Aldehyde
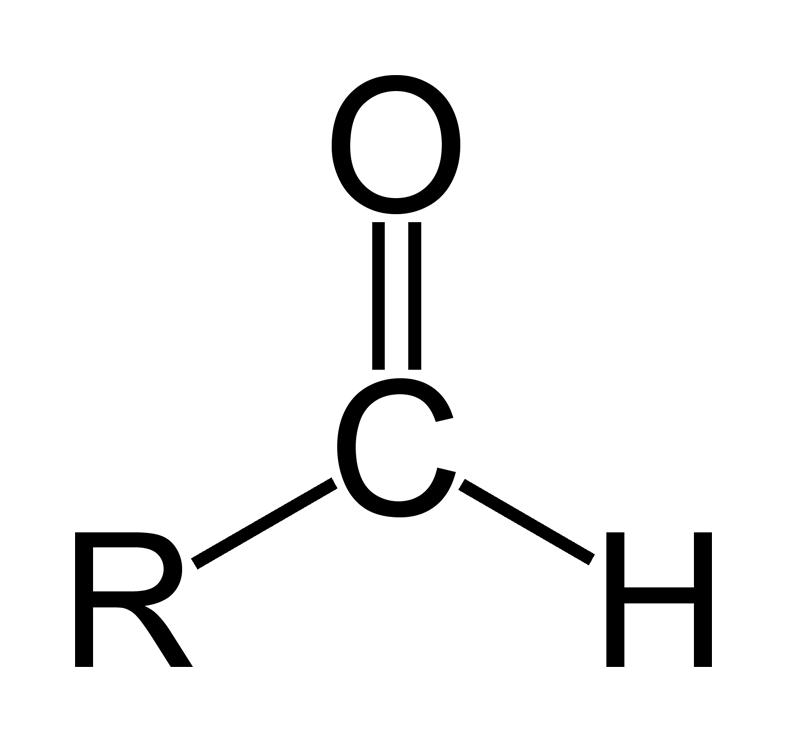
-R is the group attached to the aldehyde group.
|
WikiDoc Resources for Aldehyde |
|
Articles |
|---|
|
Most recent articles on Aldehyde |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Aldehyde at Clinical Trials.gov Clinical Trials on Aldehyde at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Aldehyde
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Aldehyde Risk calculators and risk factors for Aldehyde
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Aldehyde |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
An aldehyde is an organic compound containing a terminal carbonyl group. This functional group, which consists of a carbon atom which is bonded to a hydrogen atom and double-bonded to an oxygen atom (chemical formula O=CH-), is called the aldehyde group. The aldehyde group is also called the formyl or methanoyl group.
The word aldehyde seems to have arisen from alcohol dehydrogenated. In the past, aldehydes were sometimes named after the corresponding alcohols, for example vinous aldehyde for acetaldehyde. (Vinous is from Latin vinum = wine, the traditional source of ethanol; compare vinyl.)
The aldehyde group is polar. Oxygen, more electronegative than carbon, pulls the electrons in the carbon-oxygen bond towards itself, creating an electron deficiency at the carbon atom.
Owing to resonance stabilization of the conjugate base, an α-hydrogen in an aldehyde is more acidic than a hydrogen atom in an alkane, with a typical pKa of 17.
Nomenclature
IUPAC names for aldehydes

IUPAC prescribes the following nomenclature for aldehydes:[1][2][3]
- Acyclic aliphatic aldehydes are named as derivatives of the longest carbon chain containing the aldehyde group. Thus, HCHO is named as a derivative of methane, and CH3CH2CH2CHO is named as a derivative of butane. The name is formed by changing the suffix -e of the parent alkane to -al, so that HCHO is named methanal, and CH3CH2CH2CHO is named butanal.
- In other cases, such as when a -CHO group is attached to a ring, the suffix -carbaldehyde may be used. Thus, C6H11CHO is known as cyclohexanecarbaldehyde. If the presence of another functional group demands the use of a suffix, the aldehyde group is named with the prefix formyl-. This prefix is preferred to methanoyl-.
- If the compound is a natural product or a carboxylic acid, the prefix oxo- may be used to indicate which carbon atom is part of the aldehyde group; for example, CHOCH2COOH is named 3-oxopropanoic acid.
- If replacing the aldehyde group with a carboxyl (-COOH) group would yield a carboxylic acid with a trivial name, the aldehyde may be named by replacing the suffix -ic acid or -oic acid in this trivial name by -aldehyde. For example:
- HCHO may be called formaldehyde.
- CH3CHO may be called acetaldehyde.
- C6H5CHO may be called benzaldehyde.
Other nomenclature
The carbon atom adjacent to a carbonyl group is called the α carbon. Carbon atoms further away from the group may be named β for the carbon atom bonded to the α carbon, γ for the next, and so on. Hydrogen atoms bonded to these carbon atoms are named likewise: an α hydrogen is a hydrogen atom bonded to the α carbon and so on.
A reaction that introduces an aldehyde group is known as a formylation reaction.
Chemistry
Synthesis
There are several methods for preparing aldehydes:
- Reacting a primary alcohol with an oxidizing agent. In the laboratory this may be achieved by heating the alcohol with a chromium(VI) reagent an acidified solution of potassium dichromate, which is reduced to green Cr3+ during the reaction. Excess dichromate will further oxidise the aldehyde to form a carboxylic acid, so either the aldehyde is distilled out as it forms (if volatile), or milder methods and reagents such as PCC oxidation, IBX acid, Dess-Martin periodinane or Swern oxidation are used. The reaction is illustrated below with propan-1-ol being oxidised to form propionaldehyde, and again with pentan-1-ol being oxidized to form pentanal.
- Reacting an alkene (if there is a vinylic hydrogen) with ozone will form an ozonide (an unstable, explosive intermediate) which yields an aldehyde upon reduction with zinc and acid at reduced temperatures. This process is called ozonolysis.
- Reacting an ester with diisobutyl aluminium hydride (DIBAL-H) or sodium aluminium hydride can cause reduction, yielding an aldehyde.
- Reduction of an acid chloride using the Rosenmund reduction or using lithium tri-t-butoxyaluminium hydride (LiAlH(O-t-C4H9)3).
- Reaction of ketones with methoxymethylenetriphenylphosphine in a modified Wittig reaction.
- Various formylation reactions, such as the Vilsmeier-Haack reaction, can be used to introduce an aldehyde group.
- In the Nef reaction, aldehydes form by hydrolysis of salts of primary nitro compounds.
- Zincke aldehydes form by reaction of pyridinium salts with secondary amines followed by hydrolysis.
- In the Stephen aldehyde synthesis aldehydes form from nitriles, tin(II) chloride and hydrochloric acid.
Keto-enol tautomerism
Aldehydes can exist in either the keto or enol tautomers. Keto-enol tautomerism is catalyzed by either acid or base.
Common reactions
Reduction and oxidation
- The aldehyde group can be reduced to the group -CH2OH, changing the aldehyde into a primary alcohol.
- The aldehyde group can be oxidized to the group -COOH, yielding a carboxylic acid. Suitable oxidizing agents include potassium permanganate, nitric acid, chromium(VI) oxide, and acidified potassium dichromate.
- Another oxidation reaction is the silver mirror test. In this test, an aldehyde is treated with Tollens' reagent, which is prepared by adding a drop of sodium hydroxide solution into silver nitrate solution to give a precipitate of silver(I) oxide, and then adding just enough dilute ammonia solution to redissolve the precipitate in aqueous ammonia to produce [Ag(NH3)2]+ complex. This reagent will convert aldehydes to carboxylic acids without attacking carbon-carbon double-bonds. The name silver mirror test arises because this reaction will produce a precipitate of silver whose presence can be used to test for the presence of an aldehyde.
Nucleophilic addition reactions
In nucleophilic addition reactions a nucleophile can add to the carbon atom in the carbonyl group, yielding an addition compound where this carbon atom has tetrahedral molecular geometry. Together with protonation of the oxygen atom in the carbonyl group (which can take place either before or after addition), this yields a product where the carbon atom in the carbonyl group is bonded to the nucleophile, a hydrogen atom, and a hydroxyl group.
In many cases, a water molecule is removed after the addition takes place; in this case, the reaction is classed as an addition-elimination or addition-condensation reaction.
There are various examples of nucleophilic addition reactions.
- In the acetalisation reaction, under acidic or basic conditions, an alcohol adds to the carbonyl group and a proton is transferred to form a hemiacetal. Under acidic conditions, the hemiacetal and the alcohol can further react to form an acetal and water. Simple hemiacetals are usually unstable, although cyclic ones such as glucose can be stable. Acetals are stable, but revert to the aldehyde in the presence of acid.
- Aldehydes can react with water (under acidic or basic conditions) to form hydrates, R-C(H)(OH)(OH), although these are only stable when strong electron withdrawing groups are present, as in chloral hydrate. The mechanism is identical to hemiacetal formation.
- In alkylimino-de-oxo-bisubstitution, a primary or secondary amine adds to the carbonyl group and a proton is transferred from the nitrogen to the oxygen atom to create a carbinolamine. In the case of a primary amine, a water molecule can be eliminated from the carbinolamine to yield an imine. This reaction is catalyzed by acid.
- The cyano group in HCN can add to the carbonyl group to form cyanohydrins, R-C(H)(OH)(CN).
- In the Grignard reaction, a Grignard reagent adds to the group, eventually yielding an alcohol with a substituted group from the Grignard reagent.
- In the aldol reaction, the metal enolates of ketones, esters, amides and carboxylic acids will add to aldehydes to form β-hydroxycarbonyl compounds (aldols). Acid or base-catalyzed dehydration will then lead to α,β-unsaturated carbonyl compounds. The combination of these two steps is known as the aldol condensation.
- Hydroxylamine (NH2OH) can add to the carbonyl group. After the elimination of water, this will result in an oxime.
- An ammonia derivative of the form H2NNR2 such as hydrazine (H2NNH2) or 2,4-dinitrophenylhydrazine can add to the carbonyl group. After the elimination of water, this will result in the formation of a hydrazone. This forms the basis of a test for aldehydes and ketones.
More complex reactions
- If an aldehyde is converted to a simple hydrazone (RCH=NHNH2) and this is heated with a base such as KOH, the terminal carbon is fully reduced via the Wolff-Kishner reaction to a methyl group. The Wolff-Kishner reaction may be performed as a one-pot reaction, giving the overall conversion RCH=O → RCH3.
- Reaction of aldehydes with reducing agents such as magnesium gives diols in a Pinacol coupling reaction.
- The Wittig reaction takes aldehydes to alkenes and the Corey-Fuchs reaction takes aldehydes to alkynes. Both use a triphenylphosphine reagent. The Corey-Chaykovsky reagent is a sulfonium ylide which converts aldehydes to epoxides.
Examples of aldehydes
- Methanal (Formaldehyde)
- Ethanal (Acetaldehyde)
- Propanal (Propionaldehyde)
- Butanal (butyraldehyde)
- Glucose
- Benzaldehyde
- Cinnamaldehyde
Related compounds
Other kinds of organic compounds containing carbonyl groups include
References
- ↑ Short Summary of IUPAC Nomenclature of Organic Compounds, web page, University of Wisconsin Colleges, accessed on line August 4, 2007.
- ↑ §R-5.6.1, Aldehydes, thioaldehydes, and their analogues, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
- ↑ §R-5.7.1, Carboxylic acids, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
ar:ألدهيد bg:Алдехид cs:Aldehydy da:Aldehyd de:Aldehyde et:Aldehüüdid eo:Aldehido fa:آلدهید fo:Aldehyd ko:알데하이드 id:Alkanal it:Aldeidi he:אלדהיד la:Aldehydum lv:Aldehīdi mk:Алдехид nl:Aldehyde no:Aldehyd nn:Aldehyd sk:Aldehyd sr:Алдехид su:Aldehida fi:Aldehydi sv:Aldehyd uk:Альдегіди