:10.1371/journal.pone.0009505. PMC 2831066. PMID 20209079.
- ↑ Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (August 2012). "A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β". Science Translational Medicine. 4 (147): 147ra111. doi:10.1126/scitranslmed.3003748. PMC 3551275. PMID 22896675.
- ↑ Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (October 2013). "Sleep drives metabolite clearance from the adult brain". Science. 342 (6156): 373–7. Bibcode:2013Sci...342..373X. doi:10.1126/science.1241224. PMC 3880190. PMID 24136970.
- ↑ Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ (August 2015). "Clearance systems in the brain-implications for Alzheimer disease". Nature Reviews. Neurology. 11 (8): 457–70. doi:10.1038/nrneurol.2015.119. PMC 4694579. PMID 26195256.
- ↑ Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J (2014). "Amyloid-beta: a crucial factor in Alzheimer's disease". Medical Principles and Practice. 24 (1): 1–10. doi:10.1159/000369101. PMID 25471398.
- ↑ Parker MH, Reitz AB (2000). "Assembly of β-Amyloid Aggregates at the Molecular Level". Chemtracts-Organic Chemistry. 13 (1): 51–56.
- ↑ Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ (August 2008). "Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory". Nature Medicine. 14 (8): 837–42. doi:10.1038/nm1782. PMC 2772133. PMID 18568035. Lay summary – Fox News.
- ↑ Prelli F, Castaño E, Glenner GG, Frangione B (August 1988). "Differences between vascular and plaque core amyloid in Alzheimer's disease". Journal of Neurochemistry. 51 (2): 648–51. doi:10.1111/j.1471-4159.1988.tb01087.x. PMID 3292706.
- ↑ 26.0 26.1 Zhao LN, Long H, Mu Y, Chew LY (2012). "The toxicity of amyloid β oligomers". International Journal of Molecular Sciences. 13 (6): 7303–27. doi:10.3390/ijms13067303. PMC 3397527. PMID 22837695.
- ↑ Arispe N, Rojas E, Pollard HB (January 1993). "Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum". Proceedings of the National Academy of Sciences of the United States of America. 90 (2): 567–71. Bibcode:1993PNAS...90..567A. doi:10.1073/pnas.90.2.567. PMC 45704. PMID 8380642.
- ↑ Abramov AY, Canevari L, Duchen MR (December 2004). "Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture". Biochimica et Biophysica Acta. 8th European Symposium on Calcium. 1742 (1–3): 81–7. doi:10.1016/j.bbamcr.2004.09.006. PMID 15590058.
- ↑ Ekinci FJ, Linsley MD, Shea TB (March 2000). "Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation". Brain Research. Molecular Brain Research. 76 (2): 389–95. doi:10.1016/S0169-328X(00)00025-5. PMID 10762716.
- ↑ Pannuzzo M, Milardi D, Raudino A, Karttunen M, La Rosa C (June 2013). "Analytical model and multiscale simulations of Aβ peptide aggregation in lipid membranes: towards a unifying description of conformational transitions, oligomerization and membrane damage". Physical Chemistry Chemical Physics. 15 (23): 8940–51. Bibcode:2013PCCP...15.8940P. doi:10.1039/c3cp44539a. PMID 23588697.
- ↑ Pannuzzo M (June 2016). "On the physiological/pathological link between Aβ peptide, cholesterol, calcium ions and membrane deformation: A molecular dynamics study". Biochimica et Biophysica Acta. 1858 (6): 1380–9. doi:10.1016/j.bbamem.2016.03.018. PMID 27003127.
- ↑ 32.0 32.1 32.2 Ghiso J, Frangione B (December 2002). "Amyloidosis and Alzheimer's disease". Advanced Drug Delivery Reviews. 54 (12): 1539–51. doi:10.1016/S0169-409X(02)00149-7. PMID 12453671.
- ↑ 33.0 33.1 Selkoe DJ (October 2001). "Clearing the brain's amyloid cobwebs". Neuron. 32 (2): 177–80. doi:10.1016/S0896-6273(01)00475-5. PMID 11683988.
- ↑ Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M (September 1998). "Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau". Nature Neuroscience. 1 (5): 355–8. doi:10.1038/1565. PMID 10196523.
- ↑ Roses AD (February 1998). "Alzheimer diseases: a model of gene mutations and susceptibility polymorphisms for complex psychiatric diseases". American Journal of Medical Genetics. 81 (1): 49–57. doi:10.1002/(SICI)1096-8628(19980207)81:1<49::AID-AJMG10>3.0.CO;2-W. PMID 9514588.
- ↑ Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R (May 2002). "Alzheimer's beta-amyloid peptides compete for insulin binding to the insulin receptor" (PDF). The Journal of Neuroscience. 22 (10): RC221. doi:10.1523/JNEUROSCI.22-10-j0001.2002. PMID 12006603.
- ↑ Ray WJ, Yao M, Mumm J, Schroeter EH, Saftig P, Wolfe M, Selkoe DJ, Kopan R, Goate AM (December 1999). "Cell surface presenilin-1 participates in the gamma-secretase-like proteolysis of Notch". The Journal of Biological Chemistry. 274 (51): 36801–7. doi:10.1074/jbc.274.51.36801. PMID 10593990.
- ↑ Roberts SB (December 2002). "Gamma-secretase inhibitors and Alzheimer's disease". Advanced Drug Delivery Reviews. 54 (12): 1579–88. doi:10.1016/S0169-409X(02)00155-2. PMID 12453675.
- ↑ Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (October 1999). "Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE". Science. 286 (5440): 735–41. doi:10.1126/science.286.5440.735. PMID 10531052.
- ↑ Vassar R (December 2002). "Beta-secretase (BACE) as a drug target for Alzheimer's disease". Advanced Drug Delivery Reviews. 54 (12): 1589–602. doi:10.1016/S0169-409X(02)00157-6. PMID 12453676.
- ↑ Zlokovic BV, Frangione B (2003). Transport-clearance hypothesis for Alzheimer's disease and potential therapeutic implications. Landes Bioscience. pp. 114–122.
- ↑ Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (June 1985). "Amyloid plaque core protein in Alzheimer disease and Down syndrome". Proceedings of the National Academy of Sciences of the United States of America. 82 (12): 4245–9. Bibcode:1985PNAS...82.4245M. doi:10.1073/pnas.82.12.4245. PMC 397973. PMID 3159021.
- ↑ Castaño EM, Prelli F, Soto C, Beavis R, Matsubara E, Shoji M, Frangione B (December 1996). "The length of amyloid-beta in hereditary cerebral hemorrhage with amyloidosis, Dutch type. Implications for the role of amyloid-beta 1-42 in Alzheimer's disease". The Journal of Biological Chemistry. 271 (50): 32185–91. doi:10.1074/jbc.271.50.32185. PMID 8943274.
- ↑ Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ (November 1993). "beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease". Proceedings of the National Academy of Sciences of the United States of America. 90 (22): 10836–40. Bibcode:1993PNAS...9010836R. doi:10.1073/pnas.90.22.10836. PMC 47873. PMID 8248178.
- ↑ Shinkai Y, Yoshimura M, Ito Y, Odaka A, Suzuki N, Yanagisawa K, Ihara Y (September 1995). "Amyloid beta-proteins 1-40 and 1-42(43) in the soluble fraction of extra- and intracranial blood vessels". Annals of Neurology. 38 (3): 421–8. doi:10.1002/ana.410380312. PMID 7668828.
- ↑ Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS (December 2001). "Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP". Nature. 414 (6864): 643–8. doi:10.1038/414643a. PMID 11740561.
- ↑ Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J (September 1999). "Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease". The American Journal of Pathology. 155 (3): 853–62. doi:10.1016/S0002-9440(10)65184-X. PMC 1866907. PMID 10487842.
- ↑ Schirinzi T, Di Lazzaro G, Sancesario GM, Colona VL, Scaricamazza E, Mercuri NB, Martorana A, Sancesario G (December 2017). "Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer's disease". Journal of Neural Transmission. 124 (12): 1621–1625. doi:10.1007/s00702-017-1786-8. PMID 28866757.
- ↑ Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (April 2003). "Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis". Science. 300 (5618): 486–9. Bibcode:2003Sci...300..486K. doi:10.1126/science.1079469. PMID 12702875.
- ↑ Gandy S, Simon AJ, Steele JW, Lublin AL, Lah JJ, Walker LC, Levey AI, Krafft GA, Levy E, Checler F, Glabe C, Bilker WB, Abel T, Schmeidler J, Ehrlich ME (August 2010). "Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers". Annals of Neurology. 68 (2): 220–30. doi:10.1002/ana.22052. PMC 3094694. PMID 20641005. Lay summary – Drug Discovery and Development.
- ↑ Jin WS, Bu XL, Liu YH, Shen LL, Zhuang ZQ, Jiao SS, Zhu C, Wang QH, Zhou HD, Zhang T, Wang YJ (February 2017). "Plasma Amyloid-Beta Levels in Patients with Different Types of Cancer". Neurotoxicity Research. 31 (2): 283–288. doi:10.1007/s12640-016-9682-9. PMID 27913965.
- ↑ Lim S, Yoo BK, Kim HS, Gilmore HL, Lee Y, Lee HP, Kim SJ, Letterio J, Lee HG (December 2014). "Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer". BMC Cancer. 14: 928. doi:10.1186/1471-2407-14-928. PMID 25491510.
- ↑ Hartley SL, Handen BL, Devenny D, Mihaila I, Hardison R, Lao PJ, Klunk WE, Bulova P, Johnson SC, Christian BT (October 2017). "Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome". Neurobiology of Aging. 58: 68–76. doi:10.1016/j.neurobiolaging.2017.05.019. PMID 28715661.
- ↑ Olsson F, Schmidt S, Althoff V, Munter LM, Jin S, Rosqvist S, Lendahl U, Multhaup G, Lundkvist J (January 2014). "Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions". The Journal of Biological Chemistry. 289 (3): 1540–50. doi:10.1074/jbc.M113.498246. PMC 3894335. PMID 24225948.
- ↑ Hartmann T, Bieger SC, Brühl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K (September 1997). "Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides". Nature Medicine. 3 (9): 1016–20. doi:10.1038/nm0997-1016. PMID 9288729.
- ↑ Yin YI, Bassit B, Zhu L, Yang X, Wang C, Li YM (August 2007). "{gamma}-Secretase Substrate Concentration Modulates the Abeta42/Aβ40 Ratio: IMPLICATIONS FOR ALZHEIMER DISEASE". The Journal of Biological Chemistry. 282 (32): 23639–44. doi:10.1074/jbc.M704601200. PMID 17556361.
- ↑ Kikuchi K, Kidana K, Tatebe T, Tomita T (December 2017). "Dysregulated Metabolism of the Amyloid-β Protein and Therapeutic Approaches in Alzheimer Disease". Journal of Cellular Biochemistry. 118 (12): 4183–4190. doi:10.1002/jcb.26129. PMID 28488760.
- ↑ Nalivaeva NN, Belyaev ND, Zhuravin IA, Turner AJ (2012). "The Alzheimer's amyloid-degrading peptidase, neprilysin: can we control it?". International Journal of Alzheimer's Disease. 2012: 383796. doi:10.1155/2012/383796. PMC 3412116. PMID 22900228.
- ↑ "2008 Alzheimer's disease facts and figures". Alzheimer's & Dementia. 4 (2): 110–33. March 2008. doi:10.1016/j.jalz.2008.02.005. PMID 18631956.
- ↑ Glenner GG, Wong CW (August 1984). "Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein". Biochemical and Biophysical Research Communications. 122 (3): 1131–5. doi:10.1016/0006-291X(84)91209-9. PMID 6236805.
- ↑ Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, Lu Y, Felix AM, Maggio JE, Lee JP (June 2000). "The Alzheimer's peptide a beta adopts a collapsed coil structure in water". Journal of Structural Biology. 130 (2–3): 130–41. doi:10.1006/jsbi.2000.4288. PMID 10940221.
- ↑ Yang M, Teplow DB (December 2008). "Amyloid beta-protein monomer folding: free-energy surfaces reveal alloform-specific differences". Journal of Molecular Biology. 384 (2): 450–64. doi:10.1016/j.jmb.2008.09.039. PMC 2673916. PMID 18835397.
- ↑ Sgourakis NG, Merced-Serrano M, Boutsidis C, Drineas P, Du Z, Wang C, Garcia AE (January 2011). "Atomic-level characterization of the ensemble of the Aβ(1-42) monomer in water using unbiased molecular dynamics simulations and spectral algorithms". Journal of Molecular Biology. 405 (2): 570–83. doi:10.1016/j.jmb.2010.10.015. PMC 3060569. PMID 21056574.
- ↑ Sgourakis NG, Yan Y, McCallum SA, Wang C, Garcia AE (May 2007). "The Alzheimer's peptides Aβ40 and 42 adopt distinct conformations in water: a combined MD / NMR study". Journal of Molecular Biology. 368 (5): 1448–57. doi:10.1016/j.jmb.2007.02.093. PMC 1978067. PMID 17397862.
- ↑ Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO (May 2010). "Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils". Nature Structural & Molecular Biology. 17 (5): 561–7. doi:10.1038/nsmb.1799. PMC 2922021. PMID 20383142.
- ↑ Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, Miesbauer L, Solomon L, Bartley D, Walter K, Johnson RW, Hajduk PJ, Olejniczak ET (March 2009). "Structural characterization of a soluble amyloid beta-peptide oligomer". Biochemistry. 48 (9): 1870–7. doi:10.1021/bi802046n. PMID 19216516.
- ↑ Strodel B, Lee JW, Whittleston CS, Wales DJ (September 2010). "Transmembrane structures for Alzheimer's Aβ(1-42) oligomers". Journal of the American Chemical Society. 132 (38): 13300–12. doi:10.1021/ja103725c. PMID 20822103.
- ↑ Mattson MP (August 2004). "Pathways towards and away from Alzheimer's disease". Nature. 430 (7000): 631–9. Bibcode:2004Natur.430..631M. doi:10.1038/nature02621. PMC 3091392. PMID 15295589.
- ↑ Flagmeier P, De S, Wirthensohn DC, Lee SF, Vincke C, Muyldermans S, Knowles TP, Gandhi S, Dobson CM, Klenerman D (June 2017). "2+ Influx into Lipid Vesicles Induced by Protein Aggregates". Angewandte Chemie. 56 (27): 7750–7754. doi:10.1002/anie.201700966. PMID 28474754.
- ↑ Citron M (September 2004). "Strategies for disease modification in Alzheimer's disease". Nature Reviews. Neuroscience. 5 (9): 677–85. doi:10.1038/nrn1495. PMID 15322526.
- ↑ 71.0 71.1 Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K (September 2017). "Alzheimer's disease drug development pipeline: 2017". review. Alzheimer's & Dementia. 3 (3): 367–384. doi:10.1016/j.trci.2017.05.002. PMC 5651419. PMID 29067343.
- ↑ Schilling S, Rahfeld JU, Lues I, Lemere CA (May 2018). "Passive Aβ Immunotherapy: Current Achievements and Future Perspectives". review. Molecules. 23 (5). doi:10.3390/molecules23051068. PMID 29751505.
- ↑ Wang Y, Yan T, Lu H, Yin W, Lin B, Fan W, Zhang X, Fernandez-Funez P (2017). "Lessons from Anti-Amyloid-β Immunotherapies in Alzheimer Disease: Aiming at a Moving Target". review. Neuro-Degenerative Diseases. 17 (6): 242–250. doi:10.1159/000478741. PMID 28787714.
- ↑ Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ (November 2002). "New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer's disease". The Journal of Biological Chemistry. 277 (45): 42881–90. doi:10.1074/jbc.M206593200. PMID 12167652.
- ↑ Sharma S, Nehru B, Saini A (September 2017). "Inhibition of Alzheimer's amyloid-beta aggregation in-vitro by carbenoxolone: Insight into mechanism of action". primary. Neurochemistry International. 108: 481–493. doi:10.1016/j.neuint.2017.06.011. PMID 28652220.
- ↑ Parker MH, Chen R, Conway KA, Lee DH, Luo C, Boyd RE, Nortey SO, Ross TM, Scott MK, Reitz AB (November 2002). "Synthesis of (-)-5,8-dihydroxy-3R-methyl-2R-(dipropylamino)-1,2,3,4-tetrahydronaphthalene: an inhibitor of beta-amyloid(1-42) aggregation". Bioorganic & Medicinal Chemistry. 10 (11): 3565–9. doi:10.1016/S0968-0896(02)00251-1. PMID 12213471.
- ↑ Finder VH, Vodopivec I, Nitsch RM, Glockshuber R (February 2010). "The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42". Journal of Molecular Biology. 396 (1): 9–18. doi:10.1016/j.jmb.2009.12.016. PMID 20026079.
- ↑ "State of aggregation". Nature Neuroscience. 14 (4): 399. April 2011. doi:10.1038/nn0411-399. PMID 21445061.
- ↑ Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE (October 2001). "A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease". Neurobiology of Disease. 8 (5): 890–9. doi:10.1006/nbdi.2001.0422. PMID 11592856.
- ↑ Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY (May 2002). "Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice". Proceedings of the National Academy of Sciences of the United States of America. 99 (11): 7705–10. Bibcode:2002PNAS...99.7705L. doi:10.1073/pnas.092034699. PMC 124328. PMID 12032347.
- ↑ Schneider JS, Pioli EY, Jianzhong Y, Li Q, Bezard E (April 2013). "Effects of memantine and galantamine on cognitive performance in aged rhesus macaques". Neurobiology of Aging. 34 (4): 1126–32. doi:10.1016/j.neurobiolaging.2012.10.020. PMID 23158762.
- ↑ Heurling K, Leuzy A, Zimmer ER, Lubberink M, Nordberg A (February 2016). "Imaging β-amyloid using [(18)F]flutemetamol positron emission tomography: from dosimetry to clinical diagnosis". European Journal of Nuclear Medicine and Molecular Imaging. 43 (2): 362–73. doi:10.1007/s00259-015-3208-1. PMID 26440450.
- ↑ Ito H, Shimada H, Shinotoh H, Takano H, Sasaki T, Nogami T, Suzuki M, Nagashima T, Takahata K, Seki C, Kodaka F, Eguchi Y, Fujiwara H, Kimura Y, Hirano S, Ikoma Y, Higuchi M, Kawamura K, Fukumura T, Böö ÉL, Farde L, Suhara T (June 2014). "Quantitative Analysis of Amyloid Deposition in Alzheimer Disease Using PET and the Radiotracer ¹¹C-AZD2184". Journal of Nuclear Medicine. 55 (6): 932–8. doi:10.2967/jnumed.113.133793. PMID 24732152.
- ↑ Schmidt SD, Nixon RA, Mathews PM (2012). "Tissue processing prior to analysis of Alzheimer's disease associated proteins and metabolites, including Aβ". Methods in Molecular Biology. Methods in Molecular Biology. 849: 493–506. doi:10.1007/978-1-61779-551-0_33. ISBN 978-1-61779-550-3. PMID 22528111.
- ↑ Schmidt SD, Mazzella MJ, Nixon RA, Mathews PM (2012). "Aβ measurement by enzyme-linked immunosorbent assay". Methods in Molecular Biology. Methods in Molecular Biology. 849: 507–27. doi:10.1007/978-1-61779-551-0_34. ISBN 978-1-61779-550-3. PMID 22528112.
- ↑ Stine WB, Dahlgren KN, Krafft GA, LaDu MJ (March 2003). "In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis". The Journal of Biological Chemistry. 278 (13): 11612–22. doi:10.1074/jbc.M210207200. PMID 12499373.
- ↑ Gengler S, Gault VA, Harriott P, Hölscher C (June 2007). "Impairments of hippocampal synaptic plasticity induced by aggregated beta-amyloid (25-35) are dependent on stimulation-protocol and genetic background". Experimental Brain Research. 179 (4): 621–30. doi:10.1007/s00221-006-0819-6. PMID 17171334.
- ↑ Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA (December 2007). "Monitoring the prevention of amyloid fibril formation by alpha-crystallin. Temperature dependence and the nature of the aggregating species". The FEBS Journal. 274 (24): 6290–304. doi:10.1111/j.1742-4658.2007.06144.x. PMID 18005258.
- ↑ Sanghera N, Swann MJ, Ronan G, Pinheiro TJ (October 2009). "Insight into early events in the aggregation of the prion protein on lipid membranes". Biochimica et Biophysica Acta. 1788 (10): 2245–51. doi:10.1016/j.bbamem.2009.08.005. PMID 19703409.
- ↑ Yoo YK, Kim J, Kim G, Kim YS, Kim HY, Lee S, Cho WW, Kim S, Lee SM, Lee BC, Lee JH, Hwang KS (August 2017). "A highly sensitive plasma-based amyloid-β detection system through medium-changing and noise cancellation system for early diagnosis of the Alzheimer's disease". Scientific Reports. 7 (1): 8882. doi:10.1038/s41598-017-09370-3. PMID 28827785.
External links
|
Amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Amyloid beta On the Web |
|
American Roentgen Ray Society Images of Amyloid beta |