Chemical polarity
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
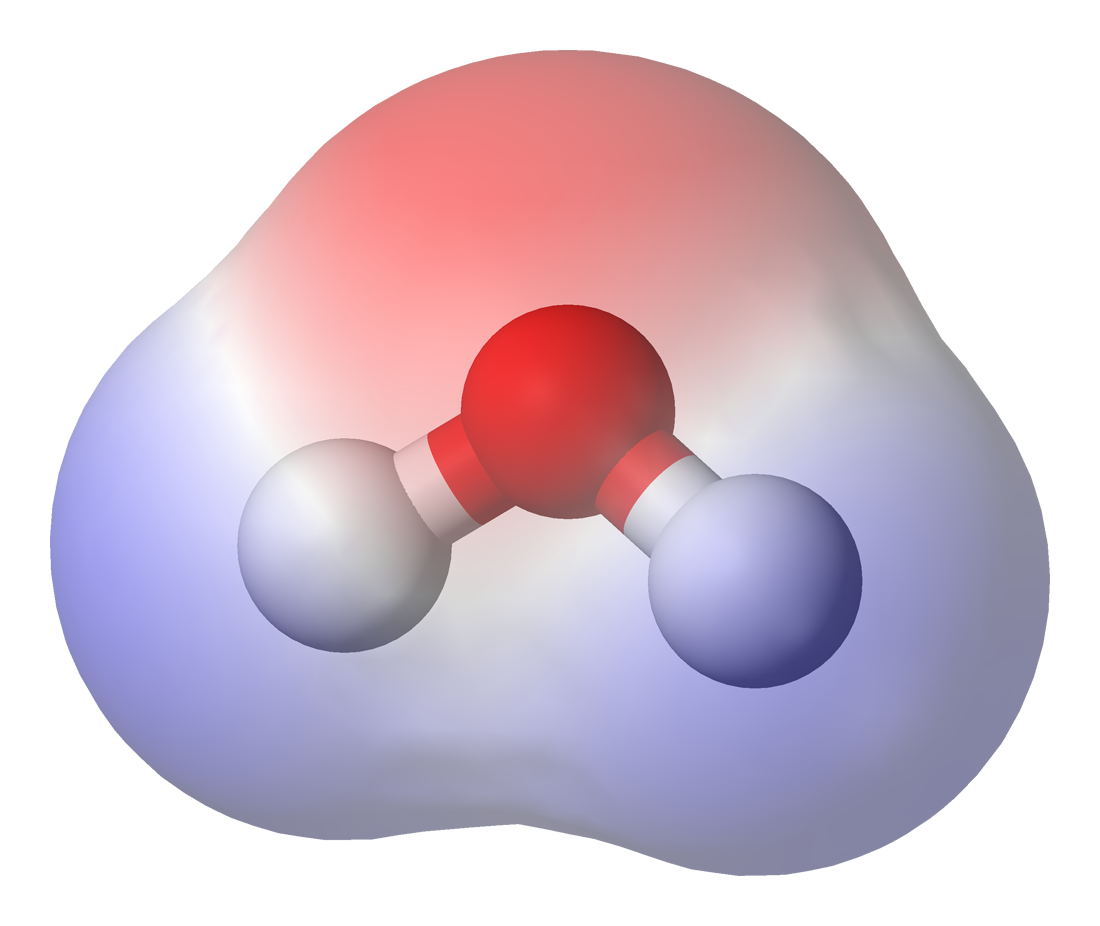
Chemical polarity, also known as bond polarity or simply polarity, is a concept in chemistry which describes how equally bonding electrons are shared between atoms. It is a physical property of compounds and affects other physical properties such as solubility, melting points and boiling points. Polarity also affects intermolecular forces, leading to some compounds or molecules within compounds being labelled as polar or non-polar.
Polarity refers to the dipole-dipole intermolecular forces between the slightly positively-charged end of one molecule to the negative end of another or the same molecule. Molecular polarity is dependent on the difference in electronegativity between atoms in a compound and the asymmetry of the compound's structure. For example, water is thought to be polar because of the uneven sharing of its electrons. However, methane is considered non-polar because the carbon shares the hydrogen molecules uniformally.
Theory
Electrons are not always shared equally between two bonding atoms: one atom might exert more of a force on the electron cloud than the other. This "pull" is termed electronegativity and measures the attraction for electrons a particular atom has. The unequal sharing of electrons within a bond leads to the formation of an electric dipole: a separation of positive and negative electric charge.
Atoms with high electronegativities - such as fluorine, oxygen, and nitrogen - exert a greater pull on electrons than atoms with lower electronegativities. In a bonding situation this can lead to unequal sharing of electrons between atoms as electrons will spend more time closer to the atom with the higher electronegativity.
Bonds can fall between one of two extremes - being completely non-polar or completely polar. A completely non-polar bond occurs when the electronegativities are identical and therefore possess a difference of zero. A completely polar bond is more correctly termed ionic bonding and occurs when the difference between electronegativities is large enough that one atom takes an electron from the other. The terms "polar" and "non-polar" bonds usually refer to covalent bonds. To determine the polarity of a covalent bond using numerical means, the difference between the electronegativity of the atoms is taken, if the result is between 0.5 and 2 then, generally, the bond is polar.
Polarity of molecules
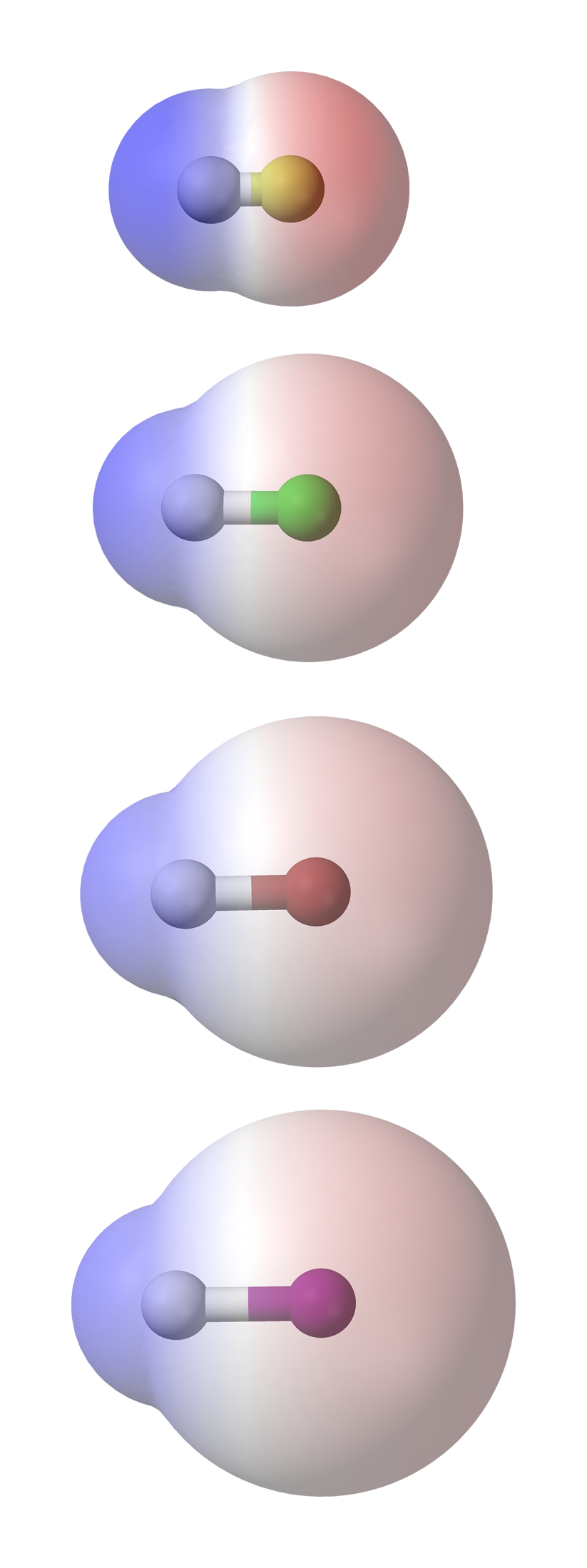
A compound is composed of one or more chemical bonds between different atoms. The polarity of each bond within the compound may determine the overall polarity of the compound: how polar or non-polar it is.
A polar molecule may be polar as a result of polar bonds or as a result of an asymmetric arrangement of non-polar bonds and non bonding pairs of electrons.
Example 1. A polar molecule by virtue of polar bonds (bonds which have unequal sharing of electrons between the two atoms involved in bonding), e.g hydrogen fluoride, HF, where the bonding pair of electron is displaced towards the more electronegative fluorine atom.
Example 2. In ammonia, NH3, the three N-H bonds have only a slight polarity (towards the more electronegative nitrogen atom), however the lone pair of electrons (pointing towards the fourth apex of the approximate tetrahedron, (VSEPR) is electron rich and results in a powerful dipole across the whole ammonia molecule.
A non-polar compound may be non polar because there is (almost) no polarity in the bonds or because of the symmetrical arrangement of polar bonds.
Example 3. Methane, CH4 The four C-H bonds, arranged tetrahedrally around the carbon atom, have very little polarity in the bonds and so there is no dipole in the molecule.
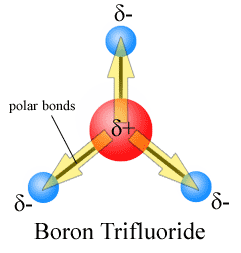
Example 4. BF3, boron trifluoride has a trigonal planar arrangement of three polar bonds at 120o This results in no overall dipole in the molecule.
Properties and examples
While molecules can be described as "polar," "non-polar," or "semi-polar," it must be noted that this is often a relative term, with one molecule simply being more polar or more non-polar than another. As such, there are no ultimate properties which can be ascribed to polar or non-polar molecules. However, the following properties are typical of such molecules.
Polar molecules
Examples of household polar molecules include ammonia and sugar (glucose). Polar molecules are generally able to dissolve in water (hydrophilic) due to the polar nature of water - like dissolves like. Polar molecules have slightly positive and slightly negatively charged ends.
Non-polar molecules
Examples of household non-polar compounds include fats, oil and petrol. Most non-polar molecules are water insoluble (hydrophobic) at room temperature. However many non-polar organic solvents, such as turpentine, are able to dissolve non-polar substances - like dissolves like.
When comparing a polar and non-polar molecule with similar molar masses, the polar molecule generally has a higher boiling point, because of the dipole-dipole interaction between their molecules.
Predicting molecule polarity
| Formula | Description | Example | |
| Polar | AB | Linear Molecules | CO |
|---|---|---|---|
| HAx | Molecules with a single H | HCl | |
| AxOH | Molecules with an OH at one end | C2H5OH | |
| OxAy | Molecules with an O at one end | H2O | |
| NxAy | Molecules with an N at one end | NH3 | |
| Nonpolar | Ax | All elements | O2 |
| CxAy | Most carbon compounds | CO2 |
Electronegativity difference predictions
Non-polar covalent bond: 0.0-0.4
Slightly polar bond: 0.5-0.9
Moderately polar bond: 1-1.3
Highly polar bond: 1.4-1.7
Slightly ionic bond: 1.8-2.2
Ionic Bond: 2.3+
See also
- Solubility
- Emulsion
- Detergent
- Dipole
- Covalent bond
- Electronegativity
- Dielectric
- Chemical bonding
de:Polarität (Chemie) he:קוטביות nl:Apolaire verbinding fi:Poolisuus